Abstract
The potential role of metabolic impairments in the pathophysiology of depression is motivating researchers to evaluate the treatment efficacy of creatine, a naturally occurring energetic and neuroprotective compound found in brain and muscle tissues. Growing evidence is demonstrating the benefit of oral creatine supplements for reducing depressive symptoms in humans and animals. A novel question is whether dietary creatine, when combined with antidepressant drug therapy, would be more effective than either compound alone. To answer this question, four studies were conducted to investigate the behavioral effects of combined creatine and low-dose fluoxetine treatment using the forced swim test in male and female rats. Sprague-Dawley rats were fed powdered rodent chow supplemented with 0%, 2% or 4% w/w creatine monohydrate for 5 weeks. Rats were injected with fluoxetine (5.0 or 10.0 mg/kg) or saline according to a sub-acute dosing schedule. Female rats maintained on a 4% creatine diet displayed antidepressant-like effects compared to non-supplemented females prior to fluoxetine treatment. In contrast, creatine did not alter behavior reliably in males. Following drug treatment and a second forced swim trial, the antidepressant-like profile of creatine remained significant only in females co-administered 5.0 mg/kg fluoxetine. Moreover, in females only, supplementation with 4% creatine produced a more robust antidepressant-like behavioral profile compared to either dose of fluoxetine alone. Estrous cycle data indicated that ovarian hormones influenced the antidepressant-like effects of creatine. Addressing the issue of sex differences in response to treatment may affect our understanding of creatine, its relationship with depressive behavior, and may lead to sex-specific therapeutic strategies.
Keywords: creatine, fluoxetine, major depressive disorder, mood, forced swim test, antidepressant, sex differences
1. Introduction
Modulation of the creatine-creatine kinase-phosphocreatine pathway is a promising target for the treatment of depressive disorders (Iosifescu et al., 2008; Lyoo and Renshaw, 2002; Moretti et al., 2003). This system is vital for maintaining steady-state equilibrium in brain bioenergetic metabolism by rapidly replenishing ATP in response to significant energy demands. An increasing number of reports link creatine and phosphocreatine, as well as the enzyme creatine kinase, with the pathophysiology of depression (Agren and Niklasson, 1988; Kato et al., 1992; Moore et al., 1997; Nery et al., 2009; Niklasson and Agren, 1984; Rezin et al., 2009; Volz et al., 1998). Moreover, antidepressants alter creatine metabolism, which may be an important mode of action for these drugs (Assis et al., 2009; Santos et al., 2009).
Daily long-term supplementation with creatine monohydrate produces measurable increases in creatine and phosphocreatine in the brain of humans and animals (Dechent et al., 1999; Ferrante et al., 2000; Ipsiroglu et al., 2001; Lyoo et al., 2003; Perasso et al., 2003; Persky et al., 2003a,b; Stöckler et al., 1996). A growing number of clinical studies are finding that creatine supplementation is an effective adjunct to conventional medicines in patients with treatment resistant depression (Amital et al., 2006a,b; Kondo et al., 2011b; Roitman et al., 2007). Converging evidence from animal studies assessing forced swim test behavior showed that creatine supplementation produced antidepressant-like effects in female rats, but not male rats (Allen et al., 2010). Moreover, in these studies, creatine supplementation selectively altered male and female rat swimming activity, a behavior that has been established as an index of brain serotonergic activity (for review, see Detke et al., 1995; Page et al., 1999).
Several lines of evidence from antidepressant research have associated clinical improvement with the normalization of cerebral metabolic function, which is often impaired in major depressive disorders (for review, see Hroudová and Fišar, 2011; Kondo et al., 2011a). Drugs prescribed to treat depression, such as paroxetine, fluoxetine, and imipramine, alter brain- and mitochondrial-type creatine kinases in the prefrontal cortex, hippocampus, and striatum (Agostinho et al., 2009; Assis et al., 2009; Santos et al., 2009), areas of the brain known to be involved with depression (Drevets et al., 2008; Maletic et al., 2007). Additionally, antidepressant drugs increase creatine and phosphocreatine concentrations in the prefrontal cortex and hippocampus in animal models of depression (Kim et al., 2010; Czéh et al., 2001; Michael-Titus et al., 2008). It is hypothesized that increased phosphocreatine stores and creatine kinase activity attenuate metabolic impairments associated with depression by increasing the rate of ATP replenishment and accordingly improving neuronal integrity or function (Agostinho et al., 2009; Kim et al., 2010; Santos et al., 2009). In support of this notion, neuroimaging work in humans found that high baseline levels of phosphocreatine predicted treatment response with 79% accuracy in depressed adults taking selective serotonin reuptake inhibitors (SSRIs) combined with triiodothyronine, a thyroid hormone that increases brain energy metabolism (Iosifescu et al., 2008).
An important question is whether supplementation with creatine monohydrate, which increases brain phosphocreatine concentrations and possibly alters serotonergic activity, could improve the likelihood of response to SSRI antidepressants in patients diagnosed with major depression. Numerous experiments have established that the modified forced swim test is sensitive to the antidepressant effects of the SSRI fluoxetine (Cryan et al., 2005a,b; Detke et al., 1995; Page et al., 1999). A sub-acute administration schedule is standardly used to test for therapeutic efficacy in the forced swim test. Using this procedure rats receive an antidepressant drug at 1, 5, and 23.5 hours prior to a second trial on the forced swim test (Cryan et al., 2005a,b). Previous studies established that a dose of approximately 20.0 mg/kg fluoxetine (or a total of 60.0 mg/kg over 1 day) is required to produce the most reliable and significant behavioral effects in the forced swim test following sub-acute administration in rats (Cryan et al., 2006b; Page et al., 1999). For the present research, lower than typical doses of fluoxetine (5.0 mg/kg and 10.0 mg/kg, administered three times) were chosen to avoid ceiling effects in active behaviors and to specifically evaluate whether combined creatine-fluoxetine treatment would produce more robust antidepressant effects than either treatment alone.
All together, mounting evidence supports the plausibility that creatine improves depressive symptoms by directly or indirectly influencing serotonergic activity in the brain. To this end, the goal of the present research was two-fold: (1) to replicate the effects of chronic creatine supplementation on forced swim behavior in males and females; and (2) to evaluate the behavioral effects of dietary creatine when combined with the serotonergic agent fluoxetine.
2. Methods
2.1. Animals and Housing
In each of the four experiments, male or female Sprague-Dawley rats, approximately six weeks old, were obtained from Charles River Laboratories (Raleigh, NC). Rats were housed individually in hanging stainless-steel cages in a climate-controlled vivarium (22 ± 1°C) on a 12:12 h reverse light-dark cycle (lights on at 1900h). Rats were given seven days to acclimate to handling and housing conditions prior to the beginning of the study.
All procedures were approved by the Tufts University Institutional Animal Care and Use Committee.
2.2. Diets
Following habituation, rats were randomly assigned to receive standard rat chow alone or chow mixed with 2% or 4% w/w creatine monohydrate for five weeks prior to behavioral testing (in each experiment, n = 24 per diet). The creatine-supplemented mixtures were made in-house using creatine monohydrate ≥ 98% (Sigma Chemical; 0 kcal/g) and ground Purina chow #5001 (3.4 kcal/g). Food was presented in Wahmann LC306 (Timonium, MD) stainless-steel food cups, which were covered with lids and clipped to the floor of the cage to reduce spillage. Glass water bottles with drip-proof stoppers were fitted at the front of each cage. Body weight, and food and water intakes were measured every other day at the same time each day.
2.3. Drug and treatment
Fluoxetine hydrochloride was provided by the National Institute of Mental Health (NIMH; Bethesda, MD). Fluoxetine was dissolved in physiological saline at a concentration of 5 mg/ml or 10.0 mg/ml. All injections were administered subcutaneously at a volume of 1.0 ml/kg.
In each experiment, half of the rats within each diet group (n = 12 per creatine × drug group) were randomly assigned to receive fluoxetine or saline. After Trial 1 of the forced swim test, animals were injected with 5.0 mg/kg fluoxetine, 10.0 mg/kg fluoxetine, or saline according to a sub-acute schedule at 1, 5, and 23.5 hours prior to the second trial of the forced swim test (Cryan et al., 2005a,b). Over the 24-hour period, rats received a total of 15.0 mg/kg (Experiments 1 and 3) or 30.0 mg/kg (Experiments 2 and 4) prior to Trial 2.
2.4. Behavioral Testing
All behavioral measures were performed between five and six weeks after initial creatine supplementation. All procedures were conducted by the same experimenters under red lights, with the exception that the open field test was conducted under dim white light (∼35 lux). All behavioral tests were set to start at the same time of day (1100 h), and animals were tested in random order. Behavioral measures on the forced swim test were video recorded for later scoring by two observers blind to the experimental conditions. All other behavioral measures were recorded using ANY-maze video tracking system (Stoelting Co.; Wood Dale IL) for both automated scoring and subsequent scoring by an observer blind to dietary and drug conditions.
The time course for behavioral testing (forced swim test, wire suspension, then open field test) was adopted to replicate previously published work, which found behavioral changes in the forced swim test following five weeks of creatine supplementation in test-naive rats (Allen et al., 2010). In addition, recent research indicates that the forced swim test is the most vulnerable behavioral assay with regards to order effects in studies that use test batteries to assess affective behavior in rats, and as such forced swim behavior was assessed first (Blokland et al., 2011).
2.4.1. Forced swim test
The forced swim test consisted of a 15-minute Trial 1, to induce learned helplessness, followed 24 hours later by a 5-minute Trial 2. During each trial, the rat was placed in a clear Plexiglas cylinder (25 cm in diameter by 65 cm in height) filled to 48 cm with 25 degree C° water (+-0.5 C°). Each rat was tested in a clean container with fresh water, dried thoroughly on trial completion and placed back in its home cage. The container was drained and cleaned after each use.
To characterize the time course of treatment effects, the time-sampling technique developed by Detke et al. (1995) was used to score the first five minutes of behavior during Trial 1 and the total five minutes of behavior during Trial 2. Raters, blind to the experimental conditions, scored one of four predominant behaviors of the rat at the end of each five-second period of time. Counts of swimming, climbing, immobility, and diving were recorded. Latency to the onset of immobility was operationally defined as at least two consecutive five-second intervals (at least 10 seconds) of immobility. Latency to immobility is an index of how vulnerable the individual is to the negative effects of force swim stress (Allen et al., 2010; Carlezon et al., 2003; Leussis and Andersen, 2008).
2.4.2. Wire Suspension
Psychomotor testing was conducted three days after the forced swim test. This interval allowed for the total clearance of fluoxetine, which has a half-life of approximately three hours in Sprague-Dawley rats administered 1 mg/kg of the drug intravenously (Hui et al., 2007). The wire suspension test was used to exclude the possibility that differences in physical ability or motor function occurred as a result of dietary manipulations. The wire suspension task measured the ability of the animal to grasp a taut horizontal wire (2 mm. in diameter, 62 cm. above the table top) with its forepaws and to remain suspended. Latency to drop from the wire was measured in seconds, with a maximum testing time of 60 sec.
2.4.3. Open Field Exploration
The open field test measured anxiety-like behavior as well as locomotor activity and exploratory behaviors. One week after the forced swim test, rats were placed in a square apparatus (50 cm length × 50 cm width). The center of the field was illuminated by a 60 watt bulb, which was the only direct source of light in the room. Each animal was placed in a randomized starting corner within the field and its behavior was recorded for five minutes. Scored behaviors included the number of rears, stretch attends, grooming, and the number of times the animal returned to its starting corner. Also recorded were average speed, average distance, latency to leave the home corner, time spent in all corners, time spent in the borders, and time spent in the center of the field.
2.5. Vaginal Smears
The week following habituation to the lab, vaginal smears were collected daily from females for the duration of the study by inserting the tip of a clean glass pipette filled with 10 μL of distilled water into the rat's vagina. One drop of fluid was collected from each rat between 1000 and 1100 hr each day, placed on a glass slide, air dried overnight, and then stained with 0.5% cresyl violet. The stained material was examined under a light microscope to determine estrous cycle phase.
2.6. Statistical Analysis
Data were analyzed using SPSS v.18.0 for MAC OSX. Body weight, calories, creatine, and water intake were analyzed using repeated measures (mixed model) ANOVA, with diet group as the between subjects factor and days as the within subjects factors. Additionally, weekly averages of daily body weight, calorie and creatine consumption, and water intake were analyzed using one-way ANOVA when significant differences were detected.
Forced swim behavior was analyzed using one-way ANOVA for Trial 1, with diet as the between subjects factor, and using two-way ANOVA for Trial 2, with diet and drug or diet and estrous cycle as the between subjects factors. In addition, separate two-way ANOVAs were also performed for open field exploration and wire suspension tests. Planned post hoc comparisons were made using least-significant differences (LSD) tests. Alpha was defined as p < 0.05.
Male and female comparisons were not made because male and female body weights and food intake differed significantly over time. Males consumed less creatine per kilogram of body weight than females over the course of the study and at the time of behavioral testing (Figures 1d and 4d). As a result, the different dosages of creatine confound behavioral analyses as a function of sex.
Fig. 1.
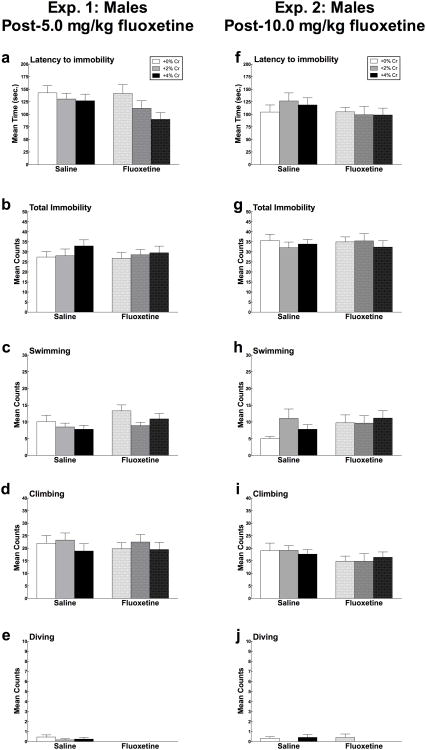
Mean (± SEM) Trial 1 latency to immobility, total immobility, swimming, climbing and diving in males in Experiment 1 (2a-e) and Experiment 2 (2f-j) as a function of creatine supplementation. Means with different letters are statistically significant (p < 0.05). In Experiment 1, no significant differences were found for any forced swim measures (2a-e). Two non-significant trends show that males fed 4% creatine displayed immobility more quickly (1a: p = 0.075) and swam less (1c: p = 0.078) compared to 0% controls. In Experiment 2, there were no significant differences found as a function of creatine supplementation for any forced swim behaviors (2f-j)
Fig. 4.
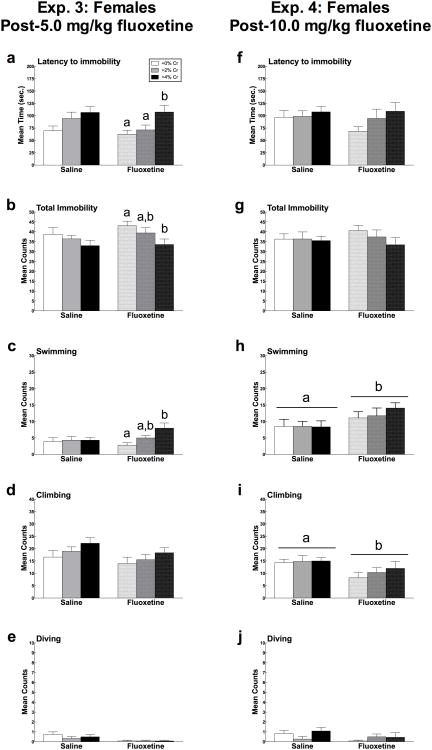
Mean (± SEM) Trial 2 latency to immobility, total immobility, swimming, and climbing in females administered creatine and/or 5.0 mg/kg fluoxetine (Experiment 3) or 10.0 mg/kg fluoxetine (Experiment 4) as a function of creatine supplementation in experiment 2. Means with different letters are statistically significant (p < 0.05). In Experiment 3 (6a-e), there were no main effects of 5.0 mg/kg fluoxetine on latency to immobility (a), total immobility (b), swimming (c), or climbing (d), but this dose reduced diving counts (e). During Trial 2, the presence of fluoxetine prolonged the antidepressant-like effects of 4% creatine, compared to saline, for latency to immobility, total immobility, and swimming (6a-c, respectively). Fluoxetine did not alter climbing or diving when combined with creatine (d, e). In Experiment 4 (6f-j), females administered 10.0 mg/kg fluoxetine swam more (h) and climbed less (g) than those administered saline, but no differences were detected in total immobility or latency to immobility (6f-h). The behavioral effects of 10.0 mg/kg fluoxetine were not altered in the presence of creatine.
3. Results
3.1. Experiment 1: Effects of creatine and 5.0 mg/kg fluoxetine on the forced swim test and locomotor performance in male rats
3.1.1. Diets and body weights
Body weight, mean daily caloric intake and water intake did not differ as a function of diet across the experiment. Male rats supplemented with 2% creatine consumed an average of 1.95 ± 0.02 g/kg creatine daily during week 1, which declined as a function of increasing body weight to an average of 1.35 ± 0.02 g/kg creatine during week 5. Male rats fed a diet supplemented with 4% creatine consumed an average of 4.07 ± 0.05 g/kg creatine daily during week 1, which declined to an average of 2.57 ± 0.04 g/kg during week 5.
3.1.2. Forced swim test
Trial 1
Diet
Rats supplemented with 4% creatine trended towards reduced latency to the onset of immobility (F(2, 68) = 2.69, p = 0.075), but this effect did not reach significance. None of the other forced swim measures were altered as a function of creatine intake (Figure 1a-e).
Trial 2
Drug
After rats had received 5.0 mg/kg fluoxetine, there were no effects of the drug on latency to the onset of immobility, mean counts of immobility, swimming, or climbing. (Figure 2a-d). Rats that received fluoxetine dove significantly less than males that received saline (F(1,65) = 1.50, p = 0.004) (Figure 2e).
Fig. 2.
Mean (± SEM) Trial 2 latency to immobility, total immobility, swimming, climbing and diving in male rats in response to creatine and/or 5.0 mg/kg fluoxetine (Experiment 1; 3a-e) and 10.0 mg/kg fluoxetine (Experiment 2; 3f-j) as a function of creatine supplementation. Means with different letters are statistically significant (p < 0.05). In Experiment 1, 5.0 mg/kg fluoxetine had no effect on latency to the onset of immobility, mean counts of immobility, swimming, or climbing when administered alone or when combined with creatine (a-e). In Experiment 2, there were no interaction effects of creatine and 10.0 mg/kg fluoxetine on any forced swim behaviors (3f-j).
Diet × Drug
Fluoxetine did not significantly interact with diet for latency to immobility, mean counts of immobility, swimming, climbing, or diving (all p > 0.05)(Figure 2a-e).
3.1.3. Wire Suspension
Latency to drop from a wire did not differ as a function of diet (average hang time ± SEM: 0% creatine, 16.29 ± 2.26 s; 2% creatine, 11.96 ± 1.09 s; 4% creatine: 14.46 ± 2.09 s or previous drug treatment (all p > 0.05).
3.1.4 Open field test
Open field activity did not vary as a function of diet or previous drug treatment (all p > 0.05).
3.2. Experiment 2: Effects of creatine and 10.0 mg/kg fluoxetine on the forced swim test and locomotor performance in male rats
3.2.1. Diets and body weights
Body weight and daily caloric intake were not altered by creatine supplementation. Male rats supplemented with 2% creatine consumed an average of 1.85 ± 0.02 g/kg creatine daily during week 1, which declined as a function of increasing body weight to an average of 1.27 ± 0.01 g/kg creatine during week 5. Male rats fed a diet supplemented with 4% creatine consumed an average of 3.80 ± 0.04 g/kg creatine daily during week 1, which declined to an average of 2.67 ± 0.08 g/kg during week 5.
Water intake differed as a function of diet over the course of the study (F(1,68) = 5.32, p = 0.007). Separate one-way ANOVAs of average daily water consumption by week showed that rats given a diet containing 4% creatine drank significantly more water than rats in all other groups during week 1 (F(2,69) = 10.63, p < 0.001), week 2 (F(2,69) = 4.90, p = 0.01), week 3 (F(2,69) = 6.24, p = 0.003), week 4 (F(2,69) = 3.95, p = 0.024), and week 5 (F(2,69) = 3.16, p = 0.049).
3.2.2. Forced swim test
Trial 1
Diet
There were no differences in behavior on the forced swim test as a function of dietary conditions (all p > 0.05; Figure 1f-j).
Trial 2
Drug
There were no main effects of 10.0 mg/kg fluoxetine treatment on latency to the onset of immobility, mean counts of immobility, swimming, climbing, or diving in male rats (all p > 0.05; Figure 2f-j).
Diet × Drug
Fluoxetine did not interact with diet condition in latency to immobility, mean counts of immobility, swimming, climbing, or diving (all p > .05) (Figure 2f-j).
3.2.3. Wire suspension
Latency to drop from the wire did not differ significantly as a function of diet (average hang time ± SEM: 0% creatine, 12.77 ± 1.39 s; 2% creatine, 11.81 ± 1.64 s; 4% creatine: 14.79 ± 1.87 s) or prior drug treatment (all p > 0.05).
3.2.4. Open field test
Open field activity did not vary as a function of diet or prior drug treatment (all p > 0.05).
3.3. Experiment 3: Effects of creatine and 5.0 mg/kg fluoxetine on the forced swim test and locomotor performance in female rats
3.3.1. Diets and body weights
Neither body weight nor caloric intake varied as a function of diet over the course of the experiment. Water intake differed significantly as a function of diet (F(2, 69) = 3.52, p = 0.035). Female rats supplemented with 4% creatine drank more water than rats consuming 0% and 2% creatine supplemented diets during week 4 (F(2,69) = 4.32, p = 0.017), week 5 (F(2,69) = 4.68, p = 0.012), and week 6 (F(2,69) = 3.30, p = 0.043). Female rats supplemented with 2% creatine consumed an average of 1.97 ± 0.03 g/kg creatine daily during week 1, which was reduced as a function of increasing body weight to an average of 1.62 ± 0.02 g/kg creatine during week 5. Female rats fed a diet supplemented with 4% creatine consumed an average of 3.88 ± 0.06 g/kg creatine daily during week 1, which declined to an average of 3.48 ± 0.08 g/kg during week 5.
3.3.2. Forced swim test
Trial 1
Diet
Latency to immobility differed significantly as a function of diet (F(2,69) = 3.30, p = 0.043) (Figure 3a). Females fed a diet supplemented with 4% creatine showed longer latencies to display immobility compared to animals fed the diet containing 0% creatine (p = 0.028) or 2% creatine (p = 0.032).
Fig. 3.
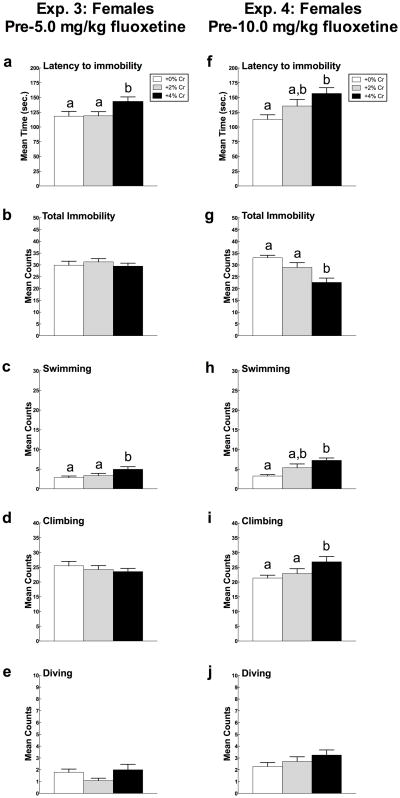
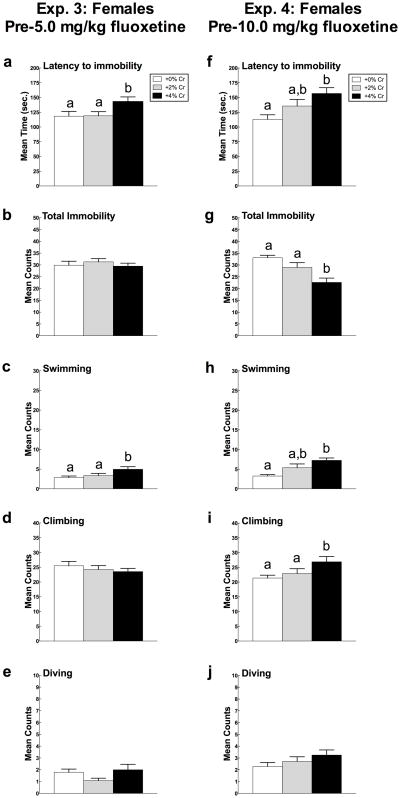
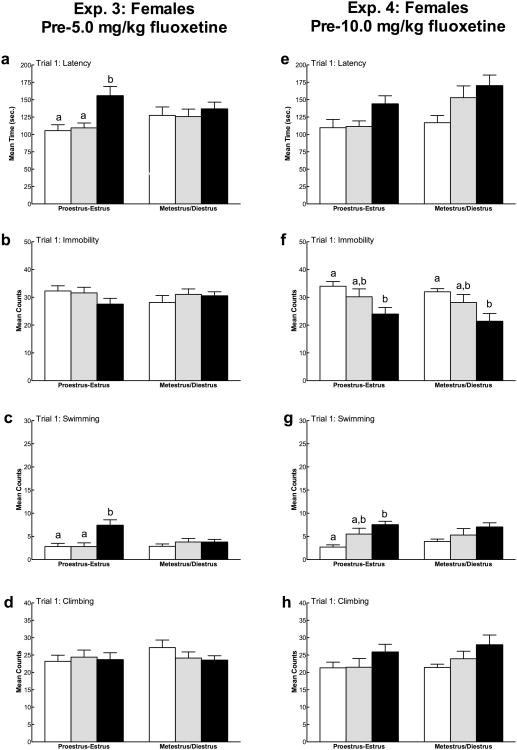
Mean (± SEM) Trial 1 latency to immobility, total immobility, swimming, and climbing in female rats in Experiment 3 (5a-e) and Experiment 4 (5f-j) as a function of creatine supplementation. Means with different letters are statistically significant (p < 0.05). In Experiment 3, females fed 4% creatine displayed immobility less rapidly (a) and swam more (c) than rats fed 0% and 2% creatine diets. In Experiment 4, female rats supplemented with 4% creatine less rapidly displayed immobility (f) and spent less total time immobile (g) than the other two diets. Additionally, females fed 4% creatine swam more (h) and climbed more (i) compared to control rats. Diving (e, j) was not influenced by creatine supplementation in either experiment.
Duration of total immobility did not differ significantly in females fed creatine during Trial 1 (p > 0.05) (Figure 3b).
Swimming counts differed significantly as a function of diet (F(2,69) = 3.99, p = 0.023) (Figure 3c). Post hoc analyses indicated that animals in the 4% creatine group swam significantly more than animals fed 0% creatine (p = 0.008) and 2% creatine (p = 0.047).
Neither climbing nor diving differed as a function of diet (all p > 0.05) (Figure 3d-e).
Trial 2
Drug
There was no main effect of fluoxetine on latency to immobility, mean counts of immobility, swimming, or climbing following 5.0 mg/kg fluoxetine treatment (Figure 4a-d). The number of dives differed significantly as a function of drug treatment (F(1,66) = 10.35, p = 0.002), such that animals receiving fluoxetine dove less than those that received saline (Figure 4e).
Diet × Drug
Fluoxetine did not significantly interact with creatine condition in latency to immobility, mean counts of immobility, swimming, climbing, or diving (all p > 0.05) (Figure 4a-e). Because there was a significant effect of diet, post hoc analyses were conducted to examine the effect of creatine within drug treatment groups. Creatine produced significant behavioral effects in the presence of fluoxetine, but not saline, for latency to immobility (F(2,35) = 4.88, p = 0.014), total immobility (F(2, 33) = 3.36, p = 0.047), and swimming (F(2, 33) = 5.85, p = 0.007) (Figure 4a-c), respectively. These effects were not observed for climbing or diving (p > 0.05; Figure 4d-e).
3.3.3. Wire suspension
Latency to drop from the wire did not differ as a function of diet (average hang time ± SEM: 0% creatine, 16.08 ± 1.63 s; 2% creatine, 18.01 ± 2.59 s; 4% creatine: 13.33 ± 1.18 s) or prior drug treatment (all p > 0.05).
3.3.4. Open field test
Open field activity did not vary as a function of diet or drug treatment (all p > 0.05).
3.3.5. Estrous cycle
The mean duration of the female estrous cycle was 4-5 days. Fisher's exact tests confirmed that there were no differences in the frequency of animals in each phase of the cycle as a function of diet on behavioral testing days. Due to the low sample size within each phase of the estrous cycle at the time of behavioral testing (n < 5 for each diet group), we cannot verify the influence of proestrus, estrus, metestrus, or diestrus on creatine's behavioral effects separately. To enable a preliminary evaluation of the influence of ovarian hormones, female rats were classified into one of two groups: proestrus-estrus (high ovarian hormones) and metestrus-diestrus (low ovarian hormones) to increase statistical power (e.g., Feng et al., 2010; Lynch, 2008).
Overall, there were no differences between the proestrus-estrus and metestrus-diestrus phases for latency to immobility, total immobility, swimming, or climbing during either forced swim test trial (Figure 5a-d). In terms of diet, the effect of creatine on forced swim behavior varied as a function of estrous cycle phase. During proestrus-estrus, there were significant effects of creatine on Trial 1 latency to immobility (F(2,25) = 7.99, p = 0.002), Trial 2 latency to immobility (F(2,32) = 3.71, p = 0.036), and Trial 1 swimming counts (F(2,25) = 7.99, p = 0.002). During proestrus-estrous, females fed 4% creatine displayed immobility less rapidly compared to those fed 0% creatine (Trial 1: p = 0.001, Trial 2: p = 0.01) or 2% creatine (Trial 1: p = 0.002; Trial 2: n.s.). Also during proestrus-estrus, females consuming the 4% creatine diet swam significantly more during Trial 1 than females given 0% creatine (p = 0.002) or 2% creatine (p = 0.002). The antidepressant-like effects of creatine were not observed during forced swim test Trial 1 in females tested during metestrus-diestrus (all p > 0.05). There was a significant effect of creatine on swimming during Trial 2 in metestrus-diestrus rats (F(2,34) = 3.53, p = 0.04), where females fed 4% creatine swam more than 0% controls (p = 0.012).
Fig. 5.
Mean (± SEM) effects of estrous cycle phase on Trial 1 forced swim behaviors in female rats. Means with different letters are statistically significant (p < 0.05). In Experiment 3 (7a-d), females given 4% creatine were less vulnerable to the negative effects of forced swim testing during proestrus-estrus phases, when ovarian hormones are highest. In particular, during proestrus-estrus but not metestrus-diestrus, 4% creatine had a positive effect on latency to immobility (a) and swimming (c). In Experiment 4 (7e-h), there was a less clear effect of diet on estrous cycle phase during forced swim testing. In females given 4% creatine, swimming was only increased during proestrus-estrus, but these animals displayed less immobility (f) during both hormonal phases.
3.4. Experiment 4: Effects of creatine and 10.0 mg/kg fluoxetine on the forced swim test and locomotor performance in female rats
3.4.1. Diets and body weights
Body weight, daily caloric intake and water intake did not differ as a function of diet across the experiment. Female rats supplemented with 2% creatine consumed an average of 1.90 ± 0.02 g/kg creatine daily during week 1, which was reduced as a function of increasing body weight to an average of 1.55 ± 0.03 g/kg creatine during week 5. Female rats fed a diet supplemented with 4% creatine consumed an average of 3.85 ± 0.04 g/kg creatine daily during week 1, which declined to an average of 3.14 ± 0.05 g/kg during week 5.
3.4.2. Forced swim test
Trial 1
Diet
Creatine supplementation significantly altered latency to immobility (F(2,69) = 5.11, p = 0.009) (Figure 3f). Post hoc analysis indicated that female rats fed a diet supplemented with 4% creatine took significantly longer to display immobility in the forced swim test than females given 0% creatine (p = 0.002) but did not differ from those supplemented with 2% creatine (p > 0.05).
Total immobility also differed significantly as a function of diet (F(2,69) = 9.73, p < 0.001) (Figure 3g). Rats fed a diet containing 4% creatine spent less time in the immobile posture than females supplemented with 0% creatine (p < 0.001) and 2% creatine (p = 0.01).
Swimming differed as a function of diet (F(2,69) = 8.59, p < 0.001) (Figure 3h). Females supplemented with 4% creatine swam more than rats given 0% creatine (p < 0.001) but not those given 2% creatine (p > 0.05).
Climbing was significantly altered by creatine supplementation (F(2, 69) = 3.59, p = 0.033) (Figure 3i). Post hoc analyses indicated that females fed 4% creatine climbed more compared to rats fed 0% creatine (p = 0.012) but not those supplemented with 2% creatine (p > 0.05).
Diving activity was not changed as a function of creatine supplementation (p > 0.05; Figure 3j).
Trial 2
Drug
Females receiving 10.0 mg/kg fluoxetine swam significantly more (F(1,63) = 5.88, p = 0.018) and climbed significantly less (F(1, 63) = 7.20, p = 0.009) than females that received saline. No differences were observed in latency to immobility, total immobility, or diving as a function of drug administration (Figure 4f-j).
Diet × Drug
Fluoxetine did not interact with diet condition in latency to immobility, mean counts of immobility, swimming, climbing, or diving (p > 0.05 for all measures; Figure 4f-j). A post hoc analysis was conducted because significant main effects of diet and drug were found. However, no differences were detected within treatment groups as a function of creatine supplementation.
3.4.3. Wire suspension
Latency to drop from the wire did not differ as a function of diet (average hang time ± SEM: 0% creatine, 16.00 ± 2.83 s; 2% creatine, 14.41 ± 2.39 s; 4% creatine: 19.40 ± 1.67 s) or prior drug treatment (all p > 0.05).
3.4.4. Open field test
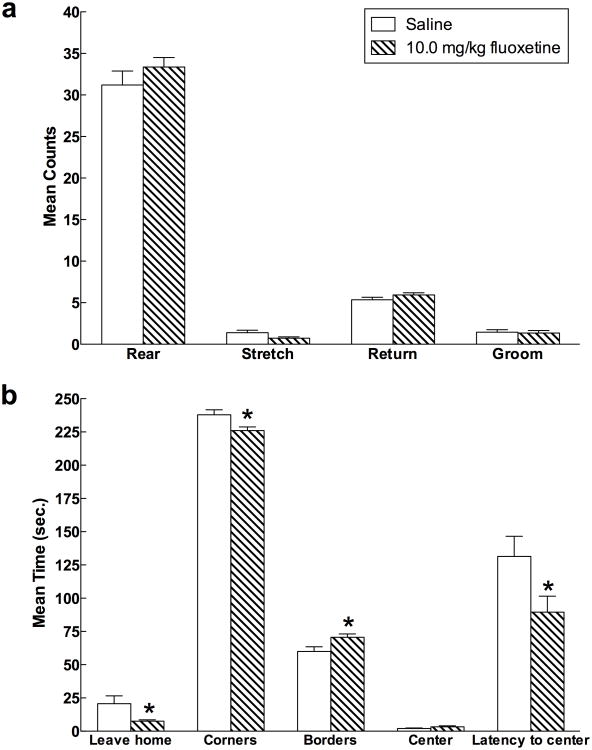
Open field activity did not vary significantly as a function of diet. However, there was a significant effect of prior antidepressant drug administration on a number of open field behaviors (Figure 6a-b). Specifically, relative to rats given saline, females that had previously received 10.0 mg/kg fluoxetine left the home corner more quickly (F(1,63) = 4.214, p = 0.044), spent less time in the corners (F(1,63) = 5.317, p = 0.024), more time in borders (F(1,63) = 4.759, p = 0.033), moved more quickly (F(1,63) = 4.67, p = 0.035), and traveled a greater distance (F(1,63) = 4.22, p = 0.044). There were no interaction effects between drug and diet for any open field behaviors (all p > 0.05).
Fig. 6.
Mean (± SEM) open field behaviors as a function of 10.0 mg/kg fluoxetine treatment in female rats (Experiment 4). Treatment with 10.0 mg/kg fluoxetine during the forced swim test produced differences in open field behavior one week later. Specifically, the drug reduced the latency to leave the home (starting) corner, decreased time spent in corners, increased time spent exploring borders, and reduced the latency to enter the illuminated center of the field.
3.4.5. Estrous cycle
The mean duration of female estrous cycle was 4-5 days. Fisher's exact tests confirmed that there were no differences in the frequency of animals in each phase of the cycle as a function of diet on behavioral testing days. As in Experiment 3, females were categorized as proestrus-estrus and metestrus-diestrus to evaluate the activational effects of ovarian hormones at the time of forced swim testing.
The proestrus-estrus and metestrus-diestrus phases did not differ for latency to immobility, total immobility, swimming, or climbing during either forced swim test trial (all p > .05; Figure 5e-h). In terms of diet, the effect of creatine on forced swim behavior varied as a function of estrous cycle phase. During proestrus-estrus, there was a significant effect of creatine on Trial 1 total immobility (F(2,32) = 5.31, p = 0.01) and Trial 1 swimming counts (F(2,32) = 9.05, p = 0.001). Proestrus-estrus females fed 4% creatine displayed less immobility compared to those fed 0% creatine (Trial 1: p = 0.003), and 4% creatine-fed females swam significantly more during Trial 1 than 0% creatine-fed rats (p < 0.001). In females tested during metestrus-diestrus, there was a significant effect of creatine on Trial 1 immobility (F(2,32) = 4.26, p = 0.022), where females fed 4% creatine were less immobile than 0% controls (p = 0.007). No differences were observed for creatine in metestrus-diestrus rats testing during Trial 2 (all p > .05).
4. Discussion
The findings of the present study underscore the importance of creatine in modulating antidepressant-like behavior in females. Consistent with previous work (Allen et al., 2010), intake of dietary creatine (4% added) increased anti-immobility behaviors in the forced swim test in female rats only, and this effect was dependent upon levels of ovarian hormones. Importantly, during Trial 2, antidepressant-like effects were only observed in females administered combined creatine-fluoxetine treatment, particularly 4% creatine supplemented rats treated with 5.0 mg/kg doses of fluoxetine. A similar trend was observed for 10.0 mg/kg fluoxetine, but this did not reach significance. Moreover, 4% creatine alone produced a greater anti-immobility effect in females than 5.0 and 10.0 mg/kg fluoxetine alone. Male responses to creatine were not compatible with previous research, as no differences in either trial of the forced swim test were observed as a function of creatine supplementation or fluoxetine in male rats.
Although the outcome that rats did not display significant changes in immobility counts in response to fluoxetine alone may seem surprising, this result was anticipated based on published research. As mentioned previously, when the aim of an experiment requires reliable and robust changes in forced swim behaviors following sub-acute fluoxetine treatment, investigators use a dose of at least 20.0 mg/kg over a 24-hour period (Cryan et al., 2006b; Page et al., 1999). Lower than typical doses of fluoxetine were chosen to examine whether the combination of dietary creatine and fluoxetine would augment the antidepressant effects of either compound administered individually. The potential benefits of the approach of combining creatine with low-dose SSRI treatment would be to provide clinical relief to patients resistant to conventional drug treatments and/or to reduce side effects associated with higher doses of fluoxetine. More practically speaking, in the design of this research, the doses of 5.0 mg/kg and 10.0 mg/kg (or a total of 15.0 mg/kg and 30.0 mg/kg) were specifically chosen to avoid ceiling effects in forced swim activity since both creatine and fluoxetine are known to increase swimming and reduce immobility in the forced swim test (Allen et al., 2010; Cryan et al., 2006b). A sub-acute schedule of fluoxetine was chosen because chronic fluoxetine administration is known to reduce food intake in male and female rats (Heisler et al., 1997, 1999) and therefore would confound the behavioral results because creatine is supplemented orally.
The precise mechanisms underlying the antidepressant-like action of creatine in females is not clearly understood. It is evident that the creatine-creatine kinase-phosophocreatine pathway plays a critical and multifaceted role in the brain. First, phosphocreatine, the stored form of creatine, works together with creatine kinase to rapidly buffer ATP resynthesis in brain tissue to reduce mitochondrial dysfunction and neuronal loss (Walliman et al., 1992). Second, creatine promotes antioxidant effects at the level of neural cell membranes (Lawler et al., 2002; Sestilli et al., 2006, 2011). Third, creatine is a neuromodulator of GABAA and NMDA receptor activity (Almeida et al., 2006; Koga et al., 2005; Oliveira et al., 2008), and possibly interacts with serotonergic and dopaminergic systems (Allen et al., 2010; Agren and Niklasson, 1988).
Antidepressant agents are hypothesized to affect cerebral creatine-phosphocreatine pathways to prevent large fluctuations in ATP/ADP ratios, diminished metabolic capacity, and neuronal impairment associated with depression (Assis et al., 2009; Santos et al., 2009). Stress, which has been studied extensively as a precipitating factor in depression, is one force that challenges the creatine system to work harder to maintain energy balance. For instance, chronic psychosocial stress produces a ∼15% decline in total creatine (creatine + phosphocreatine) levels, reduced hippocampal volume (-7%), and impaired neurogenesis (-33%) in subordinate animals exposed to conflict with a dominant animal for one week (Czéh et al., 2001; Fuchs et al., 2002; van der Hart et al., 2002). Treatment with antidepressant drugs opposes these negative effects on the creatine system and neuroplasticity factors (Czéh et al., 2001; Fuchs et al., 2002; Kim et al., 2010; van der Hart et al., 2002). Alterations in energy metabolism produced by animal models of depression, which are based on stress, correspond with impairments in the creatine-phosphocreatine pathway in severely depressed humans. In particular, imaging studies report decreased total creatine in the ACC (Mirza et al., 2006), left caudate (Gabbay et al., 2007), and medial prefrontal cortex (Venkatraman et al., 2009) in depressed patients. More severe episodes of depression have been associated with reduced total creatine in the frontal lobe (Kato et al., 1992) and the left dorsolateral prefrontal cortex (Michael et al., 2003). Similarly, reductions in total white matter creatine have been associated with bipolar depression (Dager et al., 2004). In addition, depressive episodes have been linked with reduced creatine kinase activity (Segal et al., 2007, Sora et al., 1986). Given that creatine supplementation may be most optimal when the brain is energetically impaired, future studies are planned to evaluate the biochemical and behavioral effects of creatine after repeated episodes of unpredictable chronic mild stress or social defeat.
It is plausible that pharmaceutical agents that increase creatine kinase activity or buffer phosphocreatine levels provide therapeutic relief for depression. However, the evidence is mixed for the effects of antidepressant treatments on creatine kinase activity. Some pharmacological studies have found that antidepressant and antipsychotic drugs increase brain creatine kinase activity, concluding that these drugs attenuate metabolic dysregulation associated with depression by increasing creatine kinase metabolism. On the other hand, other studies have found that antidepressant and antipsychotic drugs decrease creatine kinase activity, and have attributed impairments in enzyme metabolism to drug side effects. For instance, Santos et al. (2009) recently demonstrated that chronic administration of the SSRI paroxetine (10 mg/kg) increases CK activity in the prefrontal cortex, hippocampus, and striatum of rats. In contrast, Agostinho et al. (2009) found, in these same brain regions and species, that acute and chronic treatment with fluoxetine decreased creatine kinase activity. Additionally, Erakovic et al. (2001) reported that single and chronic electroconvulsive shock (ECS) increased creatine kinase activity in the frontal cortex and hippocampus of rats, whereas Búrigo et al. (2006) found the opposite, in particular that ECS inhibited creatine kinase activity in the hippocampus, striatum, and frontal cortex. These inconsistencies in the treatment literature give emphasis to the convoluted relationships between psychoactive therapies and the creatine-phosphocreatine system.
A compelling outcome of the present work is the influence sex has on the depressive phenotype displayed by rats in response to creatine supplementation. Our findings of antidepressant effects of creatine in females add to a growing body of data that have found a sex-dependent relationship between creatine and depression. For instance, Renshaw et al. (2001) reported that alterations in high energy phosphates in depressed women, but not men, were predictive of SSRI treatment response, indicating that creatine may be more beneficial for treating depressed females. Furthermore, an examination of brain metabolite levels in healthy and depressed patients recently found that total creatine varied as a function of sex and diagnosis in the dorsolateral prefrontal cortex (Nery et al., 2009). In particular, the investigators found that total creatine was reduced in depressed males compared to healthy controls whereas total creatine in depressed females was higher than healthy females.
A point worthy of note is that the antidepressant-like effects of creatine have been replicated three times in female rats, and a recent clinical intervention study indicates that this positive outcome translates to women. In an open-label, add-on clinical study, Kondo et al. (2011) found that daily creatine supplementation significantly improved depressive symptoms in treatment-resistant female adolescents (Kondo et al., 2011b). Sex differences produced by creatine supplementation, as discussed previously (Allen et al., 2010), may be attributable to the activational or organizational effects of sex hormones, which influence brain energy metabolism, neural transmission, synaptic plasticity, and stress vulnerability. There is evidence that creatine kinase activity and gene expression rise and fall in sync with levels of estradiol in females, but not males (Malnick et al., 1983; Ramirez et al., 2002; Sömjen et al., 1991). Estrogens (e.g., estradiol, estrone, and estriol) combined with dietary creatine may exert an antidepressant-like effect in females by stimulating creatine kinase activity, similar to how antidepressants such as paroxetine increase creatine activity and normalize brain energy metabolism (Santos et al., 2009).
We hypothesize that the cyclic variations in ovarian hormones, particularly estradiol, stimulate creatine kinase activity every 4-5 days to amplify the rate at which ATP is replenished by phosphocreatine in female brain cells. Estrous cycle data from the present studies suggest that the antidepressant-like effects of creatine are augmented in the presence of ovarian hormones. In experiment 3, females fed creatine showed robust anti-immobility effects (increased latency to immobility and swimming counts) when tested during proestrus-estrus, when levels of estrogens are highest. These effects were not observed during metestrus-diestrus, when levels of ovarian hormones reach nadir. These effects were less consistently observed in experiment 4, with the exception that swimming counts increased only in females fed 4% creatine during proestrus-estrus. The selective alterations in swimming counts, but not climbing counts, in the presence of high levels of ovarian hormones indicates that serotonin could mediate the effects of creatine and estrogens.
The finding that the behavioral effects of creatine are influenced by the presence of ovarian hormones is potentially of major significance. It is well known that creatine and ovarian hormones, particularly estrogens, are neuromodulators with antioxidant, anti-inflammatory, and anti-apoptotic actions (for review, see Andres et al., 2007; Arnold and Beyer, 2009). In addition, both compounds have demonstrated neuroprotective effects (Andres et al., 2007; Behl, 2003; Spence and Voskuhl, 2012) as well as antidepressant-like effects in human and animal studies of various brain-related disorders (Allen et al, 2010; Walf and Frye, 2009). The cellular substrate that most likely bridges the interaction between creatine and estrogens is the energy-producing mitochondrial compartment found within neurons and glia. Estrogens can directly affect mitochondrial physiology and protein activity via estrogen receptors (particularly ERβ) located within the mitochondrial compartment (Arnold and Beyer, 2009). Likewise, creatine and creatine kinase can also influence mitochondrial properties and energy balance from within the compartment. The plausible mechanisms underlying the shared effects of creatine and estrogens include the ability of both compounds to increase energy availability (via improving aerobic glycolysis, respiratory efficiency, or regeneration of ATP), to promote neuronal proliferation and survival, and to protect mitochondria against damaging reactive oxygen species and inflammatory processes (for review, see Andres et al., 2005; Arnold and Beyer, 2009; Sestili et al., 2006; Spence and Voskuhl, 2012). Further study of the relationship between creatine and estrogens has the potential to provide a wealth of insight into the mechanisms underlying sex differences in the prevalence and treatment of mood disorders.
Nevertheless, we must recognize two important issues in the present research that limit our understanding of sex differences in creatine's behavioral effects. The first is that this study was not designed to directly investigate the role of ovarian hormones. These results should be considered with caution because the small sample size for each estrous cycle phase made it difficult to assess the effects of creatine during each of the four phases of the estrous cycle separately. Ongoing studies in our laboratory are thoroughly investigating the cause-and-effect relationship between gonadal steroids (e.g., estradiol, progesterone, and testosterone) and creatine supplementation on brain chemistry and forced swim behavior (e.g., F31AT006292). Second, sex differences in the behavioral effects observed in the present research could be due to differential food intake, as males consumed less creatine per kilogram of body weight over the 5-week course of treatment. It remains to be known whether documented differences in brain creatine concentrations or the expression and activity levels of creatine kinase and creatine transporters influence behavioral responses to creatine (Gledhill et al, 1988; Hamakawa et al, 1999; Ramirez and Jimenez, 2002; Wong et al, 1983). Thus, it is premature to conclude that creatine could not be beneficial for brain function in males.
Reports describing sexually dimorphic neurochemical and behavioral responses to antidepressant treatments are becoming increasingly common in human and animal research (Dalla et al., 2009). Many clinical trials have found drugs that affect serotonin systems (e.g., SSRIs) to be more successful in treating females, but drugs that act on both serotonin and norepinephrine systems (e.g., tricyclics) to be more favorable in treating males (Higuchi et al., 2009; Kornstein et al., 2000; Vermeiden et al., 2009; Young et al., 2009). Diverging serotonergic neurochemical alterations have been demonstrated in at least five animal models of depression and may explain sex differences frequently observed in these behavioral assays (for review, see Dalla et al., 2009). Of significance, the differential behavioral responses observed in males and females fed creatine may point to differences in the effects of creatine on serotonin. Consistent with previous work (Allen et al., 2010), creatine increased swimming behavior in females given 4% creatine in Experiments 1 and 2, and this behavioral change is robustly predictive of serotonergic neurochemical alterations (Detke et al., 1995). Specifically, it is assumed that creatine enhances serotonergic activity to buffer against the negative effects of forced swim stress. However, in the second study of females (Experiment 4), creatine also increased climbing behavior in females, which is associated with changes in noradrenergic neurotransmission. This effect has not been observed in the two previous studies of creatine and forced swim behavior but nonetheless deserves attention as creatine may have multiple upstream or downstream effects that influence other neurotransmitter systems.
Equally interesting is the finding that, in females but not males, exposure to fluoxetine (10.0 mg/kg, sub-acute) during the forced swim test significantly reduced anxiety-like behaviors in the open field test one week later without altering general locomotor activity. This result is supportive of a greater benefit of serotonergic drugs in females than males, and perhaps suggests that sub-acute administration of fluoxetine during an acutely stressful situation attenuates anxiety-like behavior in future stressful situations. A review of the literature has suggested that the open field is not sensitive to the effects of SSRIs, including fluoxetine, in male rats (Prut and Belzung, 2003), however, few studies have examined the effects of fluoxetine on open field behavior in female rats. It has been demonstrated that uncontrollable stress is more detrimental to females than males, which may confer differences in response to treatment with fluoxetine (Leuner et al., 2004). More precise research is needed because it is difficult to draw conclusions from the present study as fluoxetine was not administered directly before open field testing.
In conclusion, long-term dietary creatine reliably induces an antidepressant-like effect in female rats. If cerebral metabolic dysfunction is a reversible correlate of depressive pathophysiology, in accordance with the bioenergenic hypothesis, the creatine-phosphocreatine pathway is a plausible novel therapeutic target for antidepressant action (for review, see Kondo et al., 2011a). Supportive of this premise, ongoing clinical studies in treatment-resistant depressed adults (Amital et al., 2006a,b; Roitman et al., 2007) and female adolescents (Kondo et al., 2011b) are yielding positive findings for the use of dietary creatine as an adjunct to antidepressant treatment. Consistent with this clinical evidence, the present study showed that low dose (5.0 mg/kg) fluoxetine, administered on a sub-acute schedule, buffered the antidepressant-like effects of creatine in females during the second forced swim trial. Moreover, the interaction between creatine and ovarian hormones may have significant clinical implications in light of sex differences in the prevalence and treatment of depressive disorders. The question of whether creatine has potential to enhance treatment efficacy for depression or reduce drug side effects, particularly in females, should be addressed in future studies that assess chronic administration of varying doses and classes of antidepressant drugs on biochemistry and behavior in male and female rats. Behavioral and mechanistic insight of this translational research will add to the growing clinical knowledge base for creatine and antidepressant drugs to directly inform current and future human clinical trials.
Acknowledgments
This present research was supported in part by grants MH58681, DA015116, and DA031247, as well as the Utah Science, Technology and Research (USTAR) initiative. Financial support was also provided by award number F31AT006292 from the National Center of Complementary and Alternative Medicine (NCCAM). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NCCAM, NIMH, NIDA or the National Institutes of Health.
Footnotes
Disclosure/Conflict of Interest: PJ Allen, KE D'Anci, and RB Kanarek have no financial or competing interests to declare. PF Renshaw is a consultant for Novartis, GSK, Roche, Ridge Diagnostics, and Kyowa Hakko. He receives or has received research support from GSK, Roche, and Eli Lilly. PF Renshaw owns stock in Ridge Diagnostics and has received royalty payments from Repligen Corporation. Dr. Renshaw is an inventor on a patent applications filed by McLean Hospital (Belmont, MA, USA) in 2001 that describes the use of creatine as a treatment for depression. No patent has issued, and this application has not been licensed.
References
- Agostinho FR, Scaini G, Ferreira GK, Jeremias IC, Réus GZ, Rezin GT, et al. Effects of olanzapine, fluoxetine and olanzapine/fluoxetine on creatine kinase activity in rat brain. Brain Res Bull. 2009;80:337–40. doi: 10.1016/j.brainresbull.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Agren H, Niklasson F. Creatinine and creatine in CSF: indices of brain energy metabolism in depression. Short note. J Neural Transm. 1988;74:55–9. doi: 10.1007/BF01243575. [DOI] [PubMed] [Google Scholar]
- Allen PJ, D'Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2010;35:534–46. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN. Exocytotic release of creatine in rat brain. Synapse. 2006;60:118–23. doi: 10.1002/syn.20280. [DOI] [PubMed] [Google Scholar]
- Amital D, Vishne T, Roitman S, Kotler M, Levine J. Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J Clin Psychiatry. 2006a;67:836–7. doi: 10.4088/jcp.v67n0521c. [DOI] [PubMed] [Google Scholar]
- Amital D, Vishne T, Rubinow A, Levine J. Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry. 2006b;163:1840–1. doi: 10.1176/ajp.2006.163.10.1840b. [DOI] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Assis LC, Rezin GT, Comim CM, Valvassori SS, Jeremias IC, Zugno AI, et al. Effect of acute administration of ketamine and imipramine on creatine kinase activity in the brain of rats. Rev Bras Psiquiatr. 2009;31:247–52. doi: 10.1590/s1516-44462009000300010. [DOI] [PubMed] [Google Scholar]
- Behl C. Estrogen can protect neurons: modes of action. The Journal of Steroid Biochemistry and Molecular Biology. 2002;83:195–7. doi: 10.1016/s0960-0760(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Blokland A, ten Oever S, van Gorp D, van Draanen M, Schmidt T, Nguyen E, Krugliak A, Napoletano A, Keuter S, Klinkenberg I. The use of a test battery assessing affective behavior in rats: Order effects. Behav Brain Res. doi: 10.1016/j.bbr.2011.11.042. in press. [DOI] [PubMed] [Google Scholar]
- Búrigo M, Roza CA, Bassani C, Feier G, Dal-Pizzol F, Quevedo J, et al. Decreased Creatine Kinase Activity Caused by Electroconvulsive Shock. Neurochem Res. 2006;31:877–81. doi: 10.1007/s11064-006-9091-1. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–7. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005a;182:335–44. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005b;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Sciences; 2001; pp. 12796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–8. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol. 1999;277:R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erakovic V, Zupan G, Varljen J, Laginja J, Simonic A. Altered activities of rat brain metabolic enzymes in electroconvulsive shock-induced seizures. Epilepsia. 2001;42:181–9. doi: 10.1046/j.1528-1157.2001.30499.x. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000;20:4389–97. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Michaelis T, de Biurrun G, Watanabe T, Frahm J. Synaptic plasticity and tianeptine: structural regulation. Eur Psychiatry. 2002;17(3):311–7. doi: 10.1016/s0924-9338(02)00652-1. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Hess DA, Liu S, Babb JS, Klein RG, Gonen O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am J Psychiatry. 2007;164:1881–9. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill RF, Van der Merwe CA, Greyling M, Van Niekerk MM. Race-gender differences in serum creatine kinase activity: a study among South Africans. J Neurol Neurosurg Psychiatry. 1988;51:301–4. doi: 10.1136/jnnp.51.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamakawa H, Kato T, Shioiri T, Inubushi T, Kato N. Quantitative proton magnetic resonance spectroscopy of the bilateral frontal lobes in patients with bipolar disorder. Psychol Med. 1999;29:639–44. doi: 10.1017/s0033291799008442. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997;58:767–73. doi: 10.1016/s0091-3057(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav. 1999;63:377–85. doi: 10.1016/s0091-3057(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Sato K, Naito S, Yoshida K, Takahashi H, Kamata M, et al. Differential clinical effects of fluvoxamine by the effect of age in Japanese female major depressive patients. Neuropsychiatr Dis Treat. 2009;5:151–5. doi: 10.2147/ndt.s4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová J, Fišar Z. Connectivity between mitochondrial functions and psychiatric disorders. Psychiatry Clin Neurosci. 2011;65:130–41. doi: 10.1111/j.1440-1819.2010.02178.x. [DOI] [PubMed] [Google Scholar]
- Hui Y, Huang N, Ebbert L, Bina H, Chiang A, Maples C, et al. Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. J Pharmacol Toxicol Methods. 2007;56:256–64. doi: 10.1016/j.vascn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–34. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stöckler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001;69:1805–15. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–30. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Lee Y-J, Kim H, Lee D-W, Woo D-C, Choi C-B, et al. Desipramine attenuates forced swim test-induced behavioral and neurochemical alterations in mice: An in vivo 1H-MRS study at 9.4T. Brain Res. 2010;1348:105–13. doi: 10.1016/j.brainres.2010.05.097. [DOI] [PubMed] [Google Scholar]
- Koga Y, Takahashi H, Oikawa D, Tachibana T, Denbow D, Furuse M. Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience. 2005;132:65–71. doi: 10.1016/j.neuroscience.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung Y-H, Kim N, Jeong E-K, DelMastro KK, et al. Review: Magnetic Resonance Spectroscopy Studies of Pediatric Major Depressive Disorder. Depress Res Treat. 2011a:1–13. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Sung Y-H, Hellem TL, Fiedler KK, Shi X, Jeong E-K, et al. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord. 2011b;135:354–61. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Lawler J. Direct Antioxidant Properties of Creatine. Biochem Biophys Res Commun. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolialoffredo S, Shors T. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–70. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, et al. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Renshaw PF. Magnetic resonance spectroscopy: current and future applications in psychiatric research. Biol Psychiatry. 2002;51:195–207. doi: 10.1016/s0006-3223(01)01313-0. [DOI] [PubMed] [Google Scholar]
- Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract. 2007;61:2030–40. doi: 10.1111/j.1742-1241.2007.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnick SD, Shaer A, Soreq H, Kaye AM. Estrogen-induced creatine kinase in the reproductive system of the immature female rat. Endocrinology. 1983;113:1907–9. doi: 10.1210/endo-113-5-1907. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–84. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael-Titus A, Albert M, Michael G, Michaelis T, Watanabe T, Frahm J, et al. SONU20176289, a compound combining partial dopamine D2 receptor agonism with specific serotonin reuptake inhibitor activity, affects neuroplasticity in an animal model for depression. Eur J Pharmacol. 2008;598:43–50. doi: 10.1016/j.ejphar.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Mirza Y, O'Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J Child Neurol. 2006;21:106–11. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–8. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–85. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- Nery FG, Stanley JA, Chen H-H, Hatch JP, Nicoletti MA, Monkul ES, et al. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: A single voxel 1H spectroscopy study. Psychiatry Research: Neuroimaging. 2009;174:177–83. doi: 10.1016/j.pscychresns.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol Psychiatry. 1984;19:1183–206. [PubMed] [Google Scholar]
- Oliveira M, Furian A, Fighera M, Fiorenza N, Ferreira J, Rubin M, et al. The involvement of the polyamines binding sites at the NMDA receptor in creatine-induced spatial learning enhancement. Behav Brain Res. 2008;187:200–4. doi: 10.1016/j.bbr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–7. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Perasso L. Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain Res. 2003;974:37–42. doi: 10.1016/s0006-8993(03)02547-2. [DOI] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA, Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Clin Pharmacokinet. 2003a;42:557–74. doi: 10.2165/00003088-200342060-00005. [DOI] [PubMed] [Google Scholar]
- Persky AM, Müller M, Derendorf H, Grant M, Brazeau GA, Hochhaus G. Single- and Multiple-Dose Pharmacokinetics of Oral Creatine. The Journal of Clinical Pharmacology. 2003b;43:29–37. doi: 10.1177/0091270002239703. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ramirez O, Jimenez E. Sexual dimorphism in rat cerebrum and cerebellum: different patterns of catalytically active creatine kinase isoenzymes during postnatal development and aging. Int J Dev Neurosci. 2002;20:627–39. doi: 10.1016/s0736-5748(02)00102-8. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, Frederick Bde B, et al. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–55. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial Dysfunction and Psychiatric Disorders. Neurochem Res. 2008;34:1021–9. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Roitman S, Green T, Osher Y, Karni N, Levine J. Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord. 2007;9:754–8. doi: 10.1111/j.1399-5618.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Santos PM, Scaini G, Rezin GT, Benedet J, Rochi N, Jeremias GC, et al. Brain creatine kinase activity is increased by chronic administration of paroxetine. Brain Res Bull. 2009;80:327–30. doi: 10.1016/j.brainresbull.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Segal M, Avital A, Drobot M, Lukanin A, Derevenski A, Sandbank S, et al. Serum creatine kinase level in unmedicated nonpsychotic, psychotic, bipolar and schizoaffective depressed patients. Eur Neuropsychopharmacol. 2007;17:194–8. doi: 10.1016/j.euroneuro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40:837–49. doi: 10.1016/j.freeradbiomed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, et al. Creatine as an antioxidant. Amino Acids. 2011;40:1385–96. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- Sömjen DWY, Mor Z, Harell A, Kaye AM. Regulation of proliferation of rat cartilage and bone by sex steroid hormones. J Steroid Biochem Mol Biol. 1991;40:717–23. doi: 10.1016/0960-0760(91)90296-h. [DOI] [PubMed] [Google Scholar]
- Sora I, Nishimon K, Otsuki S. Dexamethasone suppression test and noradrenergic function in affective and schizophrenic disorders. Biol Psychiatry. 1986;21:621–31. doi: 10.1016/0006-3223(86)90123-x. [DOI] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckler S, Hanefeld F, Frahm J. Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet. 1996;348:789–90. doi: 10.1016/s0140-6736(96)04116-5. [DOI] [PubMed] [Google Scholar]
- van der Hart MGC, Czéh B, de Biurrun G, Michaelis T, Watanabe T, Natt O, et al. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–41. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- Venkatraman TN, Krishnan RR, Steffens DC, Song AW, Taylor WD. Biochemical abnormalities of the medial temporal lobe and medial prefrontal cortex in late-life depression. Psychiatry Research: Neuroimaging. 2009;172:49–54. doi: 10.1016/j.pscychresns.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiden M, van den Broek W, Mulder P, Birkenhager T. Influence of gender and menopausal status on antidepressant treatment response in depressed inpatients. J Psychopharmacol (Oxf) 2009;24:497–502. doi: 10.1177/0269881109105137. [DOI] [PubMed] [Google Scholar]
- Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, et al. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–95. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gend Med. 2009;6:300–11. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ET, Cobb C, Umehara MK, Wolff GA, Haywood LJ, Greenberg T, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol. 1983;79:582–6. doi: 10.1093/ajcp/79.5.582. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, et al. Sex differences in response to citalopram: A STAR∗D report. J Psychiatr Res. 2009;43:503–11. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]