Abstract
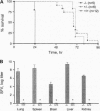
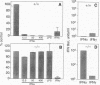
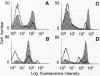
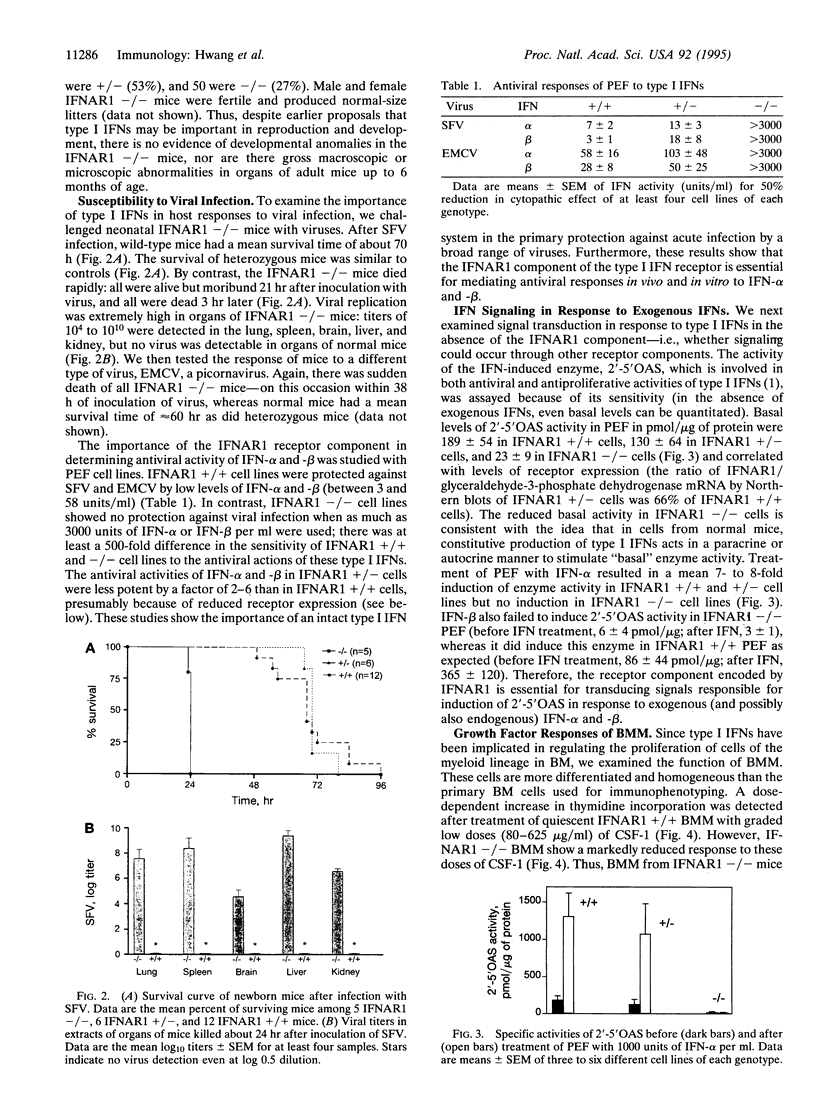
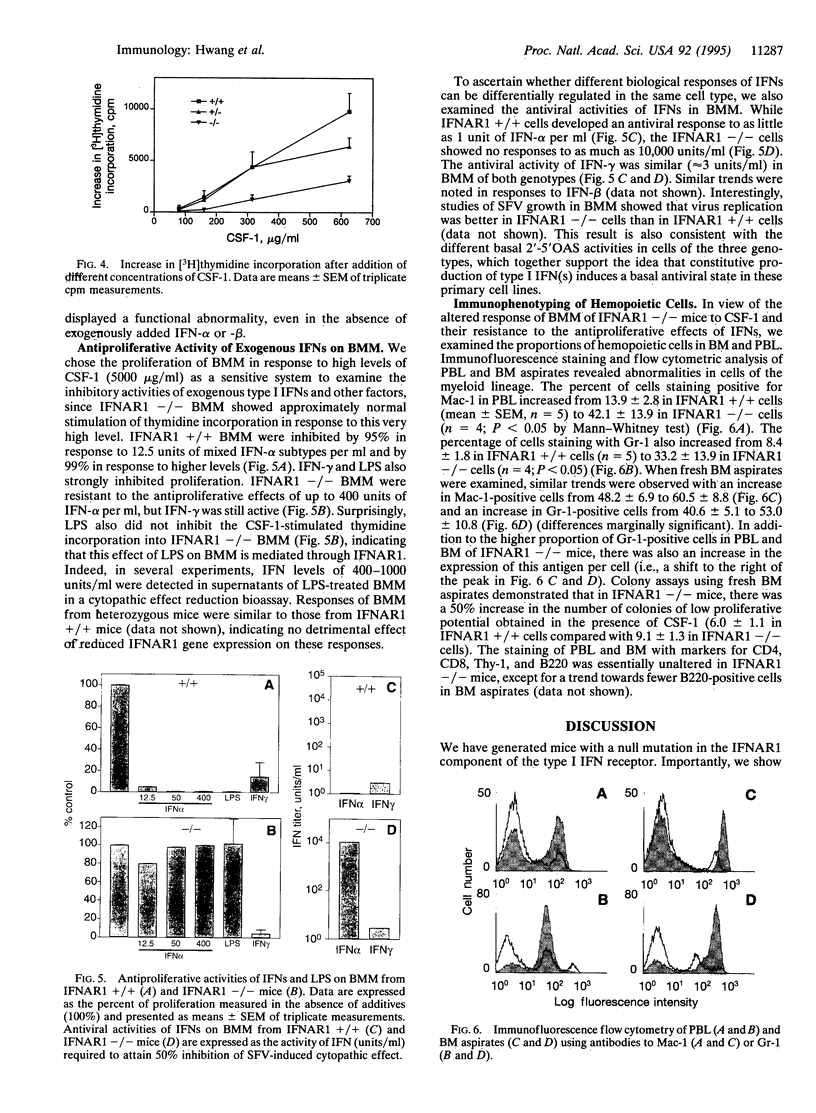
To examine the in vivo role(s) of type I interferons (IFNs) and to determine the role of a component of the type I IFN receptor (IFNAR1) in mediating responses to these IFNs, we generated mice with a null mutation (-/-) in the IFNAR1 gene. Despite compelling evidence for modulation of cell proliferation and differentiation by type I IFNs, there were no gross signs of abnormal fetal development or morphological changes in adult IFNAR1-/- mice. However, abnormalities of hemopoietic cells were detected in IFNAR1 -/- mice. Elevated levels of myeloid lineage cells were detected in peripheral blood and bone marrow by staining with Mac-1 and Gr-1 antibodies. Furthermore, bone marrow macrophages from IFNAR1 -/- mice showed abnormal responses to colony-stimulating factor 1 and lipopolysaccharide. IFNAR1 -/- mice were highly susceptible to viral infection: viral titers were undetected 24 hr after infection of IFNAR1 +/+ mice but were extremely high in organs of IFNAR1 -/- mice, demonstrating that the type I IFN system is a major acute antiviral defence. In cell lines derived from IFNAR1 -/- mice, there was no signaling in response to IFN-alpha or -beta as measured by induction of 2'-5' oligoadenylate synthetase, antiviral, or antiproliferative responses. Importantly, these studies demonstrate that type I IFNs function in the development and responses of myeloid lineage cells, particularly macrophages, and that the IFNAR1 receptor component is essential for antiproliferative and antiviral responses to IFN-alpha and -beta.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., von Gabain A. An Epstein-Barr virus immortalization associated gene segment interferes specifically with the IFN-induced anti-proliferative response in human B-lymphoid cell lines. EMBO J. 1990 Jan;9(1):147–152. doi: 10.1002/j.1460-2075.1990.tb08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S., Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann C. M., Fleischmann W. R., Jr Differential antiproliferative activities of IFNs alpha, beta and gamma: kinetics of establishment of their antiproliferative effects and the rapid development of resistance to IFNs alpha and beta. J Biol Regul Homeost Agents. 1988 Oct-Dec;2(4):173–185. [PubMed] [Google Scholar]
- Greenway A. L., Overall M. L., Sattayasai N., Rowley M. J., Hertzog P. J., McMullen G. L., Cheetham B. F., Marzuki S. Selective production of interferon-alpha subtypes by cultured peripheral blood mononuclear cells and lymphoblastoid cell lines. Immunology. 1992 Jan;75(1):182–188. [PMC free article] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Emery P., Cheetham B. F., Mackay I. R., Linnane A. W. Interferons in rheumatoid arthritis: alterations in production and response related to disease activity. Clin Immunol Immunopathol. 1988 Aug;48(2):192–201. doi: 10.1016/0090-1229(88)90083-9. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Hwang S. Y., Holland K. A., Tymms M. J., Iannello R., Kola I. A gene on human chromosome 21 located in the region 21q22.2 to 21q22.3 encodes a factor necessary for signal transduction and antiviral response to type I interferons. J Biol Chem. 1994 May 13;269(19):14088–14093. [PubMed] [Google Scholar]
- Hertzog P. J., Hwang S. Y., Kola I. Role of interferons in the regulation of cell proliferation, differentiation, and development. Mol Reprod Dev. 1994 Oct;39(2):226–232. doi: 10.1002/mrd.1080390216. [DOI] [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Hwang S. Y., Greenway A. L., McMullen G. L., Hertzog P. J. Detection of cells producing murine interferon-alpha using antipeptide antibodies. Immunol Cell Biol. 1994 Jun;72(3):234–240. doi: 10.1038/icb.1994.35. [DOI] [PubMed] [Google Scholar]
- Lutfalla G., Uzé G. Structure of the murine interferon alpha/beta receptor-encoding gene: high-frequency rearrangements in the interferon-resistant L1210 cell line. Gene. 1994 Oct 21;148(2):343–346. doi: 10.1016/0378-1119(94)90710-2. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Michalevicz R., Revel M. Interferons regulate the in vitro differentiation of multilineage lympho-myeloid stem cells in hairy cell leukemia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2307–2311. doi: 10.1073/pnas.84.8.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994 Jun 24;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Novick D., Cohen B., Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994 May 6;77(3):391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B., Harvey C., Osheroff P., Pan Y. C., Kelder B., Pestka S. A species of human alpha interferon that lacks the ability to boost human natural killer activity. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4926–4929. doi: 10.1073/pnas.81.15.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Quesada J. R., Gutterman J. U. Clinical study of recombinant DNA-produced leukocyte interferon (clone A) in a intermittent schedule in cancer patients. J Natl Cancer Inst. 1983 Jun;70(6):1041–1046. [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Soh J., Mariano T. M., Lim J. K., Izotova L., Mirochnitchenko O., Schwartz B., Langer J. A., Pestka S. Expression of a functional human type I interferon receptor in hamster cells: application of functional yeast artificial chromosome (YAC) screening. J Biol Chem. 1994 Jul 8;269(27):18102–18110. [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Streuli M., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Vignaux F., Gigou M. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5038–5042. doi: 10.1073/pnas.84.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Bandu M. T., Proudhon D., Mogensen K. E. Behavior of a cloned murine interferon alpha/beta receptor expressed in homospecific or heterospecific background. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990 Jan 26;60(2):225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- Vairo G., Hamilton J. A. CSF-1 stimulates Na+K+-ATPase mediated 86Rb+ uptake in mouse bone marrow-derived macrophages. Biochem Biophys Res Commun. 1985 Oct 15;132(1):430–437. doi: 10.1016/0006-291x(85)91040-x. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Weber H. The interferon genes. Prog Nucleic Acid Res Mol Biol. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]