Abstract
Background:
Glucova Active Tablet is a proprietary Ayurvedic formulation with ingredients reported for anti-hyperglycemic, anti-hyperlipidemic activity and antioxidant properties.
Objective:
Evaluation of anti-diabetic activity of Glucova Active Tablet on Type I and Type II diabetic model in rats.
Materials and Methods:
Experimental Type I diabetes was induced in 24 albino rats with intra-peritoneal injection of streptozotocin (50 mg/kg). Type II diabetes was induced in 18 albino rats by intra-peritoneal injection of streptozotocin (35 mg/kg) along with high fat diet. The rats were divided in 5 groups for Type I model and 4 groups for Type II model. Normal control group was kept common for both experimental models. Glucova Active Tablet (108 mg/kg) treatment was provided for 28 days twice daily orally. Fasting blood glucose level, serum lipid profile and liver anti-oxidant parameters like superoxide dismutase and reduced glutathione was carried out in both experimental models. Pancreas histopathology was also done. Statistical analysis were done by ‘analysis of variance’ test followed by post hoc Tukey's test, with significant level of P < 0.05.
Results and Discussion:
Glucova Active Tablet showed significant effect on fasting blood glucose level. It also showed significant alteration in lipid profile and antioxidant parameters. Histopathology study revealed restoration of beta cells in pancreas in Glucova Active Tablet treated group.
Conclusion:
Finding of this study concludes that Glucova Active Tablet has shown promising anti-diabetic activity in Type I and Type II diabetic rats. It was also found showing good anti-hyperlipidemic activity and anti-oxidant property.
Keywords: Anti-hyperlipidemic, anti-oxidant, Glucova Active Tablet, Type I and Type II diabetic model
INTRODUCTION
Diabetes mellitus (DM) is the most common metabolic disorder. It is characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both.[1] As per World Health Organization (WHO), at least 171 million people (2.8% of worldwide population) were suffered from diabetes in year of 2000.[2] It is estimated that by the year 2030, this number may almost be doubled.[2] The greatest increase in prevalence is expected to occur in Asia and Africa.[2] The increase in incidence of diabetes in developing countries follows trend of urbanization and lifestyle changes, perhaps most importantly a “Western-style” diet.
Diabetes mellitus is classified into two types, insulin-dependent diabetes mellitus (IDDM, Type I) and non-insulin-dependent diabetes mellitus (NIDDM, Type II). Type I diabetes is an autoimmune disease characterized by a local inflammatory reaction in and around islets of pancreas that is followed by selective destruction of insulin-secreting β cells. Type II diabetes is characterized by peripheral insulin resistance and impaired insulin secretion.[1]
Management of diabetes without any side effects is still a challenge in the medical field, as presently available drugs for diabetes have one or more adverse effects.[3] Since the existing drugs for the treatment of DM do not satisfy our need completely, the search for new drugs will continue. In recent years, herbal remedies for the unsolved medical problems have been gaining importance in the research field.
Glucova Active Tablet is a such proprietary Ayurvedic formulation which contains extract of Enicostemma littorale (Mamajjak) whole plant,[4,5] Pterocarpus marsupium (Vijayasar) Bark,[6,7] Momordica charantia (Karela) Fruit,[8] Tinospora cordifolia (Guduchi) Stem,[3] Gymnema sylvestre (Madhunashini) Aerial,[9] Syzygium cuminii (Jamun) Seed,[10] Azadirachta indica (Neem) Leaves,[11] Caesalpinia crista (Latakaranj) Seed,[12] Swertia chirata (Kiratatikta) Aerial,[13] Curcuma longa (Haridra) Rhizome,[14] Emblica officinalis (Amalaki) Fruit[15] and powder of Pramehahar Kwath,[16] Piper nigrum (Marich) Fruit,[17] Zingiber officinale (Shunthi) Rhizome,[17] Piper longum (Pippali) Fruit.[17] Research article supported that Glucova Active Tablet is quality-based proprietary ayurvedic formulation.[18] It is a proprietary Ayurvedic medicine manufactured and marketed by Vasu Healthcare Pvt. Ltd., Vadodara. Majority of ingredients of Glucova Active Tablet are well reported in Ayurvedic texts and scientific research publications for anti-hyperglycemic, anti-hyperlipidemic activity and antioxidant property. However, no such evidence was found which proves safety and efficacy of such combination.
In the present study, an attempt was made to investigate the toxicity and anti-diabetic activity of Glucova Active Tablet on Type I and Type II diabetic model in rats.
MATERIALS AND METHODS
Experimental animals
Albino Wistar rats (160-180 g) were procured from the Flair Lab, Surat, India. The animals were housed in standard polypropylene cages (three rats/cage) and maintained under controlled room temperature (22 ± 2°C) and humidity (55 ± 5%) with 12:12 h light and dark cycle. All the rats were provided with commercially available rat normal pellets diet (NPD) and water ad libitum, prior to the dietary manipulation. The guidelines of committee for the purpose of control and supervision of experiments on animals (CPCSEA), Govt. of India were followed. Prior permission was taken from the institutional animal ethics committee IAEC Protocol No. 984/11/03 for conducting the study.
Drugs and chemicals
Glucova Active Tablet (Batch No. 102) was received from Vasu Healthcare Pvt. Ltd., Baroda, Gujarat, India. Streptozotocin (STZ) was purchased from Sigma Eldrich Lab, Mumbai. The feed ingredients such as casein and cholesterol (Sigma Eldrich Lab, Mumbai, India), dl-methionine (Jubilant Pharma Company, Vadodara), vitamin and mineral mix (Sarabhai chemicals, Baroda, India) and yeast powder (Jubilant Pharma Company, Vadodara, India) were procured from the commercial sources. Lard, insulin (Eli Lilly, Gurgaon, India) and sodium carboxy methyl cellulose (Na-CMC) (Sigma Eldrich Lab, Mumbai) were also obtained from commercial sources. Sample of metformin was received from Intas Pharmaceutical, India. The compound was administered orally as suspension by mixing with vehicle 1% Na-CMC. Dose of the test drug was fixed by extrapolating the human dose to laboratory animals, based on body surface area ratio as per the table of Paget and Barnes.
Acute toxicity study
Healthy female Wistar albino rats (210-240 g) were divided into 2 groups of 3 animals each. The animals had free access to water and food throughout the experiment, except for the fasting period before the oral administration of the single dose of the formulation. The formulation was suspended in distilled water and administered by gavages (orally) at single doses of 2000 mg/kg to 1st group and single doses of 5000 mg/kg 2nd group. The general behavior and mortality of the rats was continuously monitored for 1 h after dosing periodically during first 24 h (with special attention given during the first 4 h.) and then daily for a total of the 14 days. Changes in the normal activity of rats, sign and symptoms of toxicity and mortality were monitored and recorded. Acute toxicity study was carried out as per OECD Guidelines 423.[19]
Induction of Type I diabetes by STZ
Rats were fasted overnight. Then they were injected with STZ at a dose of 50 mg/kg (citrate buffer pH 4.4, i.p.). The induction of diabetes was confirmed by determination of high fasting blood glucose level with polydipsia and polyuria on the fifth day of STZ administration. Rats with fasting blood glucose level >200 mg/dL were selected for experimentation.[20]
Induction of Type II diabetes by high fat diet-fed and STZ
The rats were kept on dietary regimens consisting HFD (58% fat, 25% protein and 17% carbohydrate, as a percentage of total kcal) ad libitum, for the initial period of 2 weeks. After 2 weeks, the rats were injected with low dose of STZ (35 mg/kg in citrate buffer pH 4.4, i.p.). Before and after 7 days of STZ injection, body weight and plasma glucose (PGL) were measured. The rats with the non-fasting blood glucose level of 300 mg/dL were considered as diabetic. Food and water intake of the animals were also measured. The rats were allowed to continue to feed on their respective diets till the end of the study.[21]
Experimental design for Type I diabetes
The experimental animals were divided into five groups, six animals in each group. Normal control group was common for Type I and Type II diabetic model.
Group-1 served as Normal control
Group-2 served as Type I Diabetic control [50 mg/kg once (i.p.) of STZ]
Group-3 served as Type I Diabetic rats treated with Glucova Active Tablet [108 mg/kg twice daily, p.o., for 28 days]
Group-4 served as Type I Diabetic rats treated with Metformin [100 mg/kg twice daily, p.o., for 28 days]
Group-5 served as Type I Diabetic rats treated with insulin [6 unit/kg daily, i.v., for 28 days].
Experimental design for Type II diabetes
The experimental animals were divided into four groups, six animals in each group. Normal control group was common for Type I and Type II diabetic model.
Group-1 served as Normal control
Group-2 served as Type II Diabetic Control [HFD for 28 days + STZ (35 mg/kg once i.p.) on 15th day of HFD]
Group-3 served as Type II Diabetic rats treated with Glucova Active Tablet [108 mg/kg twice daily, p.o., for 28 days]
Group-4 served as Type II Diabetic rats treated with Metformin [100 mg/kg twice daily, p.o., for 28 days].
Blood sample collection
After treatment, leaving 12 hours fasting period, blood samples were collected from rats under light ether anesthesia from retro orbital plexuses in clean dry centrifuge tubes. Samples were allowed to clot for 30 min at room temperature. Sera from the samples were obtained by centrifugation after 30 min at 4000 rpm, then divided into aliquots and kept at 2-8°C for further assay of lipid profile and other biochemical parameters while serum glucose was determined immediately.
Biochemical parameters
Biochemical parameters such as fasting blood glucose (FBG) level, serum cholesterol, serum triglyceride, serum high density lipoprotein (HDL), serum low density lipoprotein (LDL) and serum very low density lipoprotein (VLDL) were carried out using auto analyzer (Merck, Germany).
Anti-oxidant parameters of liver homogenates like super oxide dismutase (SOD)[22] and reduced glutathione (GSH)[23] were also carried out.
Estimation of Super oxide dismutase
0.5 mL of tissue homogenate, 0.5 mL of cold distilled water, 0.25 mL of ice-cold ethanol and 0.15 mL of ice-cold chloroform were mixed well using cyclomixer for 5 min and centrifuged at 2500 rpm for 15 min at 4°C. To 0.5 mL of supernatant, 1.5 mL of carbonate buffer (pH 10.2) and 0.5 mL of 0.4 M ethylenediamine tetra-acetic acid (EDTA) solutions were added. The reaction was initiated by the addition of 0.4 mL of epinephrine bitartrate (3 mM) and the change in optic density/min was measured at 480 nm against reagent blank. SOD activity was expressed as units/mg of protein. Change in optical density/minute at 50% inhibition of epinephrine to adrenochrome transition by the enzyme was taken as the enzyme unit. Calibration curve was prepared by using 1-12 units of SOD.[22]
Estimation of reduced glutathione
GSH contents were measured after precipitation of protein with 10% (w/v) chilled trichloroacetic acid and equal volumes (0.2:0.2 mL) of supernatant and 10% trichloroacetic acid were centrifuged at 5000 rpm for 30 min. by using cooling centrifuge. The supernatant thus obtained was used to estimate GSH content. 0.25 mL supernatant was mixed with 1.0 mL, 0.3 M disodium hydrogen phosphate solution (pH 8). 0.125 mL DTNB- 5,5 – dithiobis-2-nitrobenzoic acid (prepared in 1% w/v sodium citrate) was added just before measuring the absorbance at 412 nm using UV spectrophotometer. Different concentrations of GSH were also processed similarly to prepare a standard curve.[23]
Histopathology of pancreas
Whole pancreas from each animal was removed after sacrificing the animal under anesthesia and it was collected in 10% formalin solution and immediately processed by paraffin technique. Section of approximately 5 μm thickness was cut and stained by hematoxylin and eosin (HandE). Then sections were examined under microscope to evaluate structural changes in beta cells.
Statistical analysis
Analysis was done with the help of standard statistical software, Graph pad prism version 5. Data was expressed as Mean ± Standard Error of Mean. Different groups were compared with analysis of variance (ANOVA) and same group in different time points with repeated measures ANOVA followed by post hoc Tukey's test. A p < 0.05 was considered as statistically significant.
RESULTS
Acute toxicity study
The animals were observed for mortality and other toxic symptoms for 14 days of observation period. No toxic symptoms and mortality were found in both the dose level during this study.
Evaluation of anti-diabetic potential of Glucova Active Tablet on Type I and Type II diabetes
Effect on fasting blood glucose level
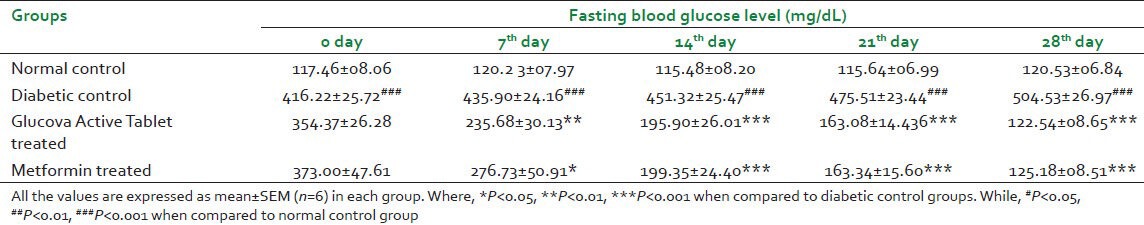
Disease control animals of both experimental models showed statistically significant (P < 0.001) increase in blood glucose level as compared to normal control group. It indicates diabetes was effectively induced. After 28 days of pretreatment of Glucova Active Tablet in both experimental models i.e., STZ and High Fat Diet + STZ induced Type I and Type II diabetic, respectively, showed significant reduction in elevated blood glucose levels. Data represent that Glucova Active Tablet showed statistically significant decreases (P < 0.001) in blood glucose level in Type I and Type II diabetic models. As expected, in metformin-treated rats, blood glucose level was found significantly decreased in experimental models. Insulin treatment also significantly reduced blood glucose level when administered in Type I diabetic animals. Results are found to be statistically significant in comparison with diabetic control group [Tables 1 and 2].
Table 1.
Effect of Glucova Active Tablet on fasting blood glucose level in Type I diabetic rats
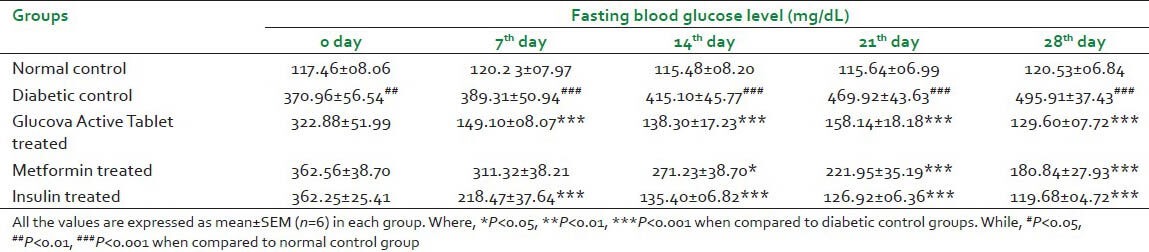
Table 2.
Effect of Glucova Active Tablet on fasting blood glucose level in Type II diabetic rats
Effect on lipid profile
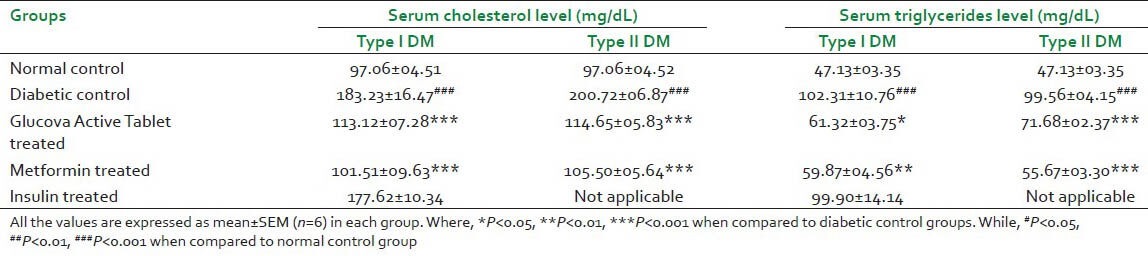
Disease control group in both experimental models exhibited statistically significant increase in serum cholesterol and serum triglycerides as compared to normal control group. Glucova Active Tablet significantly reduced elevated level of serum cholesterol and serum triglycerides in comparison to disease control group of Type I and Type II diabetic model [Table 3]. Metformin treatment also significantly reversed elevated level of serum cholesterol and serum triglycerides. Insulin treatment did not show any significant alteration in both parameters with respect to disease control group [Table 3].
Table 3.
Effect of Glucova Active Tablet on serum cholesterol and serum triglycerides level in Type I and Type II diabetic rats
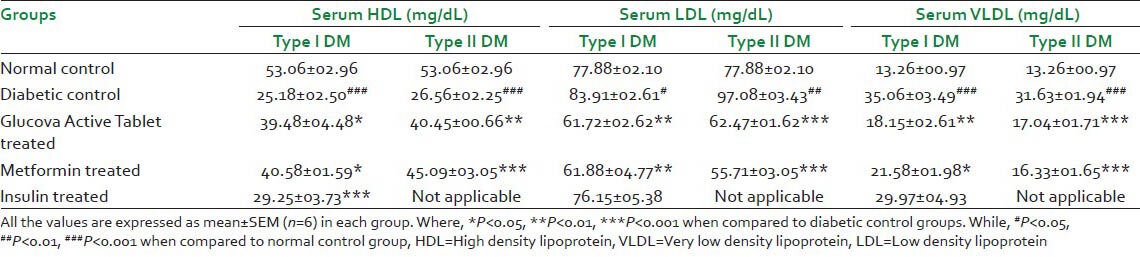
Serum HDL significantly increased where serum LDL and VLDL level significantly decreased in Glucova Active Tablet treated groups of Type I and Type II diabetic models [Table 4]. Comparison was carried out with respect to disease control groups of respective model. More significant effect was observed in Glucova Active Tablet treated Type II diabetic rats in comparison to Glucova Active Tablet treated Type I diabetic rats [Table 4].
Table 4.
Effect of Glucova Active Tablet on serum HDL, LDL and VLDL in Type I and Type II diabetic rats
Effect on anti-oxidant biochemical parameters of liver
Antioxidant parameters like SOD level and reduced glutathione of liver was significantly increased in Glucova Active Tablet treated groups in Type I and Type II diabetic models. No significant change was observed in SOD level of Glucova Active Tablet treated Type I diabetic rats [Table 5].
Table 5.
Effect of Glucova Active Tablet on anti-oxidant biochemical parameters of liver
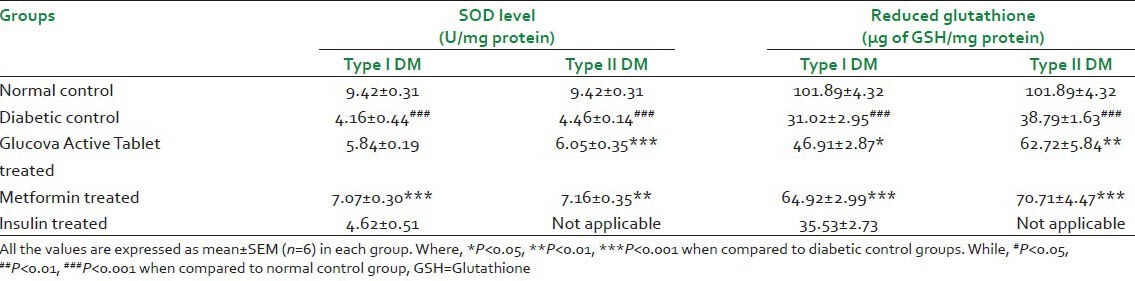
Histopathological findings
Histopathology of pancreas of normal control rat showed normal cytoarchitecture of pancreatic cells [Figure 2a]. The impact of treatment of Glucova Active Tablet on the structure of the beta cells of pancreas was studied as compared to the disease control group of representative model for Type I [Figure 1a] and Type II [Figure 2b] diabetes. Glucova Active Tablet treated groups showed improvement in pancreatic islets cell histopathology in both Type I [Figure 1b] and Type II [Figure 2c] experimental models. Reference standard drug treated groups for Type I diabetic model i.e., Metformin treated [Figure 1c] and Insulin treated [Figure 1d] showed significant recovery. In Type II diabetic model reference standard metformin-treated group [Figure 2d] also showed similar recovery like Type I diabetic model.
Figure 2.
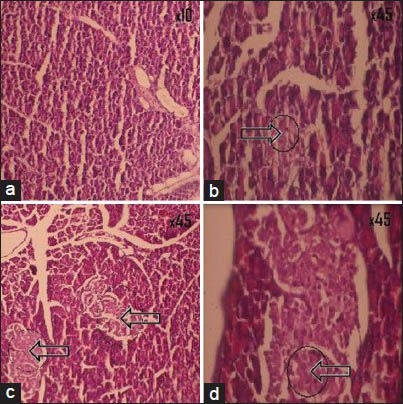
Histopathological pictures of pancreas of Type II diabetes. (a) Normal control, showing normal cyto-architecture of pancreatic cells; (b) Diabetic control, showing beta cell destruction and fat deposition as indicate by arrow; (c) Glucova Active Tablet treated, showing recovery of pancreatic beta cell destruction and fat deposition as indicate by arrow; (d) Metformin treated, showing recovery of pancreatic beta cell destruction as indicate by arrow
Figure 1.
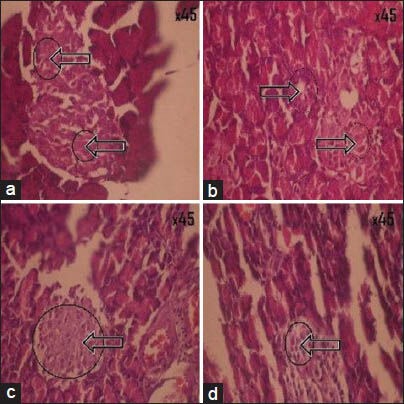
Histopathological pictures of pancreas of Type I diabetes. (a) Diabetic control, beta cell destruction as indicate by arrow; (b) Glucova Active Tablet treated, showing recovery of pancreatic beta cell destruction as indicate by arrow; (c) Metformin treated, showing recovery of pancreatic beta cell destruction as indicate by arrow; (d) Insulin treated, showing minor recovery of pancreatic beta cell destruction as indicate by arrow
DISCUSSION
The present study was undertaken to investigate toxicity as well as anti-diabetic, anti-hyperlipidemic and antioxidant effects of Glucova Active Tablet in Type I and Type II diabetic rat models. Metformin and insulin both were used as reference standard for Type I diabetic model as in conventional line of treatment they are frequently prescribed in Type I patients. In Type II model, metformin is used as reference standard because it is well known drugs for treatment of Type II diabetes.
Glucova Active Tablet showed significant decrease in fasting blood glucose levels which was near to normal control group of animals in both experimental models. Insulin deficiency may be responsible for dyslipidemia. Acute insulin deficiency initially causes an increase in free fatty acid mobilization from adipose tissue.[21] Glucova Active Tablet significantly decreased both serum cholesterol and triglyceride levels in both types of diabetic rat models. There were also significant decrease in serum LDL and VLDL levels in Glucova Active Tablet treated Type I and Type II experimental models as compared to their respective diabetic control groups. Also significant increase was observed in serum HDL level in Glucova Active Tablet treated groups as compared to diabetic control group. Metformin-treated animals have shown positive effects on complete lipid profile whereas insulin-treatment showed remarkable change in serum HDL level only.
It is now a known fact that oxidative stress plays a major role in the pathogenesis of both types of diabetes mellitus. High levels of free radicals and simultaneously declined antioxidant enzyme levels lead to cell damage. In the present study, diabetic control rats showed significant decrease in SOD and reduced glutathione levels in the rat liver homogenates compared to normal rats, indicating a dysfunction in antioxidant defensive system in diabetes mellitus.[24] Treatment with Glucova Active Tablet showed significant increase in level of antioxidant enzymes in Type I and Type II diabetic rats. An antioxidant property of Glucova Active Tablet may be due to presence of Momordica charantia (Karela), Tinospora cordifolia (Guduchi), Curcuma longa (Haridra) and Emblica officinalis (Amalaki). All these ingredients have been well reported for having antioxidant property.
Treatment with Glucova Active Tablet also showed restoration of beta cell of pancreas in both diabetic models which indicates the efficacy of formulation at cellular level.
CONCLUSION
On the basis of study data it can be concluded that Glucova Active Tablet has promising anti-diabetic activity against Type I and Type II diabetic conditions. It also has showed good anti-hyperlipidemic and anti-oxidant property which can be attributed to synergistic effect of multiple herbal ingredients of formulation.
ACKNOWLEDGEMENT
Authors are sincerely thankful of the management of Vasu Healthcare for providing product sample and technical support. Also thankful to management of Baroda College of Pharmacy for providing the necessary facilities for conducting the study.
Footnotes
Source of Support: Financial support was provided by Vasu Research Centre (A Division of Vasu Healthcare Pvt. Ltd.).
Conflict of Interest: None declared.
REFERENCES
- 1.Arora S, Ojha S, Vohora D. Characterization of streptozotocin induced diabetes mellitus in Swiss Albino Mice. Glob J Pharmacol. 2009;3:81–4. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Puranik N, Kammar KF, Devi S. Anti-diabetic activity of Tinospora cordifolia (Willd.) in streptozotocin diabetic rats; does it act like sulfonylureas? Turk J Med Sci. 2010;40:265–70. [Google Scholar]
- 4.Maroo J, Gosh A, Mathur R, Vasu VT, Gupta S. Anti-diabetic efficacy of Enicostemma littorale methanol extract in alloxan- induced diabetic rats. J Pharm Biol. 2003;41:388–91. [Google Scholar]
- 5.Murali B, Upadhyaya UM, Goyal RK. Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic (NIDDM) rats. J Ethnopharmacol. 2002;81:199–204. doi: 10.1016/s0378-8741(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 6.Sathyaraj A, Satyanarayana V, Ramakrishna, Ramakanth Anti-diabetic activity of Pterocarpus marsupium and recent advances of Anti-diabetic therapies in drug discovery technologies. Int J Res Pharm Chem. 2011;1:870–8. [Google Scholar]
- 7.Gayathri M, Kannabiran K. Ameliorative potential of aqueous extract of Pterocarpus marsupium Roxb bark on diabetes associated metabolic alterations. Curr Trend Biotechnol Pharm. 2008;2:327–33. [Google Scholar]
- 8.Fernandes N, Lagishetty C, Panda V, Naik S. An experimental evaluation of the antidiabetic and antihyperlipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement Altern Med. 2007;7:29. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persaud SJ, Al-Majed H, Raman A, Jones PM. Gymnema sylverstre stimulates insulin release in vitro by increased membrane permeability. J Endocrinol. 1999;163:207–12. doi: 10.1677/joe.0.1630207. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Saxena A. Hypoglycemic and Anti-hyperglycemic activities of Syzygium cumini (Linn.) skeels whole fruit, in normal and streptozotocin-induced diabetic rats. Asian J Pharm Biol Res. 2011;1:267–72. [Google Scholar]
- 11.Akpan HD, Ekaidem IS, Usoh IF, Ebong PE, Isong NB. Effect of aqueous extract of Azadirachta indica (Neem) Leaves on some indices of pancreatic function in alloxan-induced diabetic wistar rats. Pharmacologia. 2012;3:420–5. [Google Scholar]
- 12.Chakrabarti S, Biswas TK, Seal T, Rokeya B, Ali L, Azad Khan AK, et al. Antidiabetic activity of Caesalpinia bonducella F. in chronic Type II diabetic model in Long-Evans rats and evaluation of insulin secretagogue property of its fractions on isolated islets. J Ethnopharmacol. 2005;97:117–22. doi: 10.1016/j.jep.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekar B, Bajpai MB, Mukherjee SK. Hypoglycemic activity of Swertia chirayita (Roxb ex Flem) Karst. Indian J Exp Biol. 1990;28:616–8. [PubMed] [Google Scholar]
- 14.Rai PK, Jaiswal D, Mehta S, Rai DK, Sharma B, Watal G. Effect of Curcuma longa freeze dried rhizome powder with milk in STZ induced diabetic rats. Indian J Clin Biochem. 2010;25:175–81. doi: 10.1007/s12291-010-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirgar PR, Shah KV, Patel VP, Desai TR, Goyal RK. Investigation into mechanism of action of anti-diabetic activity of Emblica officinalis on streptozotocin induced type I diabetic rat. Res J Pharm Biol Chem Sci. 2010;1:672–82. [Google Scholar]
- 16.Vol. 1. Ajmer: Published by Krishna Gopal Ayurved Bhavan; 1980. Rastantrasar and Shiddhprayog Sangrah; pp. 619–21. [Google Scholar]
- 17.Nadkarani KM. Vol. 1. Mumbai: Popular Prakashan Pvt. Ltd; 1997. Indian Material Medica; pp. 241–3. [Google Scholar]
- 18.Pandya K, Maniar K, Soni H, Bhatt S, Patel P, Solanki B, et al. Standardization of anti-diabetic ayurvedic herbo-mineral formulation. Int J Pharm Sci Rev Res. 2011;10:174–86. [Google Scholar]
- 19.OECD 423. OECD guidelines for testing of chemicals-Acute Oral Toxicity Method. OECD 17th Dec. 2001:1–14. [Google Scholar]
- 20.Thakkar N, Patel J. Pharmacological evaluation of “Glyoherb”: A polyherbal formulation on streptozotocin-induced diabetic rats. Int J Diabetes Dev Ctries. 2010;30:1–7. doi: 10.4103/0973-3930.60001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan K, Ramarao P. Animal models in Type II diabetes research: An overview. Indian J Med Res. 2007;125:451–72. [PubMed] [Google Scholar]
- 22.Misra HP, Fridovich I. The role of Super oxide anion in the auto-oxidation of Epinephrine and a simple assay for Superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 23.Beutler E. New York, NY: Grune and Stratton; 1971. Red cell metabolism: A manual of Biochemical methods; pp. 9–16. [Google Scholar]
- 24.Georg P, Ludvik B. Lipids and diabetes. J Clin Basic Cardiol. 2000;3:159–62. [Google Scholar]


