Abstract
Background:
Ficus carica Linn. is reported to possess variety of activities, but its potential in CNS disorders is still to be explored.
Objective:
The present study was carried out to evaluate the CNS depressant activity of aqueous acetonic extract of Ficus carica Linn on different models in mice.
Materials and Methods:
The aerial parts of the plant Ficus carica L. were extracted with aqueous acetone and the solvent was removed by rotary vacuum evaporator under reduced pressure. A crude extract was given orally and its effects were tested on ketamine-induced sleeping time, muscle-coordination, anxiety (elevated-plus maze and Staircase test), convulsions [maximal electroshock (MES) and pentylenetetrazole (PTZ)-induced seizures], and nociception. In addition, we determined the levels of neurotransmitters, norepinephrine (NE) and 5-hydroxytryptamine (5-HT).
Results:
Results from the experimental models tested showed: (1) a delay on onset and prolongation of sleep of ketamine-induced sleeping time; (2) significant muscle relaxant activity; (3) a significant attenuation in the anxiety-response (4) a delay in the onset of seizures and reduction in duration of seizures and mortality induced by MES and PTZ; (5) a reduction in the licking time in nociception test and (6) increased levels of NE and 5-HT.
Conclusion:
This suggests that Ficus carica L. exerts its CNS depressive effect by modulating the neurotransmitters NE and 5-HT in the brain.
Keywords: Anti-anxiety, anticonvulsant, Ficus carica, muscle-relaxant, neurotransmitters, sedative-hypnotic
INTRODUCTION
Central Nervous System (CNS) disorders such as anxiety, insomnia, schizophrenia and epilepsy are widely seen in population worldwide. Recent figures from the Royal Society of Medicine has demonstrated that 15% of the global burden of disease is attributed to mental disorders, 30% of which is in high-income countries, 20% in middle-income countries and 10% in low-income countries.[1] The current treatment for these disorders helps to control the acute manifestations of the disease and give a symptomatic relief, yet these current treatments are far from satisfactory and there has been a need for more effective drugs with lesser side effects. This fact has stimulated a considerable research of new compounds as therapeutic alternatives for such disorders. In this regard, medicinal plants have been an important source to the development of drugs with this biological activity.[2] So far many medicinal plants have established themselves as useful in these disorders. Recently, study carried out by Saxena et al. (2013) demonstrated the nootropic (memory enhancing) potential of Ficus carica L. but its CNS depressant potential as an anti-epileptic and anxiolytic is still to be explored.[3]
Ficus carica Linn. (Moraceae) commonly known as ‘Anjir’ (Hindi) is a small deciduous tree. The tree is cultivated in many parts of North-western and South India.[4] Phytochemical screening of the leaves has characterized the presence of psoralen, bergapten, taraxasterol, β-sitosterol, lupeol, β-amyrin, coumarins, xanthotoxin, xanthotoxol, ficusin, tyrosin and marmesin. The rural people use the fresh juice of the crushed plant to cure epilepsy and insanity. The various parts of the plant are used by the rural people as a mild laxative, expectorant, anti-inflammatory and diseases in liver and spleen as deobstruent.[5,6] Phytochemical studies on this plant deal with fruits, leaves, roots and latex. The isolation of two flavonoid; quercetin and luteolin from Ficus carica L. has been reported.[7] The plant also exhibited hypoglycemic, antitumorigenic, antioxidant, hepatoprotective, antispasmodic and antiplatelet activities.[8,9,10,11,12,13]
Sedative-hypnotic as well as anticonvulsant activity of the aqueous extract of the leaves of Ficus carica L. in male mice was demonstrated, utilizing pentobarbital sodium induced sleep and nikethamide-induced convulsions. The extract showed prolongation of sleep and protection against convulsions.[8]
Based on the ethno botanical and pharmacological reports presented above, we postulate that the aqueous acetonic extracts of Ficus carica L. leaves could have potential activities on the central nervous system and the administration of these extracts could produce behavioral modifications. In order to test this, we investigated the aqueous acetonic extract of Ficus carica leaves in mice, using several behavioral tests, animal models and biochemical estimations.
MATERIALS AND METHODS
Animals
Male Swiss albino mice (25-30 g) were used. Animals were kept at constant room temperature (22 ± 10°C), relative humidity (45-55%) and maintained in a 12:12 h light/dark cycle. Experiments were carried out in accordance with the Institutional Ethical Committee Guidelines laid down by Committee for the Purpose of Supervision and Control of Experiments on Animals (CPCSEA), concerning the care and use of animals for experimental procedures (Approval No. CPCSEA/BVP/IAEC-2009-04). Animals were fed with standard diet and given water ad libitum. All mice were adapted for 7 days before extract, reference drugs or vehicle solutions were administered. For each experimental procedure, animal groups consisted of six mice. Each animal was tested only once.
The plant
Aerial parts of Ficus carica Linn (Moraceae) were collected from Pune (Phalgu variety, collected in March), India and authentication of the plant specimen was established by Head of Department of Life Sciences, Ramnarain Ruia College, Mumbai. A voucher specimen was deposited in our herbarium for future reference (Voucher No. BVFC/09/001).
Extraction procedure
The aerial parts were dried at controlled temperature and pulverized to fine powder. The powder was passed through 20-mesh sieve and exhaustively extracted with 60% (v/v) aqueous acetone using maceration method. The aqueous acetonic extract was evaporated under reduced pressure using a rotary vacuum evaporator to give an extract with a yield of 11.8%. The chemical constituents of the extract were analyzed qualitatively and found to contain steroids, flavonoids and coumarins. The extract was stored in desiccators and a weighed amount was dissolved in sodium CMC for the present investigation.
Drugs and chemicals
Diazepam (Ranbaxy Labs. Mumbai), pentylene tetrazole (Himedia Labs Mumbai), ketamine hydrochloride (Neon Labs. Mumbai), noradrenaline bitartrate (Samarth Life Sciences Ltd. Mumbai) and 5-hydroxytrytamine hydrochloride (Sigma Ltd. Mumbai) were used for the present study.
Treatment
All drugs were freshly prepared on the day of experiments; all reference drugs were injected via intraperitoneal (i.p.) and the crude extract was given orally in a volume of 10 mL/kg body weight. One hour after p.o. or 30 min after i.p. administration, the animals were submitted to the various behavioral tests. Control animals received the same volume of vehicle (0.5% sodium CMC alone) by the same route.
Toxicological study
The LD50 determination of the aqueous acetonic extract of Ficus carica L. (AAEFC) was performed in albino mice by the method of Up and Down.[14]
Behavioral evaluation
General pharmacological observation
The behavioral effect of AAEFC (250 and 500 mg/kg p.o.) was assessed by the method described by Turner (1972).[15] The effects of AAEFC on different behavioral paradigms in animals were scored with the use of nine degrees, that is, with a scale of 0-8. The base score for normal effects is 4, while a score below 4 signified subnormal responses and score above 4 signified supernormal responses. The base score for abnormal sign is 0, and the maximal score is 8. Scores below in the parentheses indicate normal activity scores. The animals were observed for 2 h after treatment for alertness (4), stereotypy (0), grooming (4), spontaneous activity (4), reactivity (4), touch response (4), startle response (0), body position (4), urination (0).
Ketamine-induced sleeping time
The effect of plant extracts on ketamine-induced sleeping time was measured as described by Mimura et al. (1990).[16] After 30-min of i.p. treatment and 1 h after p.o. administration, animals were injected with ketamine (100 mg/kg i.p.). The control group received only vehicle and ketamine. The interval between the administration of ketamine until the loss of the righting reflex was recorded as onset of sleep, while the time from the loss to regaining of the righting reflex as the duration of sleep.[17]
Muscle relaxant activity
Rotarod test
The motor coordination and performance of each male mouse was evaluated 1 h after the extract oral treatment or 30 min after i.p. administration of standard in a Rotarod apparatus (5 cm diameter, 20 rpm). Latency to fall from the rotating bar in a period of 3-min test was registered.[18]
Anti-anxiety activity
Elevated plus maze
This test has been validated to measure anxiety in rodents.[19,20] The elevated plus maze for mice consisted of two perpendicular open arms (30 × 5 cm) and two closed arms (30 × 5 × 15 cm) also in perpendicular position. The open and closed arms were connected by a central platform (5 × 5 cm). The platform and the lateral walls of the closed arms were made of black acrylic. The maze was elevated 50 cm above the floor. Thirty minutes after i.p. treatment or 1 h after the p.o. administration, each mouse was placed at the center of the maze, facing one of the closed arms. During the 5-min test period, the number of open and closed arms entries, plus the time spent in open and closed arms were recorded.[21] Entry into an arm was defined as the point when the mouse places all four paws onto the arm. All the records were taped by using a video camera located above the maze. After the test, the mazes were carefully cleaned with a wet tissue paper (10% ethanol solution).
Staircase test
Staircase is composed of five identical steps 2.5 cm high, 10 cm wide and 7.5 cm deep. The internal height of the walls is constant along the whole length of the staircase.[22] Thirty minutes after i.p. treatment or 1 h after oral administration, the mouse was placed singly on floor of the box with its back to the staircase. The number of steps climbed and the number of rears were counted over a 3-min period. A step was considered to be climbed only if the mouse had placed all four paws on the step. After each mouse had been tested, the box was cleaned to eliminate any olfactory cue which might modify the next animal's behavior.
Experimental convulsions
Pentylenetetrazole-induced seizures
PTZ was used to induce clonic-tonic convulsions.[23] A PTZ dose (80 mg/kg i.p.) was administered 1 h after oral administration or 30 min after i.p. treatment. The latency of first convulsive episode as well as its duration was recorded. If no convulsions and mortality occurred during the time limit of 30-min, animals were considered protected from PTZ-induced seizures.
Maximal electroshock-induced seizures
All the mice received current of 42 mA for 0.2 s duration through electro convulsiometer using corneal electrodes after 30-min i.p. treatment or 1 h after oral administration.[24] The incidence and duration of extensor tonus was recorded. A complete abortion of hind limb tonic extension was considered as 100% protection.
Analgesic activity
Hot plate method
Central analgesic activity was evaluated using hot plate method. After 30-min of i.p. treatment and 1 h after p.o. administration, the mice were placed individually on the hot plate maintained at 55 ± 10°C and latency of nociceptive response such as licking, flicking of a hind limb or jumping was noted. The experiment was terminated 20 s after their placement on the hot plate to avoid damage to the paws.[20]
Biochemical estimation
Brain catecholamine levels were determined using the following procedure; the whole brain was homogenized in a mixture of 2 mL of 0.01N HCl and 10 ml of butanol reagent in a glass Teflon homogenizer and the catecholamines were extracted simultaneously by the method of Brownlee and Spriggs (1965).[25] The aqueous phase/acid extract was recovered by centrifugation at 3000 g for 5 min and was used for estimation of norepinephrine (NE)[26] and 5-hydroxytryptamine (5-HT)[27] by fluorometric assays.
Statistical analysis
Graph pad prism version 5.0 was used for statistical analysis. The data obtained were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's t-test. The level of significance was set at P < 0.05 and P < 0.01.
RESULTS
Toxicity study
AAEFC was found to be safe after oral administration up to the dose of 2 g/kg. No mortality was observed up to this dose up to 24 h.
General pharmacological observation
The animals were observed for 2 h after oral administration of extract (250 and 500 mg/kg). During the observation period various behavioral scores were noted. There was decrease in alertness, spontaneous activity, touch and pain response as compared to control (0.5% Sodium CMC).
Ketamine-induced sleeping time
The absolute values of sleep latency and sleeping time showed in Figure 1 demonstrate that animals treated with AAEFC (250 and 500 mg/kg) or diazepam (0.5 mg/kg) presented a decrease in the sleep latency and prolongation of ketamine-induced sleeping time.
Figure 1.
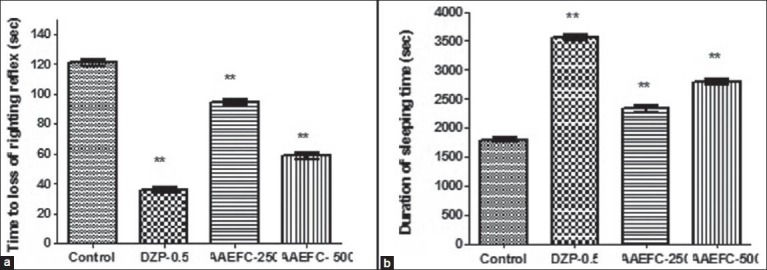
Effects of diazepam (0.5 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on (a) the latency to loss of righting reflex and (b) total sleep time. Data represents means ± S.E.M. from six mice. Comparisons were made by using a one-way ANOVA followed by Dunnett's t-test: **P < 0.05 compared with the vehicle-treated control
Muscle relaxant activity-rotarod test
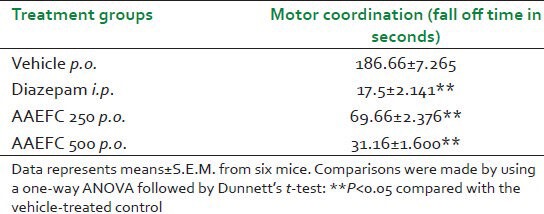
Diazepam at 2 mg/kg and the AAEFC at doses of 250 and 500 mg/kg caused significant reduction in the number of seconds spent on the rod, compared to the control group as shown in Table 1.
Table 1.
Effects of diazepam (2 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on motor coordination as evaluated on the rotarod in mice
Anti-anxiety activity
Elevated-plus maze test
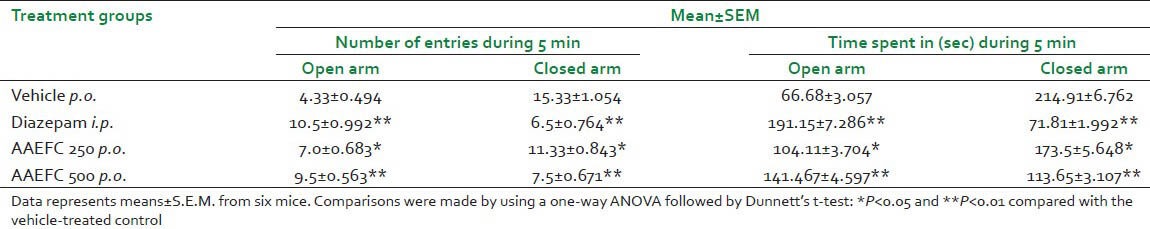
Effects of AAEFC (250 and 500 mg/kg) or diazepam (1 mg/kg) in the elevated-plus maze test are presented in Table 2. In this test, the groups treated with AAEFC at both doses and diazepam significantly modified all the parameters: The number of entries in the open arm and closed arm and the time of permanence in the open arm and closed arm as compared to control.
Table 2.
Effects of diazepam (1 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on anti-anxiety as evaluated on the elevated-plus maze in mice
Staircase test
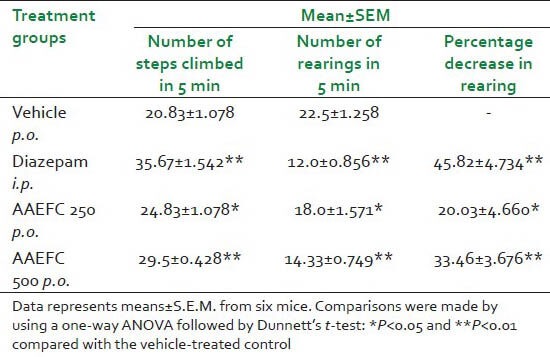
Similar to Diazepam (1 mg/kg), AAEFC at both doses [Table 3] increased the number of steps climbed and reduced the number of rearings as compared to control.
Table 3.
Effects of diazepam (1 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on anti-anxiety as evaluated on the staircase test in mice
Experimental convulsions
Pentylenetetrazole-induced seizures
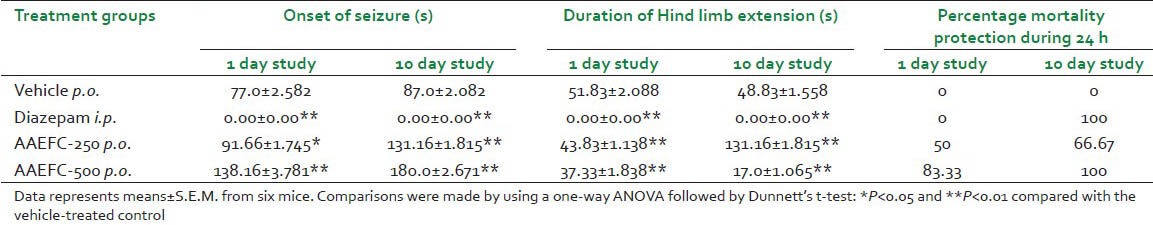
PTZ (80 mg/kg) induced clonic-tonic seizures in 100% of control mice. Pretreatment with AAEFC at 250 and 500 mg/kg significantly delayed the onset of seizures induced by PTZ. In addition, percentage of mortality was decreased [Table 4]. Diazepam showed a significant anticonvulsant activity at 4 mg/kg. Also a comparison made during a 1 day study and 10 day study showed a significant anticonvulsant activity of AAEFC.
Table 4.
Effects of diazepam (4 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on anti-convulsion as evaluated in the PTZ-induced convulsions test in mice during 1 and 10 day study
Maximal electroshock-induced seizures
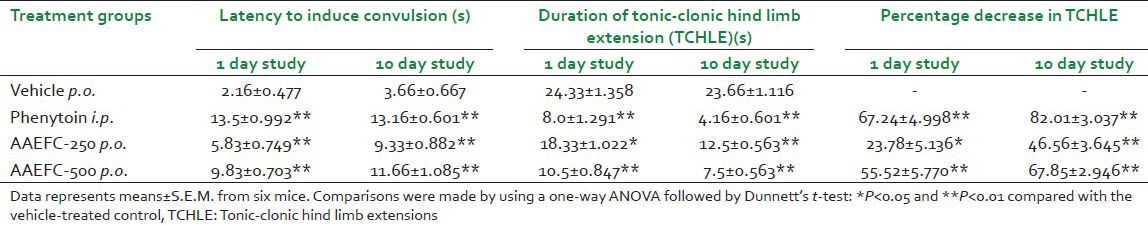
AAEFC exhibited almost dose-dependent anticonvulsant activity. Phenytoin at 25 mg/kg and AAEFC at both doses (250 and 500 mg/kg) significantly increased the latency to convulsions and decreased the duration of tonic-clonic hind limb extensions (TCHLE). The comparison between 1 day and 10 day study is given in Table 5.
Table 5.
Effects of phenytoin (25 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on anti-convulsion as evaluated in the MES-induced convulsions test in mice during 1 and 10 day study
Analgesic activity-hot plate method
Pretreatment with AAEFC at both doses showed a significant increase in pain threshold in mice. The results are shown in Table 6.
Table 6.
Effects of pentazocine (0.5 mg/kg) and aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on analgesic activity as evaluated in the hot plate test in mice

Biochemical estimations
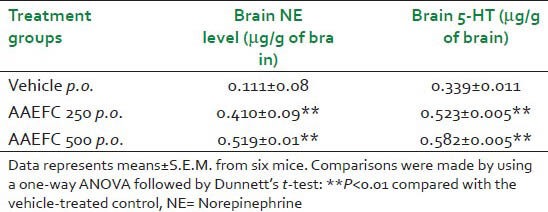
The level of norepinephrine (NE) and serotonin (5-HT) was enhanced significantly on administration of AAEFC as compared to control [Table 7].
Table 7.
Effect of aqueous acetonic extract of Ficus carica L. (250 and 500 mg/kg) on levels of norepinephrine and serotonin (5-HT) from the whole brain
DISCUSSION
In the present study, acute toxicity (LD50 for p.o. route), sedative-hypnotic potentiation, anti-anxiety, skeletal muscle relaxation, anticonvulsant and analgesic actions of aqueous acetonic extract of the aerial parts of Ficus carica L. (AAEFC) with subsequent modulation in brain monoamines such as 5-HT and NE in the whole brain were evaluated in the mice. The study demonstrated the neuropharmacological effects of the extract in mice.
In the preliminary behavioral assessment, AAEFC exhibited decreased alertness, grooming, spontaneous activity and decreased response to external touch stimuli. There was no evidence of central excitation and altered autonomic profile after the administration of AAEFC which indicated an absence of CNS stimulation and rules out any influence on sympathetic and parasympathetic activities.
The above activity was corroborated with ketamine-induced sleeping time. The ability of drugs to prolong ketamine-induced sleeping time is indicative of their sedative-hypnotic activity. Ketamine is pharmacologically related to the hallucinogen phencyclidine. It induces ‘dissociative anesthesia’, analgesia, immobility and amnesia with light sleep. Ketamine acts as an antagonist on NMDA receptors, thus explaining anesthetic effect but it also has been reported that ketamine at high doses (100 mg/kg) can activate NE and 5-HT neurons. The sedative action may be due to an increase in concentration of GABA in brain. Thus, this test was performed to evaluate sedative-hypnotic properties of test compounds. Hypnotic drugs decrease the latency to sleep and prolong the duration of sleep in ketamine-induced sleep test after single dose of ketamine (100 mg/kg).[16] Both the doses of AAEFC significantly prolonged the ketamine-induced sleeping time. Hence the probable mechanism could be either increase in the brain levels of 5-HT or NE, GABA.
In addition to sedative activity, administration of AAEFC had a profound effect on motor coordination in a dose-dependent manner, indicating a possible muscle-relaxant effect.
The EPM test is considered one of the most widely validated tests for assaying new benzodiazepine-like anxiolytic agents.[19,28] Therefore, we selected this test to investigate the anxiolytic potential of AAEFC. Anxiolytic compounds increase the number of entries into open arm as well as the time spent in the open arm. These agents are thought to act via GABAA receptor complex, justifying the use of diazepam as a positive control in this study. Diazepam enhances the frequency of Cl- channel opening and thus Cl- flux through the GABAA receptor, thus potentiating the inhibitory effect of GABA. At both doses, similar to diazepam in the EPM test, AAEFC significantly reduced the animal's aversion to the open arm and promoted the exploration thereof, indicating anxiolytic effect. It has been shown that increase in central serotonergic activity leads to anxiety whereas decrease in brain 5-HT activity results in anxiolysis.[29,30,31] But in our findings, NE and 5-HT levels increased. So the mechanism of anxiolytic action of AAEFC might involve an action on GABAergic transmission; however, further studies are needed to ascertain this.
The staircase test is another method for evaluating anti-anxiety activity. AAEFC showed antianxiety activity in a dose dependent manner by increasing the number of steps climbed and decreasing the number of rearings, similar to that of diazepam. Thus it leads to the possibility that potentiation of GABA receptor opening chloride channels could be a possible mechanism of action of AAEFC.
The most popular and widely used animal seizure models are the traditional MES and PTZ test. The present study revealed that AAEFC blocked both clonic seizures induced by PTZ and tonic seizures induced by MES. MES-induced tonic seizures can be prevented either by drugs that inhibit voltage-dependent Na+ channels or by drugs that block glutaminergic excitation mediated by the N-methyl-D-aspartate (NMDA) receptor. On the other hand, drugs that reduce T-type Ca+ 2 currents can prevent seizures induced by PTZ or by drugs that enhance GABAA receptor-mediated inhibitory neurotransmission. An antiepileptic drugs, like Valproate and Felbamate, which are effective in both types of seizure tests, possess multiple mechanisms of action and display the broadest therapeutic utility. It seems that AAEFC has multiple mechanisms for its anticonvulsant action.
AAEFC showed a significant anticonvulsant activity among other CNS depressant activities. Hence, 10 day study of AAEFC was carried out in mice to determine the long-term effect as an anticonvulsant. In 10 day study both the models MES and PTZ induced convulsions were performed. In both, significant results were observed, confirming the anticonvulsant activity of AAEFC.
AAEFC also showed a good central analgesic activity by increasing the pain threshold.
The excitation and inhibition in a physiological system is maintained by a balanced ratio of three neurotransmitters (5-HT, DA and NE). It has been reported that these catecholamines and 5-HT are involved in the etiology of depression.
Biochemical estimation of essential brain monoamine (NE and 5-HT) in single mice brain was done using solvent extraction method. AAEFC treatment has shown significant increase in the NE and 5-HT levels in the brain as compared to the control group. Therefore, sedative and hypnotic action of AAEFC may be due to the increased concentration of norepinephrine level in the brain.[32] Also, decreased activity of norepinephrine has been found in some epileptic patients.[33,34] So, the protection offered by AAEFC against chemo convulsions in mice is probably due to the increased levels of NE in brain.
Norepinephrine appears to protect against electroshock-induced convulsions, whereas 5-HT protects against PTZ-induced convulsions.[35,36] Drugs which increase brain 5-HT level usually increase sleep whereas drugs which decrease brain 5-HT level induce a state of permanent wakefulness. Serotonin is known to be major endogenous anticonvulsants.[37,38] AAEFC may exert its anticonvulsant activity by elevation of 5-HT levels.[39] Thus it can be postulated that CNS depressant action in mice induced by the AAEFC might be related to the increased concentration of NE and 5-HT in the mouse brain.
Hence, phytochemical constituents in AAEFC may be selectively interacting with central benzodiazepine receptor, acting as partial agonists in these receptors. This interaction in the complex GABAA receptor, that hyperpolarizes the neuronal membrane, promotes anxiolytic, sedative, anticonvulsant and muscle relaxant effects. Additionally, AAEFC has serotonergic action on CNS. Thus, suggesting that the alterations in NE and 5-HT may be responsible for the central inhibitory effect of AAEFC. However, role of GABA cannot be denied and further studies are needed to elucidate it.
CONCLUSION
In conclusion, this study provides evidence supporting the traditional use of Ficus carica L. as an antiepileptic drug. It also has sedative-hypnotic, skeletal muscle relaxant and anxiolytic actions on CNS. This suggests that Ficus carica L. may be useful as therapeutic tool in insomnia, anxiety, schizophrenia, migraine and epilepsy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Survey conducted by The Royal Society of Medicine. [Last accessed on 2013 Oct 05]. Available from: http://www.blogs.lse.ac.uk/healthandsocial care/2013/05/23/global-mental-health-matters .
- 2.Carlini EA. Plants and the nervous system. Pharmacol Biochem Behav. 2003;3:501–12. doi: 10.1016/s0091-3057(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 3.Saxena A, Hafsa A, Gupta R. Memory enhancing effects of Ficus carica leaves in hexane extract on interoceptive behavioral models. Asian J Pharm Clin Res. 2013;6:109–13. [Google Scholar]
- 4.Sastri BN. IV. F-G New Delhi: Publications and Information Directorate (CSIR); 1995. The Wealth of India. A Dictionary of Indian Raw materials and industrial products; pp. 26–31. [Google Scholar]
- 5.Joshi SG. New Delhi: Oxford and IBG Publishing Co. Pvt. Ltd; 2004. Medicinal Plants; p. 280. [Google Scholar]
- 6.Khare CP. New York, Berlin: Springer-Verlag, Heidelberg; 2004. Encyclopedia of Indian Medicinal Plants: Rational Western therapy, Ayurvedic and other Traditional usage; pp. 218–20. [Google Scholar]
- 7.Vaya J, Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.) Biofactors. 2006;28:169–75. doi: 10.1002/biof.5520280303. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Y, Ping J, Wang H, Li L, Sheng Y, Lian Y. Study on sedative and hypnotic effects of extract from Ficus carica leaves. Med J Wuhan Univ. 2008;29:763–5. [Google Scholar]
- 9.Amaraa A, Ei-Masry MH, Bogdady HH. Plant crude extracts could be the solution: Extracts showing in vivo antitumorigenic activity. Pak J Pharm Sci. 2008;21:159–71. [PubMed] [Google Scholar]
- 10.Yang X, Yu W, Ou Z, Ma H, Liu W, Ji X. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Food Hum Nutr. 2009;64:167–73. doi: 10.1007/s11130-009-0120-5. [DOI] [PubMed] [Google Scholar]
- 11.Krishna Mohan G, Pallavi E, Ravi Kumar B, Ramesh M, Venkatesh S. Hepatoprotective activity of Ficus carica L. leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. DARU. 2007;15:162–6. [Google Scholar]
- 12.Gond NY, Khadabadi SS. Hepatoprotective activity of Ficus carica leaf extract on Rifampicin-induced hepatic damage in rats. Indian J Pharm Sci. 2008;70:364–6. doi: 10.4103/0250-474X.43003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilani AH, Mehmood MH, Janbaz KH, Khan A, Saeed SA. Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. J Ethnopharmacol. 2008;119:1–5. doi: 10.1016/j.jep.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 14.OECD Guidelines for testing of chemicals. Acute oral toxicity – Up and Down procedure. 2001 Assessment no – 425. [Google Scholar]
- 15.Turner RA. Screening methods in pharmacology. New York: Academic Press; 1972. The organization in screening; pp. 22–41. [Google Scholar]
- 16.Mimura M, Namiki A, Kishi R, Ikeda T, Miyake H. Antagonistic effect of physostigmine on ketamine-induced anesthesia. Psychopharmacology (Berl) 1990;102:399–403. doi: 10.1007/BF02244110. [DOI] [PubMed] [Google Scholar]
- 17.Rabbani M, Sajjadi SE, Zarei HR. Anxiolytic effects of Stachys lavandulifolia Vahl on the elevated plus-maze model of anxiety in mice. J Ethnopharmacol. 2003;89:271–6. doi: 10.1016/j.jep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Emamghoreishi M, Khasaki M, Aazam MF. Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–70. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Pellow S, Chopin P, File SE, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 20.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 21.Pellow S, File S. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:526–30. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 22.Simiand J, Keane PE, Morre M. The staircase test in mice: A simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology. 1984;84:48–53. doi: 10.1007/BF00432023. [DOI] [PubMed] [Google Scholar]
- 23.Swinyard EA, Woodhead JH, White HS, Franklin MR. Experimental selection, quantification and evaluation of anticonvulsants. In: Levy R, Mattson R, Meldrum BS, Penry JK, Dreifuss FE, editors. Antiepileptic Drugs. 3rd ed. New York: Raven Press; 1989. pp. 85–102. [Google Scholar]
- 24.Swinyard EA. Laboratory assay of clinically effective antiepileptic drugs. Am Pharm Assoc. 1949;38:201–4. [PubMed] [Google Scholar]
- 25.Brownlee G, Spriggs TL. Estimation of dopamine, noradrenaline, adrenaline and 5-hydroxytryptamine from single rat brains. J Pharm Pharmacol. 1965;17:429–33. doi: 10.1111/j.2042-7158.1965.tb07698.x. [DOI] [PubMed] [Google Scholar]
- 26.Shore PA, Olin JS. Identification and chemical assay of norepinephrine in brain and other tissues. J Pharmacol. 1958;122:295–300. [PubMed] [Google Scholar]
- 27.Bogdanski DF, Pletscher A, Brodie BB, Udenfriend S. Identification and assay of serotonin in brain. J Pharmacol Exp Ther. 1956;117:82–8. [PubMed] [Google Scholar]
- 28.Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety on pathological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- 29.Argal A, Pathak AK. CNS activity of Calotropis gigantea roots. J Ethnopharmacol. 2006;10:142–5. doi: 10.1016/j.jep.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Kahn RS, Van Prag HN, Watzler S, Asnis GM, Barr G. Serotonin and anxiety revisited. Biol Psychiatry. 1988;23:189–208. doi: 10.1016/0006-3223(88)90091-1. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji M, Takeda H, Matsumiya T. Multiplicity of anxiety and serotonin in nervous system. Nippon Yakurigaku Zasshi. 2000;15:29–38. doi: 10.1254/fpj.115.29. [DOI] [PubMed] [Google Scholar]
- 32.Gupta M, Sasmal D, Gupta SD, Bagchi GK. Changes of epinephrine, norepinephrine, dopamine in mice brain and liver and 5-hydroxytrytamine in mice brain following treatment with ochratoxin A and citrinin. Indian J Pharmacol. 1984;16:102–6. [Google Scholar]
- 33.Bradely PB. Frontiers in Catecholamine Research. London, New York: Academic Press; 1973. Excitatory effect of catecholamines in the Central Nervous System; p. 653. [Google Scholar]
- 34.Mazumder UK, Gupta M, Pal DK, Bhattacharya S. CNS activities of the methanol extracts of Cuscuta reflexa Roxb. stem and Corchorous olitorius L. seed in mice. Malays J Pharm. 2006;1:190–8. [Google Scholar]
- 35.Jobe PC, Stull RE, Geiger PF. Relative significance of norepinephrine, dopamine and 5-HT in electroshock seizure in rat. Neuropharmacology. 1974;13:961–8. doi: 10.1016/0028-3908(74)90087-2. [DOI] [PubMed] [Google Scholar]
- 36.Rudzick AD, Johnson GA. Amphetamines and Related Compounds. New York: Raven press; 1970. p. 715. [Google Scholar]
- 37.Hattori H, Ito M, Mikawa H. Gamma amino butyric acid benzodiazepine binding sites in genetically seizure prone rats. Eur J Pharmacol. 1985;119:217. doi: 10.1016/0014-2999(85)90298-5. [DOI] [PubMed] [Google Scholar]
- 38.Pasini A, Tortorella A, Gale K. Anticonvulsant effect of intra nigral fluoxetin. Brain Res. 1992;119:217. doi: 10.1016/0006-8993(92)91320-e. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Guha D. CNS depressive role of aqueous extract of Spinacia oleracea L. leaves in adult male albino rats. Indian J Exp Biol. 2008;46:185–90. [PubMed] [Google Scholar]


