Abstract
The purpose of this study was to explore epigenetic changes and functions of SOX17 in human lung cancer. Five lung cancer cell lines and 88 primary lung cancer samples were examined in this study. Methylation-specific polymerase chain reaction (MSP), semi-quantitative reverse-transcription PCR, immunohistochemistry, luciferase reporter assays, colony-formation assays, and western blotting were used to analyze methylation changes and functions of SOX17 in lung cancer. SOX17 methylation was found in 60.2% of primary human lung cancer samples, and promoter region methylation of SOX17 silenced its expression. SOX17 methylation was associated with female patients and lung cancer differentiation. Colony-formation assays revealed that SOX17 suppressed lung cancer cell proliferation. Re-expression of SOX17 inhibited Wnt signaling in H23 lung cancer cell line. SOX17 acts as a Wnt signaling inhibitor.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide among both men and women (Sullivan et al., 2005; Suemitsu et al., 2008). Lung cancer comprises two major histological groups: non-small cell lung cancer (NSCLC) and small cell lung cancer, which account for 80.4% and 16.8% of cases, respectively (Travis et al., 1995). More than 60% of lung cancer patients are diagnosed at an advanced stage (Henschke et al., 2008; Spira et al., 2004), and an effective early-detection marker for lung cancer is thus desirable. Aberrant epigenetic changes are regarded as important mechanisms in lung carcinogenesis (Yang et al., 2011).
Unlike in colorectal cancer, mutations of the components in Wnt signaling pathway were relatively rare in lung cancer. Sunaga et al. (2001) found that β-catenin gene (CTNNB1) was mutated in certain lung cancer cell lines and primary lung cancers. Shigemitsu et al. (2001) reported that a small portion of lung cancers had a mutation in CTNNB1, and Ohgaki et al. (2004) demonstrated that APC (adenomatous polyposis coli) mutation occurred in a small portion of squamous cell lung cancer and small cell lung cancer. But disruption of the Wnt signaling pathway by hypermethylation of the promoter region of genes antagonizing the β-catenin/T cell factor (TCF)-signaling pathway was frequently reported in lung cancer, including RUNX3, SFRP1, WIF1, and APC (Mazieres et al., 2004; Fukui et al., 2005; Toyooka et al., 2006; Licchesi et al., 2008). Our recent study indicated that Dishevelled-associated antagonist of β-catenin 2 (DACT2) was frequently methylated in human cancer (Jia et al., 2012).
SRY-box containing gene 17 (SOX17) belongs to the high-mobility group (HMG)-box transcription factor superfamily, which is homologous to the sex-determining gene SRY (Gubbay et al., 1990). SOX17 has been reported to promote the degradation of β-catenin/TCF via a GSK3β-independent mechanism in the Wnt signaling pathway, and has been recognized as an important antagonist and inhibitor of the canonical Wnt signaling pathway (Sinner et al., 2007; Jia et al., 2010; Sinner et al., 2004). Frequent SOX17 gene methylation has been detected in colon, liver, and breast cancers (Zhang et al., 2008; Jia et al., 2010; Fu et al., 2009). However, the methylation status and effects of SOX17 on lung carcinogenesis remain unclear. We therefore investigated the epigenetic changes and functions of SOX17 in human lung cancer, using lung cancer cell lines and primary human lung cancer tissues.
Materials and Methods
Human tissue samples and cell lines
A total of 88 cases of NSCLC were collected immediately after surgical resection at the Chinese PLA General Hospital. Among these, matched cancer and adjacent normal tissue paraffin blocks were available for 29 cases. All definitive pathological diagnoses were classified as tumor stage I (n=36), stage II (n=25), stage III (n=25), or stage IV (n=2), according to UICC (International Union Against Cancer) staging criteria. All tissues were collected according to the guidelines of institutional review board of the Chinese PLA General Hospital.
Five lung cancer cell lines (H23, A549, H157, H446, and 95D) were included in this study. All lung cancer cell lines were previously established from NSCLC primary tumors, and maintained in 90% RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. Cells were passaged 1:3 after reaching 80% confluence (approximately 106 cells) in 75-cm2 culture flasks (NEST Biotechnology, Jiangsu, China).
5-Aza-2′-deoxycytidine (DAC) treatment
H23, A549, H157, H446, and 95D cells were split to low densities (30% confluence) 12 hours before treatment. Cells were treated with DAC (Sigma, St. Louis, MO) at a concentration of 2 mM. Growth medium conditioned with DAC at 2 µM was exchanged every 24 hours for a total of 96 hours of treatment. At the end of the treatment course, RNA was extracted from the cells as described below.
RNA isolation and semi-quantitative reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent (Life Technologies, Gaithersburg, MD). Agarose gel (1%) electrophoresis and spectrophotometric analysis (A260:280 nm ratio) were used to evaluate RNA quality and quantity. RNA was stored at -80°C prior to use. First-strand cDNA was synthesized according to Trizol manufacturer’s instructions. A total of 5 mg total RNA was used to synthesize first-strand cDNA using random 6-mer primers and a Superscript II-reverse transcriptase kit (Invitrogen, Carlsbad, CA). Following first-strand synthesis, the reaction mixture was diluted to 100 µl with water. Subsequently, 2.5 µl of diluted cDNA mixture was used for PCR amplification in a final reaction volume of 25 µl. PCR amplification of SOX17 was carried out using the following primers: 5′- GGCTGGCGCAGCAGAATC-3′ (forward) and 5′- AGCCCTGCTCGGGGAACT-3′ (reverse). The primer set for the SOX17 gene was designed to span intronic sequences between exons to control for genomic DNA contamination. A total of 32 cycles of amplification were performed for each RT-PCR experiment. Glyceraldehyde 3-phosphate dehydrogenase was amplified as a control using 25 cycles to ensure cDNA quality and quantity for each RT-PCR. Amplified products were analyzed on 1.5% agarose gels.
Methylation-specific PCR (MSP)
Genomic DNA from lung cancer tissue from 88 cases and five cell lines was prepared using the proteinase-K method. After chloroform/phenol extraction, DNA was precipitated in ethanol, dissolved in low-TE buffer and stored at −20°C. Genomic DNA from lung cancer tissues and cell lines was then modified with bisulfite, as previously described (Herman et al., 1996). MSP primers were designed according to genomic sequences flanking the presumed translation start sites. Primer sequences were oligo-synthesized (Invitrogen, Beijing, China) to allow bisulfite-induced changes affecting unmethylated (U) and methylated (M) alleles to be detected by MSP. MSP of SOX17 was carried out using the following primers: 5′-GGGGCGTTCGTAGTGTTA TTAGGTC-3′ (M-sense); 5′-AAACACTAAAATACC CCGAAAACTACG-3′ (M-antisense); 5′-TTAGGGGT GTTTGTAGTGTTATTAGGTT-3′ (U-sense) and 5′- TAAAACACTAAAATACCCCAAAAACTACA-3′ (U-antisense). Each MSP reaction included approximately 200 ng of bisulfite-treated DNA, 25 pmoles of each primer, 100 pmoles of dNTPs, 2.5 µl 10xPCR buffer, and 1 unit of Taq polymerase (Invitrogen, Beijing, China) in a final reaction volume of 25 µl. Cycle conditions were: 95°C for 5 minutes, 1 cycle; 35 cycles of 95°C for 30 seconds, 61°C for 30 seconds, and 72°C for 30 seconds; and 72°C for 5 minutes, 1 cycle. MSP products were analyzed using 2% agarose gel electrophoresis.
Immunohistochemistry (IHC)
IHC was performed on 4-µm-thick serial sections derived from formaldehyde-fixed paraffin blocks of lung cancer tissue and paired distant adjacent normal tissue. After deparaffinization and rehydration, endogenous peroxidase activity was blocked for 30 minutes in methanol containing 0.3% hydrogen peroxide. Antigen retrieval was performed for 45 minutes at 96°C in target retrieval solution, followed by a cooling-off period of 20 minutes. Incubation with the primary mouse antibodies [anti-β-catenin at 1:200 dilution (ZSGB Biotechnology, Beijing, China); anti-SOX17 at 1:50 dilution (OriGene Technologies, Rockville, MD), respectively] was carried out overnight at 4°C. The catalyzed signal amplification system (GBI, Mukilteo, WA) was then used to detect β-catenin and SOX17 staining, according to the manufacturer’s instructions. The correlations between SOX17 methylation and the locations of β-catenin and SOX17 were examined using χ2 tests. Values of P<0.05 were considered to be statistically significant.
cDNA construction
Full-length SOX17 cDNA (GenBank accession number NM_022454) derived from normal colon mucosa mRNA by RT-PCR was cloned into pcDNA3.1/V5-His B vector (Invitrogen, Beijing, China) via KpnI and XhoI sites, as described previously. Deletion mutants of SOX17 were generated by PCR.
Colony-formation assay
H23 cells were grown in 6-well culture plates for 24 hours before transfection. Cells were transfected with empty control vector or SOX17 expression construct, according to the manufacturer’s instructions (Roche Applied Science, San Diego, CA). Cells were diluted and reseeded at 800 cells per well in 6-well culture plates in triplicates 24 hours later. Growth medium conditioned with G418 (Invitrogen, Beijing, China) at 500 µg/ml, was exchanged every 24 hours. After 14 days, cells were fixed with 75% ethanol for 30 minutes and stained with 0.2% crystal violet for visualization and counting.
Luciferase reporter assay
H23 cells were seeded at 5×104 cells/well in 24-well culture plates 24 hours before transfection. To examine transcriptional activity driven by β-catenin/TCF, H23 cells were transfected with 200 ng/well pGL3-OT (TCF/LEF-responsive reporter containing three consensus TCF-binding sites), 30 ng/well pRL-TK control vector (Promega) as an internal control reporter, and 600 ng/well β-catenin construct. Basal transcriptional activity in H23 cells was tested by transfection with empty vectors. H23 cells were then transfected with 200 ng/well pGL3-OT, 30 ng/well pRL-TK, 150 ng/well β-catenin constructs, and 200 ng/well SOX17 constructs (wild-type and SOX17 constructs 50-414, 135-414 and 1–353) with FuGENE 6 (Roche Applied Science).
At 36 h after transfection, relative luciferase activities were measured using a GLOMAX luminometer (Promega, Madison, WI) and normalized for background Renilla luciferase activities via the Dual Luciferase Reporter Assay system (Promega), according to the manufacturer’s instructions. The luciferase assay was performed twice for each experiment.
Protein preparation and western blotting
Transfected cells were lysed in ice-cold Tris buffer (20 mmol/l Tris; pH 7.5) containing 137 mmol/l NaCl, 2 mmol/l EDTA, 1% Triton X, 10% glycerol, 50 mmol/l NaF, 1 mmol/l dithiothreitol, and a protease inhibitor cocktail (Roche Applied Science). The protein lysates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes (Hybond-P, Amersham, Pittsburgh, PA). After blocking with 5% nonfat milk and 0.1% Tween-20 in TBS, the membranes were incubated with mouse anti-SOX17 (OriGene Technologies), rabbit cyclin D1 (Bioworld Technology, Minneapolis, MN), or rabbit anti-β-actin (Beyotime Biotechnology, Shanghai, China) antibodies. The blots were visualized using enhanced chemiluminescence (Pierce Bioscience, Rockford, IL).
Statistical analysis
Statistical analysis was carried out using χ2 tests. Values of P<0.05 were considered statistically significant.
Results
Expression of SOX17 was regulated by DNA methylation in lung cancer cell lines
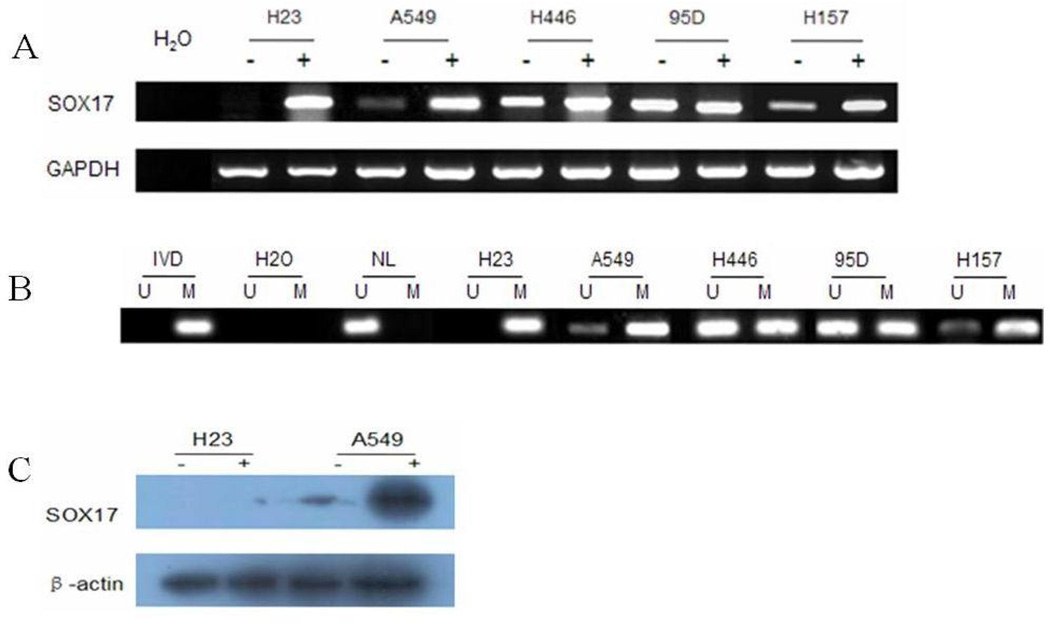
Semi-quantitative RT-PCR was employed to detect the expression levels of SOX17 in lung cancer cell lines (H23, A549, H157, H446, and 95D). Among these five lung cancer cell lines, SOX17 was weakly expressed in A549 and H157 cells, normally expressed in H446 and 95D cells, and silenced in H23 cells (Figure 1A).
Figure 1.
SOX17 expression was regulated by DNA methylation in lung cancer cell lines. A. Expression of SOX17 was detected by semi-quantitative RT-PCR in five lung cancer cell lines (H23, A549, H157, H446, and 95D) before and after treatment with 2 µmol/l DAC (+) for 96 hours. B. MSP results for SOX17 in five lung cancer cell lines. U, unmethylated; M, methylated. Amplification efficiency was verified using methylated (in vitro methylated DNA, IVD) and unmethylated controls (normal blood lymphocyte DNA, NL). C. Expression of SOX17 in H23 and A549 cells before and after treatment with 2 µmol/l DAC (+) for 96 hours was further analyzed using western blots.
Partial promoter region methylation of SOX17 was detected in four cell lines (A549, H157, H446, and95D), and complete methylation was found in H23 cells. Methylation was correlated with loss or reduction of the SOX17 gene expression (Figure 1B).
To further explore the regulation of SOX17 expression by promoter region hypermethylation, the five lung cancer cell lines were treated with DAC, a DNA methylation transferase inhibitor. Re-expression of SOX17 occurred in H23 cells, and increased expression of SOX17 was detected in A549 and H157 cells. No changes of expression were found in H446 or 95D cells (Figure 1A). The correlation between promoter methylation and reduced or absent SOX17 expression was further confirmed by western blot analysis of protein levels in H23 and A549 cells (Figure 1C). These results demonstrate that SOX17 expression was regulated by promoter region hypermethylation in lung cancer cell lines.
SOX17 was frequently methylated in human primary lung cancer and loss of SOX17 expression was related to methylation
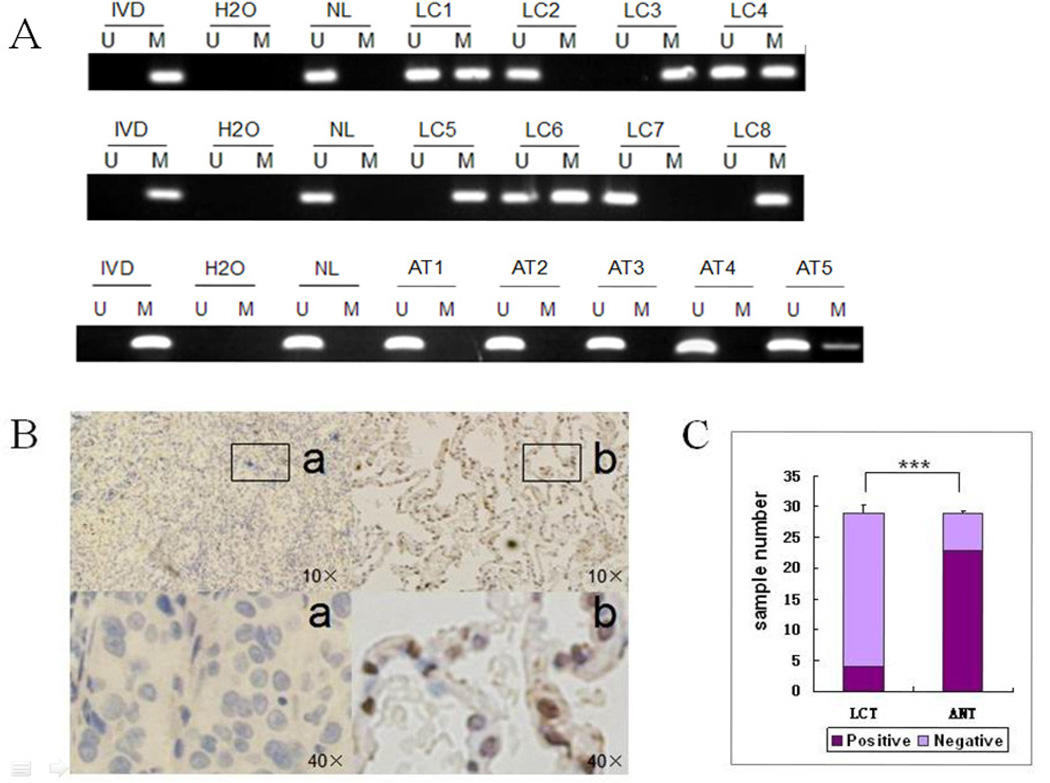
SOX17 methylation status in primary lung cancer tissues was investigated using MSP. SOX17 was methylated in 60.2% of cases (53/88) and unmethylated in 39.8% (35/88). In comparison, in 29 available adjacent normal tissue samples, SOX17 was methylated in only 17.2% (5/29) and unmethylated in 82.8% (24/29) (Figure 2A). The ratio of methylated/unmethylated SOX17 was significantly higher in lung cancer compared with adjacent normal tissue (P<0.05). IHC was performed on 29 available paraffin-embedded paired lung cancer and adjacent normal tissue samples. Reduced or absent SOX17 expression was detected in 25 cancer cases, with normal expression in only four cases. In contrast, reduced or absent SOX17 expression was found in only 6 adjacent normal tissues, while normal expression was found in 23 cases. SOX17 expression levels thus differed significantly between lung cancer and adjacent normal tissues (Figure 2B). Among the 25 cases of lung cancer with absent or reduced SOX17 expression, 22 cases were methylated (88.0%). Among the 6 cases of adjacent normal lung tissue sample with absent or reduced SOX17 expression, 4 cases were methylated (66.7%). These results suggest that promoter region hypermethylation is significantly related to absent or reduced SOX17 expression (P<0.05).
Figure 2.
SOX17 methylation and expression in primary lung cancer tissues. A. Representative SOX17 methylation results in primary lung cancer and normal lung tissues. LC1-8, primary lung cancer; AT1-AT5, adjacent normal tissues. B. SOX17 expression in primary lung cancer and adjacent normal tissues analyzed by immunohistochemistry. a. Nuclear-negative SOX17 in lung cancer tissue (LCT). b. Nuclear-positive SOX17 in adjacent normal tissue (ANT). C. Expression of SOX17 in primary lung cancer and adjacent tissues shown by histograms (χ2 tests, *** P<0.001).
SOX17 methylation was significantly associated to female sex in lung cancer patients (P<0.05), and to poor differentiation of lung cancer (P<0.05, Table 1). These results suggest that SOX17 was silenced by promoter region methylation in lung cancer, and that SOX17 methylation may induce lung carcinogenesis. SOX17 methylation may be a susceptibility factor for lung cancer in women.
Table 1.
Clinicopathological Characteristics and SOX17 Methylation Status in Human Lung Cancer
| Clinical Parameter | Number of Patients | SOX17 Methylation Status | P Value* | |
|---|---|---|---|---|
| Methylated | Unmethylated | |||
| n=53 (60.2%) | n=35 (39.8%) | |||
| Age (years) | ||||
| <65 | 60 | 37 (61.7%) | 23 (38.3%) | 0.6863 |
| ≥65 | 28 | 16 (57.1%) | 12 (42.9%) | |
| Sex | ||||
| Male | 60 | 30 (50.0%) | 30 (50.0%) | 0.0084 |
| Female | 28 | 23 (82.1%) | 5 (17.9%) | |
| Smoking | ||||
| Negative | 3 | 1 (33.3%) | 2 (67.6%) | 0.9001 |
| Positive | 40 | 22 (55.0%) | 18 (45.0%) | |
| Tumor type | ||||
| Squamous cell | 34 | 20 (58.8%) | 14 (41.2%) | 0.9908 |
| Adenocarcinoma | 46 | 27 (58.7%) | 19 (41.3%) | |
| Tumor differentiation | ||||
| Low | 38 | 29 (76.3%) | 9 (23.7%) | 0.0133 |
| Middle | 38 | 20 (52.6%) | 18 (47.4%) | |
| High | 12 | 4 (33.3%) | 8 (66.7%) | |
| Tumor stage | ||||
| I | 36 | 15 (41.7%) | 21 (58.3%) | 0.9618 |
| II | 25 | 10 (40.0%) | 15 (60.0%) | |
| III | 25 | 9 (36.0%) | 16 (64.0%) | |
| IV | 2 | 1 (50.0%) | 1 (50.0%) | |
P values were obtained from χ2 tests; P<0.05 indicates significant difference.
Colony formation was inhibited by restoration of SOX17 expression in H23 cells
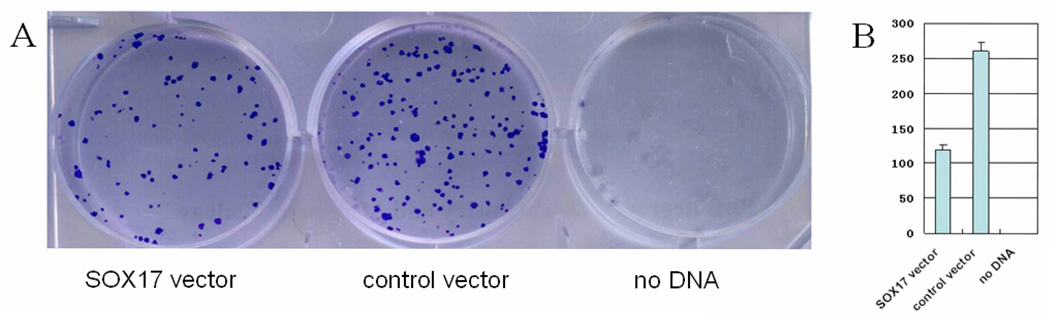
The effects of SOX17 on lung carcinogenesis were evaluated by colony-formation assays. The efficiency of colony formation was significantly inhibited by restoration of SOX17 expression in H23 cells (Figure 3), suggesting that SOX17 suppressed lung cancer cell proliferation.
Figure 3.
Restoration of SOX17 expression inhibited clonogenicity in H23 cells. A. H23 cells transfected with empty control vector or SOX17 expression vector were reseeded at 800 cells/well and selected with 500 µg/ml G418 for 14 days. Cells were observed and counted after fixing with 75% ethanol and staining with 0.2% crystal violet. B. Experiments were repeated in triplicates and the average colony number for each transfected group is expressed by a bar graph.
Wnt signaling was suppressed by re-expression of SOX17 in H23 cells
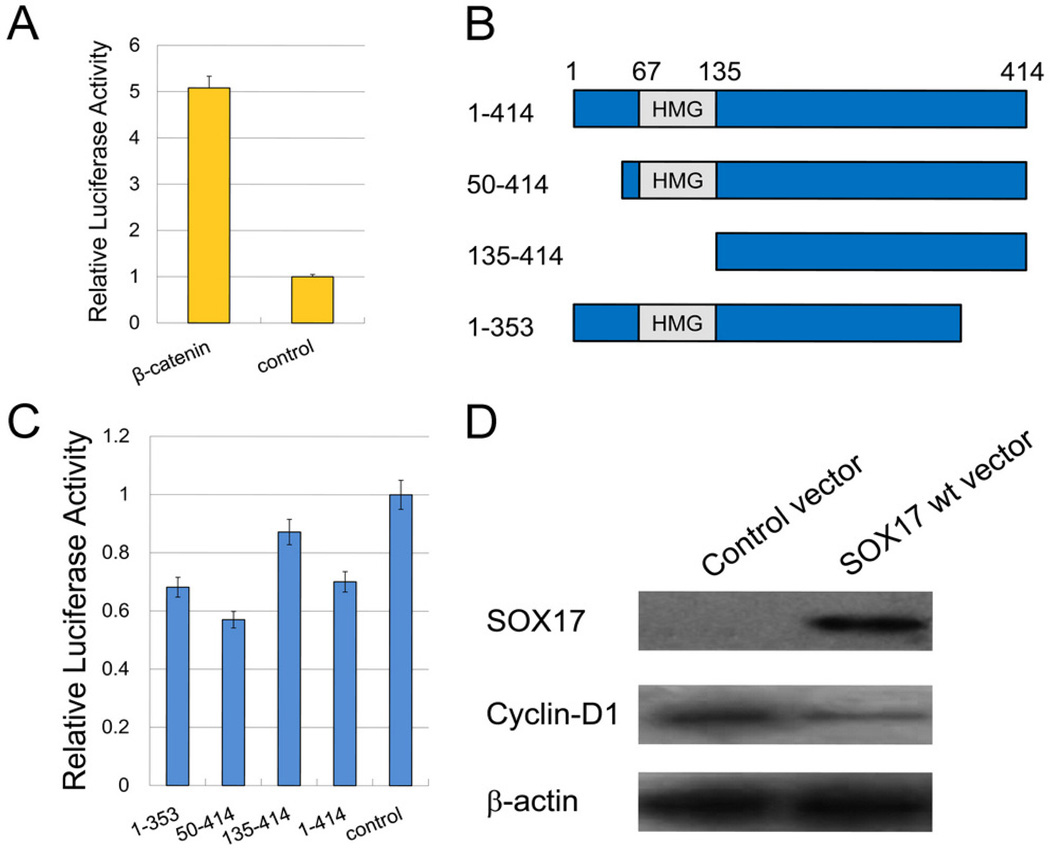
The effect of SOX17 on Wnt/β-catenin signaling was assessed using the TCF-LEF luciferase reporter system. Luciferase activity was significantly higher in H23 cells with forced β-catenin expression compared with the control empty vector group (Figure 4A). The structure of SOX17, deleted into different length fragments, is shown in Figure 4B. There was no suppressive effect on β-catenin/TCF-LEF transcription following transfection with pGL3-OT, pRL-TK, β-catenin, or SOX17 fragment 135–414 (with HMG domain deleted). Transfection with SOX17 expression vector fragments 50–414 and 1–353 produced similar inhibitory effects (Figure 4C).
Figure 4.
SOX17 influenced the transcriptional activation of β-catenin and its downstream genes in the Wnt signaling pathway. A. H23 cells were transfected with β-catenin and empty vectors, respectively, to detect relative luciferase activities. B. The architecture of SOX17 with an HMG box is depicted in schematic form. Different deletion mutants (SOX17 constructs 50–414, 135–414, and 1–353) were generated by PCR. C. H23 cells were co-transfected with β-catenin and different SOX17 expression vectors (wild-type or mutant). Transfection with wild-type and mutant SOX17 showed greater or less suppressive effects on β-catenin/TCF-LEF transcriptional activity. D. Transfection with wild-type SOX17 inhibited cyclin D1, a downstream gene of the Wnt signaling pathway.
Western blotting in H23 cells was employed to further confirm the inhibitory effect of SOX17 on the Wnt signaling pathway. The expression of cyclin D1, the downstream gene of β-catenin, was reduced in SOX17 re-expressed H23 cells (Figure 4D).
Discussion
Lung cancer is the leading cause of cancer-related deaths worldwide; however, less than 20% of patients are diagnosed at an early stage (Tanner et al., 2010). Researchers have recently made progress in identifying prospective lung cancer biomarkers (Greenberg et al., 2007). Epigenetic change is now well established as an important molecular mechanism for gene silencing, and is involved in a series of human primary carcinomas, including lung cancer (Brock et al., 2008). Our previous study demonstrated that promoter region hypermethylation of SOX17 plays an important role in colorectal cancer and hepatocellular carcinoma (Zhang et al., 2008; Jia et al., 2010). SOX17 has also been reported to be involved in the development of lung cancer (Park et al., 2006; Lange et al., 2009). However, the regulation of SOX17 expression and its function in human lung carcinogenesis remains unclear. The results of the current study showed that SOX17 was frequently methylated in human lung cancer, and that SOX17 expression was in turn regulated by promoter region hypermethylation. SOX17 methylation was associated with female sex. These results suggest that SOX17 methylation may serve as a potential marker for lung cancer detection, and also suggest that females are more susceptible than males to SOX17-related lung carcinogenesis. The mechanism of females being more susceptible to SOX17-related lung cancer remains unclear. In the sea bass, SOX17 expression was reported significantly higher in females (Navarro-Martin et al., 2009). SOX17 methylation was also related to lung cancer differentiation. Colony-formation assays indicated that SOX17 suppressed lung cancer cell proliferation, indicating a suppressive role for SOX17 in lung carcinogenesis. The results of further experiments suggest that SOX17 inhibits the Wnt signaling pathway in lung cancer cells. All the above results demonstrate that SOX17 acts as a tumor suppressor in lung cancer by inhibiting Wnt signaling.
Conclusion
Promoter region hypermethylation of SOX17 occurs frequently in lung cancer, and represents an important mechanism for downregulating gene expression. SOX17 inhibits proliferation of lung cancer cells, and plays an important role in antagonizing Wnt/β-catenin signaling in lung cancer. Epigenetic changes in SOX17 may serve as useful diagnostic and prognostic biomarkers in lung cancer.
Acknowledgments
This work was supported by grants from the NationalBasic Research Program (973 Program No. 2012CB934002, 2010CB912802, 2009CB521801), National Key Scientific Instrument Special Programme of China (Grant No. 2011YQ03013405), National Science Foundation of China (Grant No. 81121004, 81071953, 81161120432) and State High-Tech Development Plan (863 program No. SS2012AA02- A504, SS2012AA020303, SS2012AA02A209).
Footnotes
Disclosure
J.G.H. is a consultant to MDxHealth. The other authors report no conflicts of interest.
Contributor Information
Dongtao Yin, Department of Thoracic Surgery, Chinese PLA General Hospital, Beijing 100853, China and Department of Cardiothoracic Surgery, The Second Artillery General Hospital, Beijing 100088, China
Yan Jia, Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing 100853, China
Yuanzi Yu, Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing 100853, China
Malcolm V. Brock, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans Street, Baltimore, Maryland 21231, USA
James G. Herman, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1650 Orleans Street, Baltimore, Maryland 21231, USA
Chao Han, Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing 100853, China
Xiaomo Su, Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing 100853, China
Yang Liu, Department of Thoracic Surgery, Chinese PLA General Hospital, Beijing 100853, China
Mingzhou Guo, Department of Gastroenterology and Hepatology, Chinese PLA General Hospital, Beijing 100853, China
References
- Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glöckner S, Piantadosi S, Gabrielson E, Pridham G, Pelosky K, Belinsky SA, Yang SC, Baylin SB, Herman JG. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358(11):1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- Fu DY, Wang ZM, Li-Chen, Wang BL, Shen ZZ, Huang W, Shao ZM. Sox17, the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer Res Treat. 2010;119(3):601–612. doi: 10.1007/s10549-009-0339-8. [DOI] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24(41):6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- Greenberg AK, Lee MS. Biomarkers for lung cancer: clinical uses. Curr Opin Pulm Med. 2007;13(4):249–255. doi: 10.1097/MCP.0b013e32819f8f06. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Henschke CI, Yankelevitz DF. CT screening for lung cancer: update 2007. Oncologist. 2008;13(1):65–78. doi: 10.1634/theoncologist.2007-0153. [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yang Y, Liu S, Herman JG, Lu F, Guo M. SOX17 antagonizes WNT/beta-catenin signaling pathway in hepatocellular carcinoma. Epigenetics. 2010;5:743–749. doi: 10.4161/epi.5.8.13104. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M. Epigenetic regulation of DACT2, a key component of the Wnt signaling pathway in human lung cancer. J Pathol. 2012 Jul;:18. doi: 10.1002/path.4073. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA. Sox17 promotes cell cycle progression and inhibits TGF-β/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS One. 2009;4(5):e5711. doi: 10.1371/journal.pone.0005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licchesi JD, Westra WH, Hooker CM, Machida EO, Baylin SB, Herman JG. Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis. 2008;29(5):895–904. doi: 10.1093/carcin/bgn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, Reguart N, Rosell R, McCormick F, Jablons DM. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64(14):4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- Navarro-Martín L, Galay-Burgos M, Sweeney G, Piferrer F. Different sox17 transcripts during sex differentiation in sea bass, Dicentrarchus labrax. Mol Cell Endocrinol. 2009;299(2):240–251. doi: 10.1016/j.mce.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Ohgaki, Kros JM, Okamoto Y, Gaspert A, Huang H, Kurrer MO. APC mutations are infrequent but present in human lung cancer. Cancer Lett. 2004;207(2):197–203. doi: 10.1016/j.canlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006;294(1):192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Shigemitsu K, Sekido Y, Usami N, Mori S, Sato M, Horio Y, Hasegawa Y, Bader SA, Gazdar AF, Minna JD, Hida T, Yoshioka H, Imaizumi M, Ueda Y, Takahashi M, Shimokata K. Genetic alteration of the beta-catenin gene (CTNNB1) in human lung cancer and malignant mesothelioma and identification of a new 3p21.3 homozygous deletion. Oncogene. 2001;20(31):4249–4257. doi: 10.1038/sj.onc.1204557. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101(27):10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemitsu R, Yamaguchi M, Takeo S, Ondo K, Ueda H, Yoshino I, Maehara Y. Favorable surgical results for patients with non-small cell lung cancer over 80 years old: a multicenter survey. Ann Thorac Cardiovasc Surg. 2008;14(3):154–160. [PubMed] [Google Scholar]
- Sullivan V, Tran T, Holmstrom A, Kuskowski M, Koh P, Rubins JB, Kelly RF. Advanced age does not exclude lobectomy for non-small cell lung carcinoma. Chest. 2005;128(4):2671–2676. doi: 10.1378/chest.128.4.2671. [DOI] [PubMed] [Google Scholar]
- Sunaga N, Kohno T, Kolligs FT, Fearon ER, Saito R, Yokota J. Constitutive activation of the Wnt signaling pathway by CTNNB1 (beta-catenin) mutations in a subset of human lung adenocarcinoma. Genes Chromosomes Cancer. 2001;30(3):316–321. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1097>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Tanner NT, Silvestri GA. An up to date look at lung cancer screening. Cell Adh Migr. 2010;4(1):96–99. doi: 10.4161/cam.4.1.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka S, Tokumo M, Shigematsu H, Matsuo K, Asano H, Tomii K, Ichihara S, Suzuki M, Aoe M, Date H, Gazdar AF, Shimizu N. Mutational and epigenetic evidence for independent pathways for lung adenocarcinomas arising in smokers and never smokers. Cancer Res. 2006;66(3):1371–1375. doi: 10.1158/0008-5472.CAN-05-2625. [DOI] [PubMed] [Google Scholar]
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(1 Suppl):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Yang IV, Schwartz DA. Epigenetic control of gene expression in the lung. Am J Respir Crit Care Med. 2011;183(10):1295–1301. doi: 10.1164/rccm.201010-1579PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Glöckner SC, Guo M, Machida EO, Wang DH, Easwaran H, Van Neste L, Herman JG, Schuebel KE, Watkins DN, Ahuja N, Baylin SB. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 2008;68:2764–2772. doi: 10.1158/0008-5472.CAN-07-6349. [DOI] [PMC free article] [PubMed] [Google Scholar]