Abstract
Parkinson disease (PD) features profound striatal dopamine depletion and Lewy bodies containing abundant precipitated alpha-synuclein. Mechanisms linking alpha-synucleinopathy with the death of dopamine neurons remain incompletely understood. One such link may be 3,4-dihydroxyphenylacetaldehyde (DOPAL). All of the intra-neuronal metabolism of dopamine passes through DOPAL, which is toxic. DOPAL also potently oligomerizes alpha-synuclein and alpha-synuclein oligomers are thought to be pathogenic in PD. Another implicated factor in PD pathogenesis is metal ions, and alpha-synuclein contains binding sites for these ions. In this study we tested whether divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein in cell-free system and in PC12 cells conditionally over-expressing alpha-synuclein. Incubation with divalent metal ions augmented DOPAL-induced oligomerization of alpha-synuclein (Cu2+>Fe2+>Mn2+), whereas monovalent Cu1+ and trivalent Fe3+ were without effect. Other dopamine metabolites, dopamine itself, and metal ions alone or in combination with dopamine, also had no effect. Antioxidant treatment with ascorbic acid and divalent cation chelation with EDTA attenuated the augmentation by Cu2+ of DOPAL-induced alpha-synuclein oligomerization. Incubation of PC12 cells with L-DOPA markedly increased intracellular DOPAL content and promoted alpha-synuclein dimerization. Co-incubation with Cu2+ amplified (p=0.01), while monoamine oxidase inhibition prevented, L-DOPA-related dimerization of alpha-synuclein (p=0.01). We conclude that divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein. Drugs that interfere with this interaction might constitute a novel approach for future treatment or prevention approaches.
Keywords: Alpha-synuclein, Oligomerization, Dopamine, Copper, DOPAL, Parkinson disease
1. INTRODUCTION
Parkinson disease (PD) is characterized by loss of dopaminergic neurons in the nigrostriatal system and by the presence of Lewy bodies containing abundant aggregated, fibrillar alpha-synuclein [2, 18]. Substantial evidence indicates that although alpha-synuclein deposits constitute visible correlates of Lewy body diseases, it is the production of soluble oligomers that contributes to the pathogenetic process [11, 20]. Thus, there is much interest in identifying factors that promote alpha-synuclein oligomerization.
One such factor is 3,4-dihydroxyphenylacetaldehyde (DOPAL), formed from cytosolic dopamine via monoamine oxidase (MAO) [14]. DOPAL potently oligomerizes alpha-synuclein [5]. Conditions affecting the extent of DOPAL-induced oligomerization of alpha-synuclein remain incompletely understood and may be important for understanding PD pathogenesis.
Metal ions are thought to contribute to the pathogenesis of PD [8, 16, 17]. One possible mechanism for such a role is by promoting alpha-synuclein oligomerization. Alpha-synuclein contains binding sites for copper, iron, and manganese ions. Under certain conditions, metal ions oligomerize alpha-synuclein [13, 19], and it has been proposed that a unique copper-induced oligomer mediates synuclein toxicity [4, 21]; however, induction of alpha-synuclein oligomerization by exposure to metal ions requires supraphysiologic concentrations of metal ions, long reaction times, and coupling agents [12].
In this study we explored whether DOPAL and metal ions interact to promote synuclein oligomerization. Figure 1 is a concept diagram showing the main question our study was designed to address. We hypothesized that metal ions promote DOPAL-induced oligomerization of alpha-synuclein and that, conversely, in the presence of DOPAL, even brief exposure to relatively low concentrations of metal ions oligomerizes alpha-synuclein. Therefore, we compared copper, iron, and manganese in their ability to augment DOPAL-induced oligomerization of alpha-synuclein.
Fig. 1.
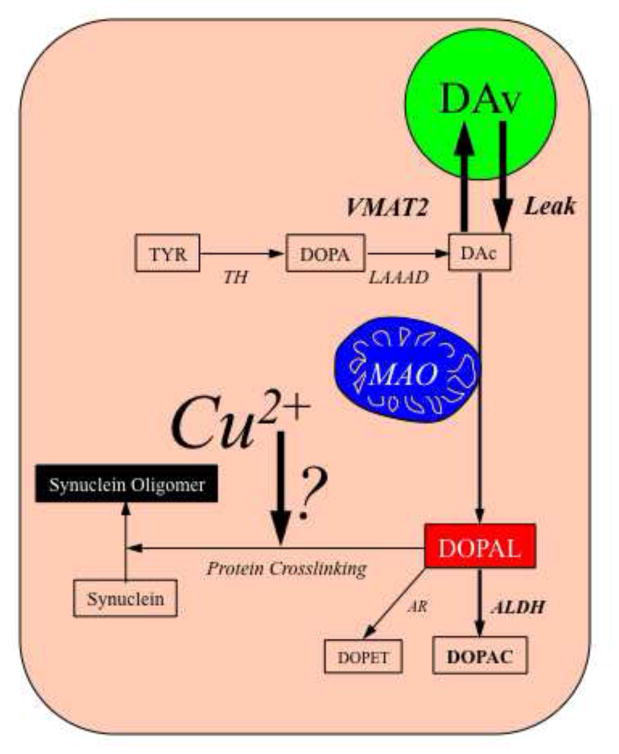
Diagram illustrating the hypothesis tested in this study.
Additionally, we compared the effect of DOPAL with dopamine and its metabolites 3,4-dihydroxyphenylethanol (DOPET) and 3,4-dihydroxyphenylacetic acid (DOPAC) in terms of synuclein oligomerization, with or without additional metal ions. We also evaluated whether an antioxidant or a divalent metal ion chelator attenuates the interaction between Cu2+ and DOPAL in oligomerizing alpha-synuclein.
Furthermore, we determined in rat pheochromocytoma PC12 cells conditionally over-expressing alpha-synuclein whether intracellular build-up of DOPAL dimerizes alpha-synuclein and if Cu 2+ enhances the DOPAL-induced dimerization.
2. EXPERIMENTAL SECTION
2.1. Chemicals and Reagents
DOPAL was prepared and provided by Dr. Kenneth L. Kirk (NIDDK/NIH).
Human recombinant alpha-synuclein was purchased from Calbiochem (La Jolla, CA, USA). Cupric chloride and ferrous sulfate were from Acros Organics (Morris Plains, NJ, USA) and manganese chloride from Fisher Science Education (Hanover Park, IL, USA). L-DOPA and cuprous chloride were obtained from Sigma Chemical Company (St Louis, MO, USA). Ferric chloride was from MP Biomedicals (Solon, OH, USA). Mouse anti-alpha-synuclein was from Invitrogen (Camarillo, CA, USA). All reagents were dissolved in deionized Type 1 water. All metal solutions were freshly prepared before incubation.
2.2. Experimental Procedures
To assess the effects of metal ions on alpha-synuclein oligomerization evoked by DOPAL, alpha-synuclein (1.6 μM) was incubated with DOPAL (30 μM) in 100 mM Tris-HCl buffer (pH 7.4) in the presence of various concentrations of metal ions (Cu2+, Cu1+, Fe2+, Fe3+ and Mn2+, 1–30 μM) at 37° C for 60 min.
To examine DOPAL’s specific effects, we compared DOPAL with dopamine and its metabolites DOPAC and DOPET. Furthermore, dopamine was incubated with MAO-A at 37° C for 60 min, to convert dopamine to DOPAL, followed by 60 min-incubation with alpha-synuclein.
In experiments to study the effects of modifiers of the DOPAL-metal ions interaction, the reducing agent ascorbic acid (10–1000 μM) or the divalent ion chelator EDTA (1000 μM) was included in the reaction and incubated with alpha-synuclein for 60 min.
Aggregation of alpha-synuclein was analyzed by Western blotting. Each reaction mixture was electrophoresed on NuPAGE 4–12% of Bis-Tris gels and transferred to a PVDF membrane using iBlot Dry Blotting system (Invitrogen, Carlsbad, CA, USA). Protein detection was performed with mouse anti-alpha-synuclein (1:200) antibody and the secondary antibody goat anti-mouse IRDye 800CW (1:10,000). The detector was an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). Band intensity was quantified by LI-COR Odyssey software.
2.3. Cell culture study
PC12 cells conditionally over-expressing alpha-synuclein was kindly provided by Drs. Ito and Nakaso, of Tottori University, Japan [9]. Cells were cultured in 5% FCS containing DMEM/F12 media with doxycycline; over-expression of alpha-synuclein was achieved by removing doxycycline. Cells with no doxycycline for 10 days were exposed to L-DOPA (100 μM) and Cu2+ (10 μM) for 6 hr, with or without pargyline (10 μM). Because alpha-synuclein is a cytosolic protein, cytosolic proteins were isolated using a Qproteome cell compartment kit (QIAGEN, Valencia, CA, USA). Cytosolic protein (500–600 μg) was purified further by immunoprecipitation (Pierce Classic kit, Pierce Biotechnology, Rockford, IL, USA). Aggregated alpha-synuclein standard was used as a positive control.
For catechol assays, PC12 cells were collected in 400 μL of 20:80 (0.04M H2PO4:0.2M acetic acid) and disrupted by freezing and thawing. The catechols were extracted by alumina adsorption and quantified by HPLC with electrochemical detection [7]. Cellular catechol concentrations were expressed in units of pg/mg protein.
2.4. Data Analysis and Statistics
All the in vitro studies were done with n ≥ 4, and all the cell studies were done in triplicate. Variability across samples is indicated by the standard error bars in the Figures.
Statistical analyses of Western blot bands and catechol contents were by one-way ANOVA with Dunnett’s or Bonferroni’s post-hoc tests, using Graph-Pad Prism Software 5.0. Statistical significance was defined by p<0.05.
3. RESULTS
3.1. Divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein
As expected, DOPAL alone evoked alpha-synuclein oligomerization (Fig. 2A,B). At 10 μM, DOPAL increased synuclein dimers to 2.7 times and at 30 μM 5.7 times control (alpha-synuclein alone). On the other hand, Cu2+ at concentrations up to 1000 μM did not augment synuclein oligomerization (Fig. 2C).
Fig. 2.
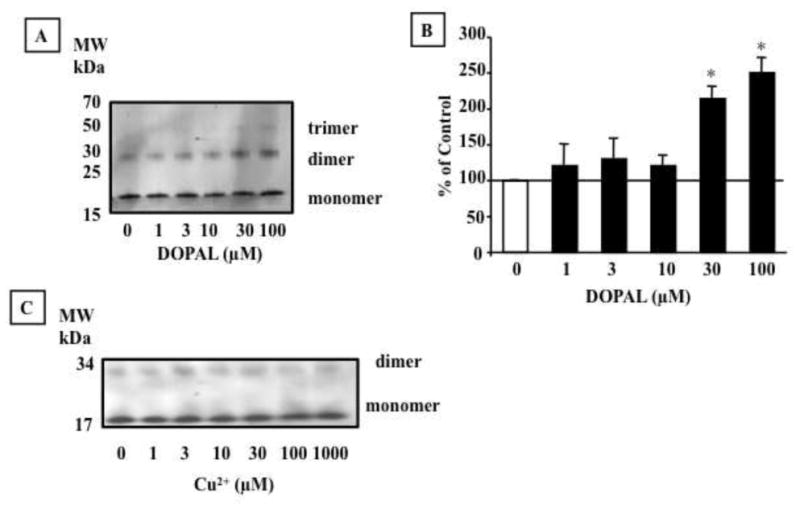
Effect of DOPAL and Cu2+ on alpha-synuclein (AS) aggregation. AS (1.6 μM) was incubated with DOPAL (1–100 μM) (A,B) and Cu2+(1 – 1000 μM) (C) for 60 min at 37 °C and the protein was detected by Western blot. Percents of control represent dimers for each treatment relative to 0 μM DOPAL. Statistical analyses were by one-way ANOVA with Dunnett’s post-hoc test. Mean values are expressed ± SEM (n=4). *P<0.05 vs. 0 μM DOPAL.
Divalent metal ions amplified DOPAL-induced alpha-synuclein oligomerization (Fig. 3A,B). Cu2+ was more potent than Fe2+ or Mn2+, with a significant increase in dimers at as low as 1 μM Cu2+. At 1 μM, Cu2+ the amount of synuclein dimerization in response to 30 μM DOPAL was almost doubled compared to no added Cu2+. At the highest Cu2+ concentration (30 μM), dimerization was increased to 4 times that seen with DOPAL alone. Higher-order oligomers (>50 kDa) were also noted in the reaction mixtures of DOPAL, alpha-synuclein, and high Cu2+ concentrations (10 and 30 μM).
Fig. 3.
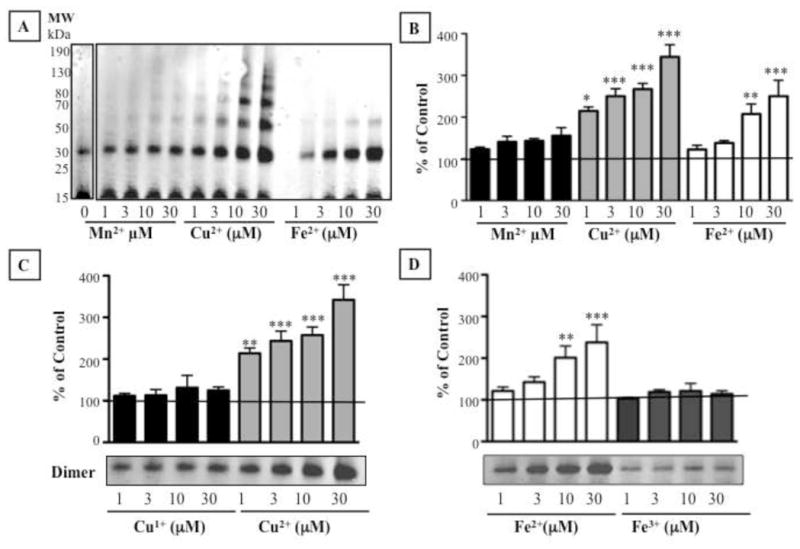
Metal ions augment DOPAL-induced alpha-synuclein oligomerization. Shown are effects of (A,B) Mn2+, Cu2+, and Fe2+ and (C,D) Cu1+ and Fe3+. Percents of control represent dimers for each treatment relative 30 μM DOPAL alone. Statistical analyses were by one-way ANOVA with Dunnett’s post-hoc test. Mean values expressed ± SEM (n=4–23). ***P<0.001, **P<0.01, *P<0.05 vs. DOPAL alone.
Fe2+ augmented DOPAL-induced alpha-synuclein dimers compared to DOPAL without added Fe2+. The effect at 30 μM was the same as Cu2+ at 3 μM, indicating a 10-fold difference between the two metals in terms of alpha-synuclein oligomerization. Formation of higher-order oligomers was also more apparent for Cu2+ than for Fe2+. Mn2+ did not enhance DOPAL-induced synuclein oligomerization.
Neither Cu1+ nor Fe3+ altered DOPAL-induced oligomerization of alpha-synuclein (Fig. 3C,D), whereas Cu2+ and Fe2+ did.
3.2. Dopamine and deaminated metabolites other than DOPAL do not oligomerize alpha-synuclein
Dopamine, DOPAC, and DOPET failed to oligomerize alpha-synuclein, even in the presence of Cu2+ (Fig. 4A). In contrast, DOPAL produced clear concentration-dependent oligomerization.
Fig. 4.
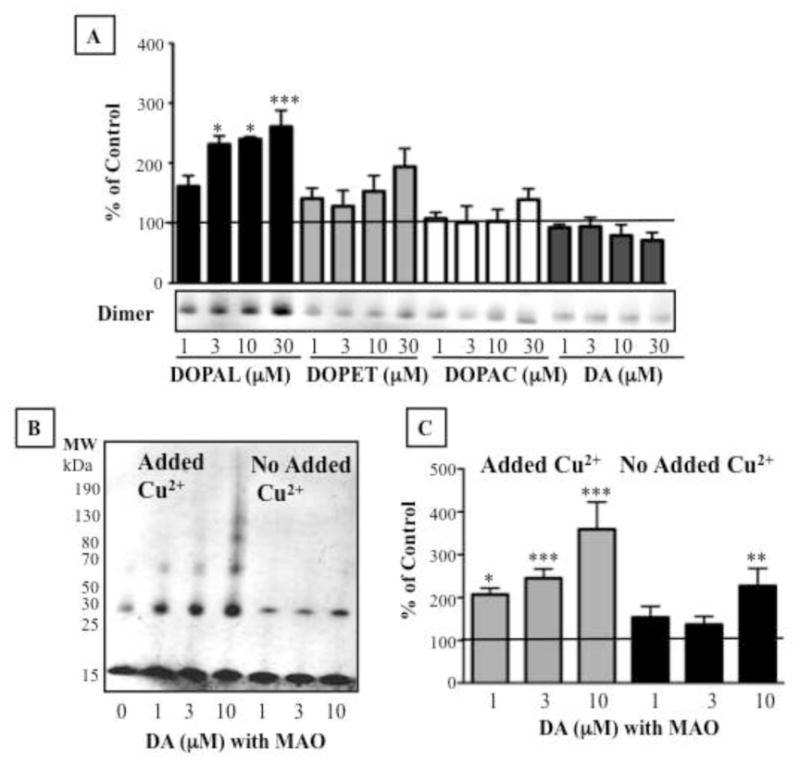
Comparison of effects of DOPAL, dopamine, DOPAC, and DOPET on alpha-synuclein (AS) oligomerization in the presence of Cu2+ (A). (B,C) Aggregation of AS by DOPAL produced from dopamine by MAO-A incubation. Percents of control represent dimers relative to AS only. Statistical analyses were by one-way ANOVA with Dunnett’s post-hoc test. Mean values expressed ± SEM (n=4–23). ***P<0.001, *P<0.05 vs. AS alone.
Incubation of dopamine with MAO-A for 60 min resulted in complete conversion of dopamine to DOPAL (data not shown). Enzymatically produced DOPAL strongly oligomerized alpha-synuclein with added Cu2+ (Fig. 4B,C).
3.3. Anti-oxidant treatment or divalent ion chelation attenuate effects of Cu2+ on DOPAL-induced oligomerization of alpha-synuclein
Ascorbic acid (10–1000 μM) reduced DOPAL-induced alpha-synuclein oligomerization, even at the lowest ascorbic acid concentration tested (10 μM, Fig. 5A,B). The divalent ion chelator EDTA also decreased the Cu2+ effect (Fig. 5A,C). Neither ascorbic acid nor EDTA affected alpha-synuclein oligomerization alone (data not shown).
Fig. 5.
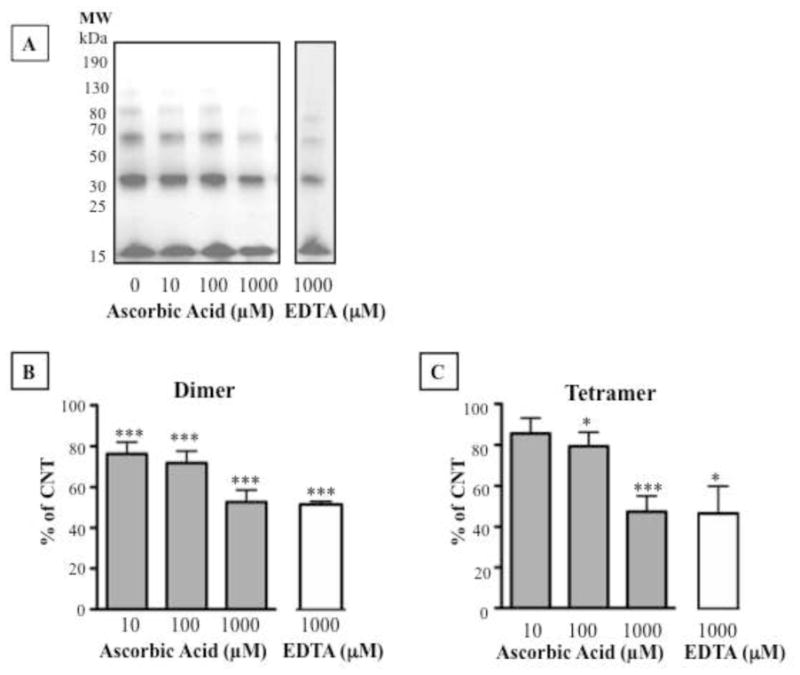
Effects of an antioxidant or metal ion chelation on DOPAL-induced alpha-synuclein oligomerization in the presence of Cu2+. (A,B,C) Effects of 10–1000 μM ascorbic acid and 1000 μM EDTA. Percent of control represents dimers (B) and tetramers (C) for each treatment relative to 30 μM DOPAL alone. Statistical analyses were by one-way ANOVA with Dunnett’s post-hoc test. Mean values expressed ±SEM (n=5). ***P<0.001,**P<0.01, *P<0.05 vs. DOPAL alone.
3.4. Cu2+ augments DOPAL-induced dimerization of alpha-synuclein in PC12 cells
Alpha-synuclein-overexpressing PC12 cells contained endogenous dopamine (12.5±1.21 ng/mg protein); endogenous DOPAL was not detected. To test for effects of building up intracellular DOPAL on alpha-synuclein aggregation, the cells were incubated for 6 hours with the dopamine precursor L-DOPA (100 μM). This augmented cellular DOPET and DOPAC levels by about 100-fold (Fig. 6B,C) and resulted in substantial intracellular DOPAL production. After 6 hours of L-DOPA treatment, cellular DOPAL averaged 4.03 ng/mg protein (Fig. 6A). When L-DOPA was combined with Cu2+, cellular concentrations of DOPAL, DOPAC, and DOPET were reduced compared to treatment with L-DOPA alone (Fig. 6A–C). Cu2+ itself had no effect on cellular catechol levels.
Fig. 6.
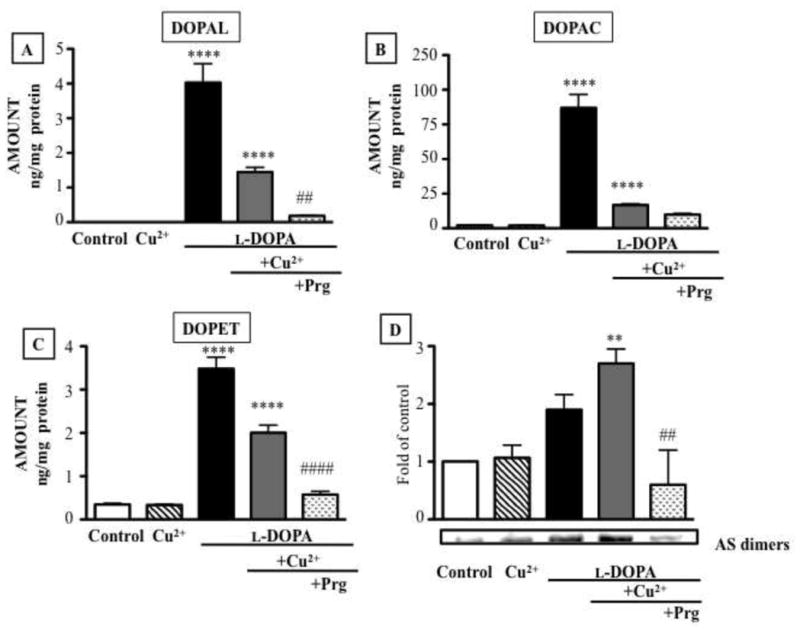
Intracellular contents of catechols (A: DOPAL; B: DOPAC; and C: DOPET) in PC12 cells over-expressing alpha-synuclein and treated with L-DOPA (100 μM), Cu2+ (10 μM), and 10 μM pargyline. Western blot of AS dimers in the cells (D). Statistical analyses were by one-way ANOVA with Bonferroni’s post-hoc test. Mean values expressed ± SEM (n=3). ***P<0.001, **P<0.01 vs. vehicle; ###P<0.001, ##P<0.01 vs. L-DOPA + Cu2+ group.
Western blotting demonstrated increased production of alpha-synuclein dimers after incubation with L-DOPA alone (Fig. 6D). Cu+2 augmented the effect, despite lower attained DOPAL concentrations, while pargyline attenuated the effect (Fig. 6D).
4. DISCUSSION
The results of this study show that divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein. It has already been reported that incubation of alpha-synuclein with Cu2+ can evoke oligomerization of the synuclein [5] and that DOPAL can dimerize synuclein in PC12 cells [7]; however, previous studies have not explored the possible interaction of these two factors, and this was the focus of the present report.
We found that neither Cu2+ nor Fe2+ alone oligomerized alpha-synuclein. At first glance this seems to conflict with previous reports [12, 13]. In order to observe direct Cu2+-induced oligomerization of alpha-synuclein, supraphysiologic concentrations, prolonged incubation, and a coupling reagent are required [12]. In the present report, we obtained evidence for a contribution of Cu2+ to alpha-synuclein oligomerization only in the setting of co-incubation with DOPAL. Even co-incubation with Cu2+ and DA did not oligomerize alpha-synuclein, unless MAO-A was added to the mixture.
The interaction between divalent metal cations and DOPAL in oligomerizing alpha-synuclein was especially prominent for Cu2+, was also noticeable for Fe2+, and was inapparent for Mn2+. These differences might be related to alpha-synuclein possessing several binding sites for Cu2+, two for Fe2+, and one for Mn2+ [3].
We separated two potential mechanisms by which metal ions could influence DOPAL-induced alpha-synuclein aggregation—redox activity and valence. Since reduced Fe2+ and oxidized Cu2+ both potentiated DOPAL-induced alpha-synuclein oligomerization, while oxidized Fe3+ and reduced Cu1+ did not, it is not the redox state of the metal ions but their divalency that determines the enhancement of DOPAL-induced alpha-synuclein oligomerization. Consistent with this explanation, EDTA chelation of divalent cations attenuated enhanced DOPAL-induced alpha-synuclein oligomerization.
We did not test effects of Fe2+ under anaerobic conditions. Because Fe2+ could easily oxidize to Fe3+ in the presence of oxygen, and since Fe3+ did not affect DOPAL-induced alpha-synuclein aggregation, the effect of Fe2+ could be more potent than what was found in this study.
The divalent cation chelator EDTA reduced DOPAL-induced oligomerization of alpha-synuclein. We speculate that Cu2+ may alter the conformation of alpha-synuclein by binding to the protein, thus rendering the protein more susceptible to cross-linking by DOPAL.
Incubation of alpha-synuclein with DOPAL was far more potent in oligomerizing alpha-synuclein than was incubation with other DA metabolites or with DA itself, confirming and extending on previously reported findings [5, 10, 15]. Further confirmation of the selective potency of DOPAL came from pre-incubating DA with MAO-A, which metabolizes DA to DOPAL. This pre-incubation induced alpha-synuclein oligomerization, whereas DA alone did not.
Alpha-synuclein aggregation by DOPAL and Cu2+ was detected in PC12 cells conditionally over-expressing alpha-synuclein. PC12 cells over-expressing alpha-synuclein contained relatively little intracellular DA and no detectable DOPAL. To augment intracellular DOPAL production, the cells were exposed to the DA precursor L-DOPA. Such exposure generated substantial amounts of cellular DA, DOPAL, DOPAC, and DOPET. Since MAO inhibition by pargyline eliminated the increase in alpha-synuclein dimerization evoked by L-DOPA, the rise in intracellular DOPAL production was responsible for the increased alpha-synuclein dimerization. Enzymatic deamination of cytosolic DA generates DOPAL and hydrogen peroxide in a 1:1 molar ratio; however, hydrogen peroxide does not oligomerize alpha-synuclein [7]. Thus, L-DOPA-induced oligomerization of alpha-synuclein is due to buildup of intracellular DOPAL.
DOPAL is susceptible to auto-oxidation to semi-quinone radicals and o-quinones [1]. Antioxidants such as ascorbic acid would be expected to inhibit this conversion. In our study, attenuation by ascorbic acid of Cu2+ effect on DOPAL-induced alpha-synuclein oligomerization would be consistent with a contribution of DOPAL quinones to the oligomerization. Ito et al. [9] noted decreased alpha-synuclein oligomerization in alpha-synuclein-overexpressing PC12 cells exposed to an inhibitor of tyrosine hydroxylase or to L-cysteine, suggesting that endogenous catecholamines or catecholamine-related quinones affect alpha-synuclein oligomerization.
It is unclear how the concentrations of Cu2+ and DOPAL used in the present study of PC12 cells relate to tissue concentrations in healthy humans or patients with PD. In human putamen, copper concentrations have been reported to average about 500 nmol/g dry tissue [6]; assuming the dry tissue weight is 1% of the wet tissue weight, the putamen Cu2+ concentration would be about 5 nmol/g wet weight, or about 5 μM. In the present study, Cu2+ concentrations from 1 to 10 μM were used for cell experiments.
In conclusion, divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein. Cu2+ is especially potent in this regard. Since alpha-synuclein oligomerization appears to play a role in the loss of catecholaminergic neurons in PD, the findings support a potential preventive or therapeutic approach based on interrupting the deleterious interaction of metal ions with DOPAL in inducing alpha-synuclein oligomerization.
Highlights.
Divalent metal ions augment DOPAL-induced oligomerization of alpha-synuclein.
Valency, not redox state, determines the augmentation of oligomerization.
DA and deaminated metabolites besides DOPAL do not oligomerize synuclein.
Anti-oxidant treatment or divalent ion chelation attenuates metal ion effect.
Cu2+ augments DOPAL-induced dimerization in PC12 cells.
Acknowledgments
We thank Dr. Kenneth L. Kirk for providing the DOPAL used in our experiments. The research was supported by the intramural research program of the NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J Biol Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 3.Bisaglia M, Tessari I, Mammi S, Bubacco L. Interaction between a-synuclein and metal ions, still looking for a role in the pathogenesis of parkinson disease. Neuromol Med. 2009;11 doi: 10.1007/s12017-009-8082-1. [DOI] [PubMed] [Google Scholar]
- 4.Brown DR. Metal binding to alpha-synuclein peptides and its contribution to toxicity. Biochemical and biophysical research communications. 2009;380:377–381. doi: 10.1016/j.bbrc.2009.01.103. [DOI] [PubMed] [Google Scholar]
- 5.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 6.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 Cells: Relevance to the pathogenesis of Parkinson disease. J Neurochem. 2012;123:932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- 9.Ito S, Nakaso K, Imamura K, Takeshima T, Nakashima K. Endogenous catecholamine enhances the dysfunction of unfolded protein response and alpha-synuclein oligomerization in PC12 cells overexpressing human alpha-synuclein. Neurosci Res. 2010;66:124–130. doi: 10.1016/j.neures.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Jinsmaa Y, Florang VR, Rees JN, Mexas LM, Eckert LL, Allen EM, Anderson DG, Doorn JA. Dopamine-derived biological reactive intermediates and protein modifications: Implications for Parkinson’s disease. Chem Biol Interact. 2011;192:118–121. doi: 10.1016/j.cbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazantsev AG, Kolchinsky AM. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch Neurol. 2008;65:1577–1581. doi: 10.1001/archneur.65.12.1577. [DOI] [PubMed] [Google Scholar]
- 12.Paik SR, Shin HJ, Lee JH. Metal-catalyzed oxidation of alpha-synuclein in the presence of Copper(II) and hydrogen peroxide. Arch Biochem Biophys. 2000;378:269–277. doi: 10.1006/abbi.2000.1822. [DOI] [PubMed] [Google Scholar]
- 13.Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340(Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 14.Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. Journal of neurochemistry. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 17.Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. Journal of neural transmission. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- 18.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Moualla D, Wright JA, Brown DR. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. Journal of neurochemistry. 2010;113:704–714. doi: 10.1111/j.1471-4159.2010.06638.x. [DOI] [PubMed] [Google Scholar]
- 20.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that {alpha}-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright JA, Wang X, Brown DR. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]


