Abstract
In the present study, we investigated the effect of simultaneous downregulation of uPAR and cathepsin B (pUC), alone or in combination with radiation, on JNK–MAPK signaling pathway in regulating the migration of non-GICs (glioma-initiating cells) and GICs. The increase in the expression of p-JNK with pUC treatment was mostly localized to nucleus whereas increase in the expression of p-JNK with radiation and overexpression of uPAR and cathepsin B was confined to cytoplasm of the cells. Depletion of cytosolic p-JNK with pUC treatment inhibited migration by downregulating the expression of the adapter proteins of the focal adhesion complex. We also observed that knockdown of uPAR and cathepsin B regulated the Ras–Pak-1 pathway to induce the translocation of p-JNK from cytosol to nucleus. In control cells, Pak-1 served as a functional inhibitor for MEKK-1, which inhibits the complex formation of MEKK-1 and p-JNK and thus inhibits the translocation of this complex into nucleus. Hence, we conclude that glioma cells utilize the availability of cytosolic p-JNK in driving the cells towards migration. Finally, treating the cells with pUC alone or in combination with radiation induced the translocation of the MEKK-1-p-JNK complex from cytosol to nucleus, thereby inhibiting the migration of glioma cells.
Introduction
Treatment for glioblastoma multiforme (GBM), the most lethal primary brain tumor, remains essentially palliative despite multimodal therapies including surgical resection, radiation and chemotherapy (Inoue et al., 2010). Aggressive infiltration of GBM cancer cells into normal brain tissue often prevents the complete removal of tumor cells through surgical resection. In addition, the existence of a small subpopulation of glioma cells that escapes radiation and chemotherapy-induced cell death makes GBM currently incurable (Gilbert and Ross, 2009). These small subpopulation of cells, referred to as glioma stem cells or glioma-initiating cells (GICs), have been shown to be highly tumorigenic, highly invasive, pro-angiogenic and resistant to therapy compared with the majority of tumor cells, suggesting the importance of targeting GICs when developing novel glioma therapies (Hjelmeland et al., 2011).
In solid malignancies, it is unusual for a single kinase abnormality or only one abnormally activated signaling pathway to be the sole cause of disease. Instead multiple signaling pathways or even a single molecular event with multiple downstream effects are dysregulated (Gossage and Eisen, 2010). One of the most exquisite examples includes the mitogen activated pathway kinases (MAPKs), which transduce signals that are involved in a multitude of cellular pathways and functions based on the cues derived from cell surface, metabolic state and environment of the cell (Lawrence et al., 2008; Owens and Keyse, 2007). Abnormalities in MAPK signaling impinge on most of the hallmark characteristics required for the development and progression of cancer (Dhillon et al., 2007). Therefore, targeting a key underlying defect in the MAPK signaling may provide a greater potential for increased efficacy by simultaneous inhibition of multiple pathways.
The c-Jun NH2-terminus kinases (JNKs) belong to the MAPK family, which also includes the extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase. JNKs are activated in response to inflammatory cytokines; environmental stresses, such as heat shock, ionizing radiation, oxidant stress and DNA damage; DNA and protein synthesis inhibition; and growth factors (Raman et al., 2007). One of the most extensively studied and well-known functions of JNK is its induction of apoptosis. Upon activation, the phosphorylated JNK translocates to nucleus where it phosphorylates and regulates the activation of transcription factors like c-Jun, ATF-2, Elk-1, p53 and c-Myc, which are involved in the induction of cell apoptosis (Dhanasekaran and Reddy, 2008; Johnson and Nakamura, 2007; Wang et al., 2010). However, it has been recently reported that the inhibition of JNK activity impairs cell migration of fibroblasts, smooth muscle cells, keratinocytes, rat bladder tumor cells, endothelial cells and Schwann cells (Chen et al., 2009; Huang et al., 2004b). In addition, JNK phosphorylates Paxillin on Ser178 and regulates the migration of NBT-II cells, MDA-MB-231 breast cancer cells and Chinese hamster ovary cells (Huang et al., 2003, 2004a, 2008). These findings emphasize the fact that the activation of JNK might be critical for the migration of cells.
Proteolytic enzymes and proteases are necessary for the degradation of surrounding proteins and other tissue components and thus play crucial roles in multiple steps of cancer invasion and metastasis (Edwards and Cancer, 1998). Among the proteases, uPAR and cathepsin B are often detected in higher amounts in malignant tumors and have been attributed to contribute major roles in the cancer progression (Alapati et al., 2012; Malla et al., 2012a; Mohamed and Sloane, 2006; Rao, 2003; Smith and Marshall, 2010). Earlier reports indicate that the blockade of uPAR and cathepsin B expression induced a significant reduction in the migration and invasion capabilities of cancer cells (Ahmed et al., 2003; Matarrese et al., 2010; Nalla et al., 2010; Veeravalli et al., 2010; Victor et al., 2011) by effectively abrogating the activation of MAPK signaling (Rabbani et al., 2010; Wegiel et al., 2009; Wu et al., 2008).
In the present study, we studied the effect of shRNA-mediated downregulation of uPAR and cathepsin B (pUC) on 5310 and 4910 non-GICs and GICs either alone or in combination with radiation treatment. Our findings indicate that treating non-GICs and GICs with pUC alone or in combination with radiation reduced the migration of these cells by regulating the JNK–MAPK signaling through the Ras-PI3K pathway in vitro and in vivo. We also observed that a major pool of p-JNK accumulated in the cytoplasm of untreated or irradiated glioma cells while the activated JNK translocated into the nucleus of the non-GICs and GICs treated with pUC alone and in combination with radiation. Further, cytoplasmic p-JNK interacted with adapter proteins of the focal adhesion complex and drove the cells towards an aggressive migratory phenotype.
Materials & methods
Ethics statement
The Institutional Animal Care and Use Committee of the University of Illinois College of Medicine at Peoria (Peoria, IL) approved all surgical interventions and post-operative animal care. The consent was written and approved. The approved protocol number is 851 and is dated November 20, 2009.
Cell culture conditions
5310 and 4910 glioma xenograft cells kindly provided by Dr. David James (University of California—San Francisco, San Francisco, CA) were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were kept at 37 °C in a humidified incubator with 5% CO2. 5310 and 4910 GICs and non-GICs were isolated with PE-conjugated CD133 antibody and cultured in their respective media as described earlier (Alapati et al., 2012; Malla et al., 2012a).
Transfection, radiation and inhibitor treatments
All transfections were carried out in 100-mm culture plates using X-tremeGENE 9 reagent as per the manufacturer's protocol (Roche, Indianapolis, IN). 5310 and 4910 non-GICs and GICs were transfected with scrambled vector (pSV) or a bicistronic shRNA construct of uPAR and cathepsin B (pUC). Either at 48 h (non-GIC) or at 24 h (GIC) after transfection, the cells were treated with 10 Gy using an RS 2000 biological irradiator (Rad Source Technologies Inc., Boca Raton, FL) X-ray unit operated at 150 kV/25 mA. Cells were then incubated for another 24 h or 48 h, respectively. Cells were transfected with a plasmid expressing full-length human cDNA clone of uPAR (FLU) (SC319092, Origene, Rockville, MD) and cathepsin B (FLC) (SC109129, Origene, Rockville, MD) for uPAR and cathepsin B overexpression studies. For inhibitor studies, cells seeded in six-well plates were treated with U0126 (10 μM, Promega, Madison, WI), SP600125 (10 μM, EMD Millipore, Billerica, MA), SB202190 (10 μM, Sigma, St. Louis, MO), or IPA3 (10 μM, SCBT, Santa Cruz, CA) for 24 h.
Immunoblotting and immunoprecipitation
Total protein (40 μg) was separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then blocked with 5% non-fat milk for 1 h, incubated with primary antibody overnight at 4 °C, washed thrice with PBS-T (PBS plus 0.1% Tween 20), and incubated with HRP-linked secondary antibody for 1 h at room temperature. The membrane was then washed and bands were visualized by chemiluminescence assay. The following antibodies were used: uPAR, cathepsin B, ERK, p-ERK, JNK, p-JNK, p38, p-p38, Vinculin, α-Actinin, Talin, PI3K, p-PI3K, Rac-1, MEKK-1, Laminin and GAPDH (all from SCBT, Santa Cruz, CA). We also used antibodies for Paxillin, p-Paxillin, Pak-1 and p-Pak-1 (all from Cell Signaling Technology, Danvers, MA). We obtained Ras10 from Millipore (Billerica, MA).
For immunoprecipitation, cell lysates (300 μg) were pre-cleared by protein A/G micro-beads (Miltenyi Biotec, Auburn, CA) and then incubated with specific antibodies at a dilution of 1:100 overnight at 4 °C. The beads were washed with lysis buffer and resuspended in sample buffer before the immunoprecipitated protein was subjected to immunoblotting.
Spheroid migration assay
5310 and 4910 non-GIC and GIC spheroids were prepared in 96-well plates coated with 1% agar by seeding 3 × 104 cells/ well. The plates were then incubated on a shaker at 100 rpm for 24 h in a humidified chamber at 37 °C with 5% CO2. The spheroids were treated with SV, pUC, 10 Gy, pUC + 10 Gy, FLU, FLC, U0126, SP600125 or SB202190 for specific time points and then transferred to 24-well plates and allowed to migrate for another 24 h. Spheroids were then fixed and stained with Hema-3, and cell migration was assessed using a light microscope. The migration of cells from spheroids to monolayers was used as an index of cell migration and was measured using a microscope calibrated with a stage and ocular micrometer. Statistical comparisons were performed using GraphPad Prism software (version 3.02). Quantitative data from migration assays was evaluated for statistical significance using Student's t-test. Differences in the values were considered significant at p < 0.5.
Extraction of nuclear and cytosolic fractions
Active Motif Nuclear Extraction Kit (Carlsbad, CA) was used to isolate the cytoplasmic and nuclear fractions from the cells as per the manufacturer's instructions. Briefly, 1× PBS washed cell pellets were resuspended in appropriate amount of hypotonic buffer and incubated at 4 °C on a rocking platform for 30 min and then centrifuged at 14,000 ×g for 30 s. The supernatant was collected and represented as cytosolic fraction. The remaining pellets were resuspended in complete lysis buffer and incubated at 4 °C for 30 min on a rocking platform. The suspension was then homogenized and the nuclear fractions were collected after centrifugation at 14,000 ×g for 10 min. Immunoblot analysis for JNK and p-JNK was performed with the cytosolic and nuclear fractions.
Immunocytochemical analysis
Glioma cells grown in 4-well chamber slides (Nalgene Nunc International, Naperville, IL) were fixed with 4% buffered formalin for 1 h at room temperature, washed, and treated with 0.1% Triton X-100 before labeling with p-JNK and p-Paxillin primary antibodies at 4 °C overnight. The cells were then stained with Alexa Fluor-conjugated secondary antibodies for 1 h at room temperature, nuclear stained with DAPI, and visualized under a confocal microscope.
Immunohistochemical analysis
Stereotactic implantation of 5310 and 4910 non-GICs and GICs was carried out as described previously (Lakka et al., 2004). Mice were treated with mock, pUC, 10 Gy, and pUC + 10 Gy using ALZET mini-osmotic pumps at the rate of 0.25 μL/h. After 5 weeks, the mice were killed by intracardiac perfusion, first with PBS and then with 4% paraformaldehyde in normal saline. Paraffin sections were prepared.
For co-localization studies, the deparaffinized sections were antigen retrieved and incubated overnight with p-Paxillin and p-JNK primary antibodies at 4 °C in a humidified chamber. They were then stained with the Alexa Fluor conjugated secondary antibodies for 1 h in the dark at room temperature, and nuclear stained with DAPI for a brief period of time before mounting. The sections were then pictured under a confocal microscope.
Results
pUC treatment decreased the radiation-induced expression of uPAR and cathepsin B
Radiation remains one of the essential therapies for treating cancer patients. To observe the effect of radiation treatment on the expression levels of uPAR and cathepsin B, 5310 and 4910 glioma cells were treated with 5 and 10 Gy at 24 h and 48 h. Radiation-treated 5310 and 4910 non-GICs and GICs showed an increase in the expression levels of uPAR and cathepsin B when compared to their controls (data not shown). Non-GICs responded to radiation treatment within 24 h whereas the response from GICs was significant only after 48 h of radiation treatment, indicating the radioresistance of GICs. Based on these results, non-GICs were treated with 10 Gy for 24 h and GICs were treated with 10 Gy for 48 h in the subsequent experiments.
An effective inhibition of uPAR and cathepsin B expression in pUC and pUC + 10 Gy treated 5310 and 4910 non-GICs and GICs in comparison to their respective non-radiated and radiated controls at 72 h was confirmed by western blotting as shown in Supplementary Fig. 1.
uPAR and cathepsin B knockdown inhibited migration of the glioma xenograft cells
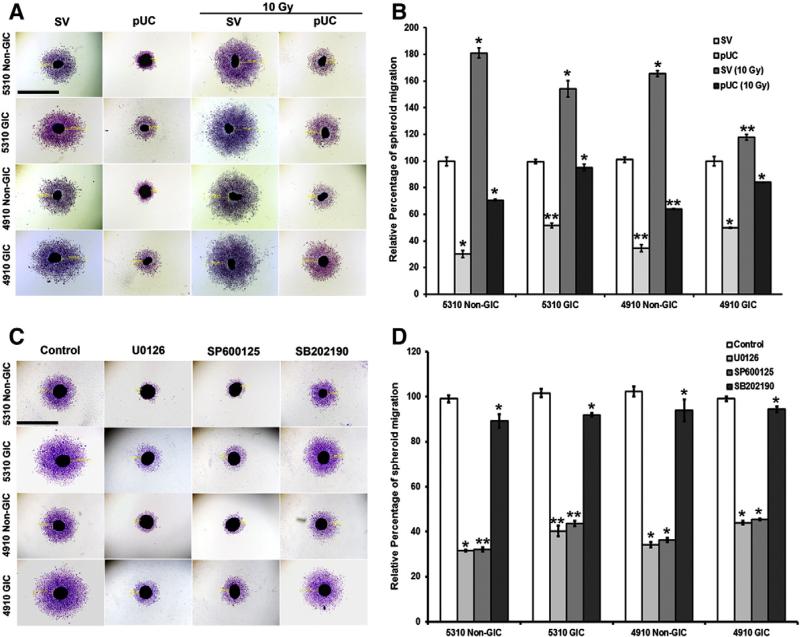
Migration of individual cells from the primary tumor mass is considered to be an essential and initial step for attaining an invasive and metastatic cancer phenotype (Friedl and Wolf, 2003). Spheroid migration assay was carried out to investigate the migrating potentials of 5310 and 4910 non-GICs and GICs with pUC and radiation alone or in combination. The xenograft cells from SV-radiated spheroids migrated more (81.32% — 5310 non-GICs, 54.56% — 5310 GICs, 64.54% — 4910 non-GICs, and 18.1% — 4910 GICs) when compared to that of their SV-treated spheroids (Figs. 1A & B). A prominent reduction in the migration of the cells from spheroids was noticed upon treatment with pUC (69.45% — 5310 non-GICs, 47.97% — 5310 GICs, 66.58% — 4910 non-GICs and 49.74% — 4910 GICs) and pUC + 10 Gy (60.96% — 5310 non-GICs, 38.27% — 5310 GICs, 59.21% — 4910 non-GICs, and 28.79% — 4910 GICs) when compared to the cells migrating from SV-treated and SV-radiated spheroids, respectively.
Figure 1.
Effect of radiation, pUC, and inhibitor treatment on migration of 5310 and 4910 non-GICs and GICs. A) Spheroids of 5310 and 4910 non-GICs and GICs were treated with SV, pUC, 10 Gy, and pUC + 10 Gy. pUC treatment was given for 72 h. For non-GIC spheroids, radiation treatment was given for 24 h, and for GIC spheroids, radiation treatment was given for 48 h. The spheroids were then allowed to migrate for another 24 h, Hema stained and pictured under a light microscope. B) Migration of the cells from the spheroids was measured using a microscope calibrated with a micrometer and percent migration was calculated from the mean of the average migration obtained from 3 independent experiments. C) 5310 and 4910 non-GIC and GIC spheroids were treated with ERK inhibitor (10 μM U0126), JNK inhibitor (10 μM SP600125) and p38 inhibitor (10 μM SB20219) for 24 h and allowed to migrate for another 24 h. The spheroids were then fixed with methanol, Hema stained and visualized under a light microscope. D) Spheroid migration into monolayers was quantified with a microscope calibrated with a micrometer and calculated as a mean of the average migration obtained from 3 independent experiments; *, p < 0.5 and **, p < 0.01. Bar = 500 μm.
MAPK inhibition reduced cell migration
Many extracellular signals converge at a family of serine/ threonine protein kinases called MAPKs. Based on the differences in the motifs within their activation loops, they can be divided into 3 groups: ERK, JNK and p38. MAPKs play well-known roles in cell proliferation, oncogenesis, differentiation, inflammation and stress response, but accumulating evidence indicates that this family is also essential for cell migration (Huang et al., 2004b). Hence, we hypothesized that MAPKs might play a role in regulating the migration of 5310 and 4910 glioma xenograft cells.
Spheroid migration assay revealed that the cells migrating from the spheroids treated with MAPK inhibitors displayed a reduced amount of migration when compared to the vehicle-treated spheroids (Figs. 1C & D). ERK inhibitor (U0126) induced 69.9%, 53.6%, 63.7% and 46.6% reduction, JNK inhibitor (SP600125) induced 65.1%, 46.1%, 60.1% and 39.5% reduction, and p38 inhibitor (SB202190) induced 32.3%, 18.1%, 24.5% and 11.1% reduction in the migration of the cells from 5310 non-GIC, 5310 GIC, 4910 non-GIC, and 4910 GIC spheroids, respectively when compared to their DMSO control spheroids. Quantification of migration of the cells from spheroids thus revealed that ERK and JNK inhibitors were very effective when compared to that of the p38 inhibitor. Thus, we continued our further studies with ERK and JNK.
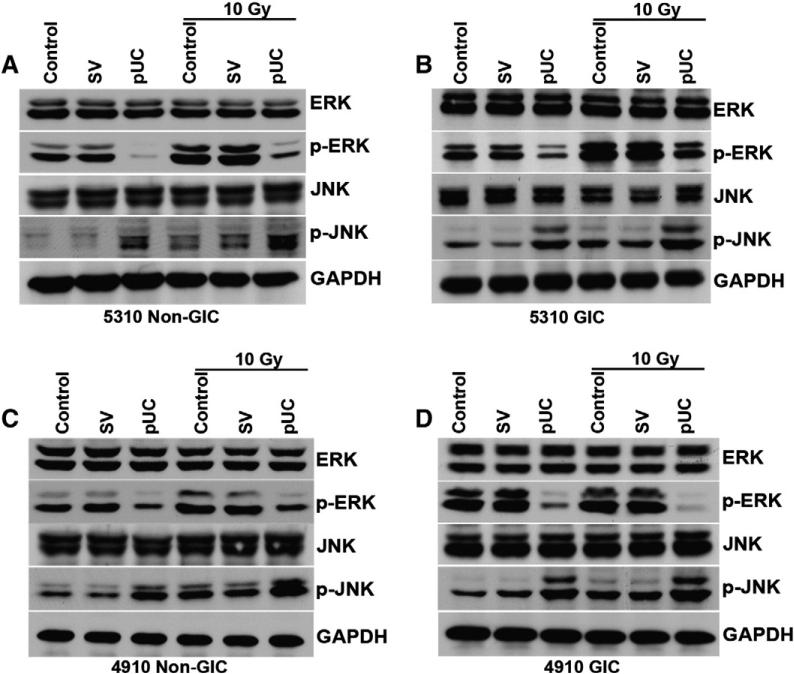
Simultaneous knockdown of uPAR and cathepsin B increased the expression of p-JNK
To observe the involvement of uPAR and cathepsin B in regulating the migration of the glioma cells through ERK and JNK, western blot analysis of 5310 and 4910 non-GICs and GICs was conducted after transfecting the cells with the bicistronic construct. pUC-treated non-GICs and GICs alone or in combination with radiation showed a decrease in the expression of p-ERK and an increase in the expression of p-JNK (Fig. 2). Earlier we observed that JNK inhibitor significantly reduced the migration of the cells (Figs. 1C and D); however, a significant increase in the protein expression of p-JNK was noticed with pUC and pUC + 10 Gy treatments. Since the above results seemed to be contradictory, we further concentrated on the role of uPAR and cathepsin B in regulating the JNK–MAPK pathway.
Figure 2.
pUC treatment alone or in combination with radiation downregulated p-ERK and upregulated p-JNK. Cell lysates were extracted and western blot analyses of A) 5310 non-GICs, B) 5310 GIC, C) 4910 non-GIC and D) 4910 GIC were performed for ERK, p-ERK, JNK and p-JNK antibodies. GAPDH served as a loading control.
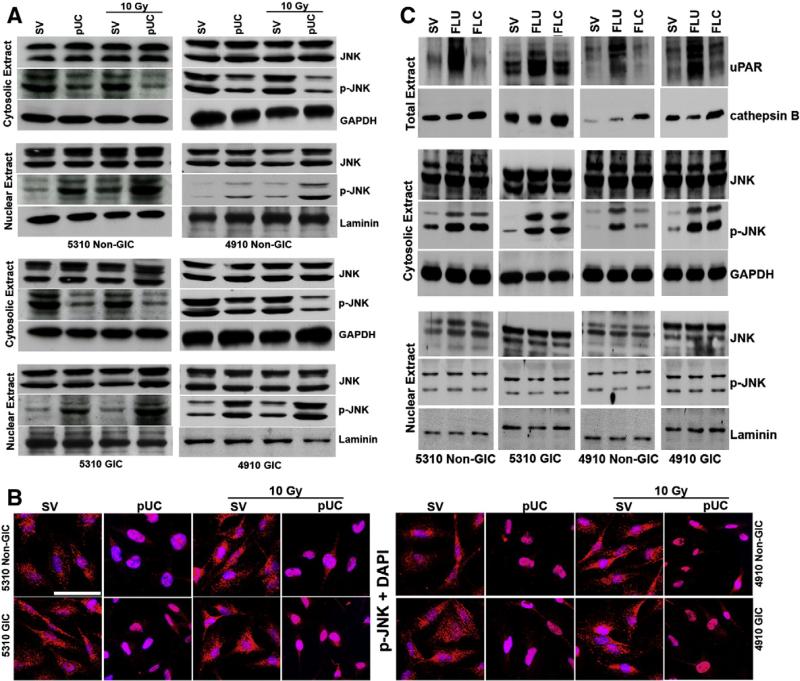
pUC treatment induced the translocation of p-JNK into the nucleus
Since, compartment-specific signaling of JNK has been reported earlier (Bogoyevitch and Kobe, 2006), we investigated the variations in the localization of p-JNK by isolating the nuclear and cytosolic extracts from pUC-treated glioma xenograft cells. Investigation of cytosolic and nuclear extracts from the cells treated with pUC, 10 Gy and their combination revealed a profound increase in the nuclear localization of activated JNK in the cells treated with pUC and pUC + 10 Gy (Fig. 3A). Nuclear localization of a minor pool of p-JNK was noticed in the glioma cells treated with radiation when compared to that of their non-irradiated controls. It was also noted that the nuclear translocation of p-JNK was more in the irradiated non-GICs when compared to that of the irradiated GICs. Nuclear localization of p-JNK in non-GICs and GICs treated with pUC alone or in combination with radiation was further confirmed by the immunocytochemical analysis as shown in Fig. 3B.
Figure 3.
pUC treatment alone or in combination with radiation induced the translocation of p-JNK into the nucleus of 5310 and 4910 glioma cells. A) Cytosolic and nuclear fractions of 5310 and 4910 non-GICs and GICs treated with pUC and radiation alone or in combination were isolated. Western blot analyses of cytosolic and nuclear fractions of non-GICs and GICs were performed and immunoblotted for JNK and p-JNK. GAPDH served as a loading control for cytosolic fractions and laminin served as a loading control for nuclear fractions. B) 5310 and 4910 non-GICs and GICs were grown in 4-well chamber slides, treated for 72 h, fixed with buffered formalin, incubated with p-JNK, stained with Alexa Fluor-conjugated secondary antibodies, nuclear stained with DAPI, and pictured under a confocal microscope. Bar = 200 μm. C) 5310 and 4910 glioma xenograft cells were treated with full-length uPAR (FLU) and full-length cathepsin B (FLC) plasmids for 48 h. Total extracts were immunoblotted for uPAR and cathepsin B. Cytosolic and nuclear extracts were isolated and western blotted for JNK and p-JNK. GAPDH served as the cytosolic loading control and laminin served as the nuclear loading control.
To observe the effect of upregulation of uPAR and cathepsin B on the activation and translocation of JNK, cytosolic and nuclear extracts from the cells transfected with FLU and FLC plasmids were isolated and immunoblotted for p-JNK. An increase in the cytosolic expression of p-JNK was observed in 5310 and 4910 non-GICs and GICs while the expression levels of p-JNK in the nuclear extracts of FLU- and FLC-treated glioma cells remained unchanged (Fig. 3C). Further, it was also observed that the FLU- and FLC-treated spheroids migrated more compared to that of their control spheroids (Supplementary Fig. 2), indicating that the cytoplasmic pool of p-JNK might be driving the cells towards a migratory phenotype.
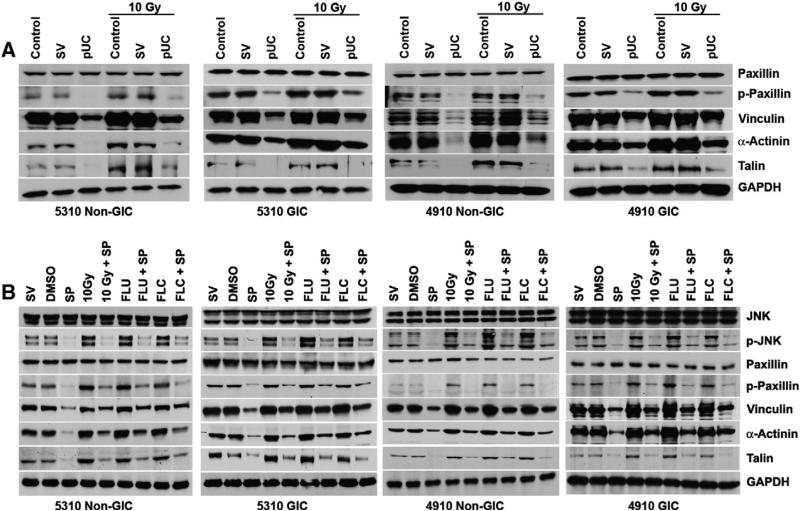
Depletion of cytosolic p-JNK by downregulating uPAR and cathepsin B reduced the expression of focal adhesion molecules
Recent findings indicate that several targets of the JNK signaling pathway include a number of focal adhesion, microtubule-associated and intermediate filament proteins that are involved in cell migration (Bogoyevitch and Kobe, 2006; Huang et al., 2004b). In our study, we observed that the protein levels of the migratory motor molecules p-Paxillin, Vinculin, α-Actinin and Talin (Fig. 4a) and the adhesion molecules Integrin αvβ3 and Integrin β1 (Supplementary Fig. 5) also increased with radiation as compared to their matched non-irradiated counterparts. pUC treatment induced the translocation of p-JNK into the nucleus and therefore reduced the availability of cytosolic p-JNK in the cells treated with pUC and pUC + 10 Gy. Western blot analysis of 5310 and 4910 non-GICs and GICs revealed that the depletion of cytosolic p-JNK in the cells treated with pUC alone or in combination with radiation reduced the expression levels of the aforementioned migratory motor molecules (Fig. 4A) and the adhesion molecules (Supplementary Fig. 5). Addition of JNK inhibitor SP600125 to cells treated with SV, DMSO, 10 Gy, FLU and FLC significantly decreased the protein expression levels of p-Paxillin, Vinculin, α-Actinin, and Talin when compared to that of their respective counterparts (Fig. 4B), indicating the importance of cytosolic p-JNK in regulating the migration of the cells.
Figure 4.
Cytosolic p-JNK aided in the migration of 5310 and 4910 non-GICs and GICs. A) 5310 and 4910 non-GICs and GICs were treated with pUC and 10 Gy radiation alone or in combination. Cell lysates were isolated and western blotted for Paxillin, p-Paxillin, Vinculin, α-Actinin and Talin. GAPDH served as a loading control. B) 5310 and 4910 xenograft cells were treated with SV, DMSO, 10 Gy, FLU and FLC alone and in combination with JNK inhibitor (10 μM SP600125 represented as SP). Cell lysates were isolated and western blotted for JNK, p-JNK, Paxillin, p-Paxillin, Vinculin, α-Actinin and Talin. GAPDH served as a loading control.
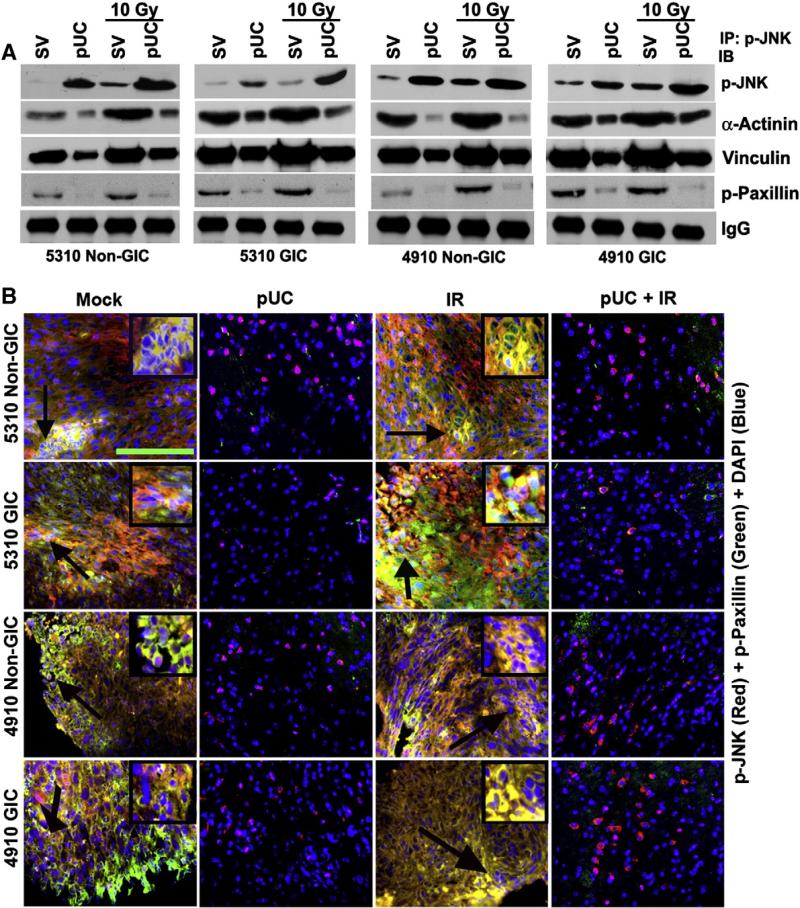
Downregulation of uPAR and cathepsin B reduced the interaction of p-JNK with migratory motor molecules
A direct interaction between p-JNK and p-Paxillin, Vinculin and α-Actinin was observed in the immunoprecipitated control samples of 5310 and 4910 non-GICs and GICs (Fig. 5A). This interaction was further augmented in the cells treated with radiation against their non-irradiated counterparts (Fig. 5A). When non-GICs and GICs were treated with pUC and pUC + 10 Gy, a significant reduction in the precipitation of p-Paxillin, Vinculin and α-Actinin with p-JNK was evident when compared to that of their SV-treated and SV-irradiated controls, respectively (Fig. 5A). Immunocytochemical analysis also confirmed the co-localization of p-JNK with p-Paxillin at the leading edge of the migrating SV-treated and SV-irradiated non-GICs and GICs (Supplementary Fig. 3). It is noteworthy that the pUC and pUC + 10 Gy treated cells displayed an increase in the expression of nuclear p-JNK and a decrease in the expression of p-Paxillin and its co-localization with p-JNK.
Figure 5.
p-JNK interacted with the migratory motor molecules. A) 5310 and 4910 non-GICs and GICs were treated with pUC and radiation alone or in combination. The cell lysates were collected and immunoprecipitated for p-JNK antibody and then western blotted for p-JNK, α-Actinin, Vinculin and p-Paxillin. B) 5310 and 4910 non-GICs and GICs were implanted intracranially and treated with mock, pUC, and radiation alone or in combination. When chronic symptoms were observed, mice were sacrificed and their brains were removed and embedded in paraffin. Deparaffinized sections were incubated with p-JNK (red) and p-Paxillin (green), stained with Alexa Fluor-conjugated secondary antibodies, and nuclear stained with DAPI. The sections were then visualized for co-localization of p-JNK and p-Paxillin under a confocal microscope. Arrows indicate the enlarged inset pictures. Bar = 50 μm.
In accordance with the in vitro studies, p-JNK and p-Paxillin significantly co-localized in the tissue sections of the mice implanted with glioma xenograft cells (Fig. 5B). The interaction between p-JNK and p-Paxillin further increased in the irradiated tissue sections. pUC treatment alone or in combination with radiation efficiently inhibited the interaction between p-JNK and p-Paxillin. Nuclear localization of p-JNK in the tissue sections of mice treated with pUC and pUC + 10 Gy was also evident.
uPAR and cathepsin B regulate p-JNK through Ras–PI3K pathway
Alterations in Ras–MAPK pathway have been reported to play critical roles in tumorigenesis by regulating the proliferation, differentiation and migration of the tumor cells (Santarpia et al., 2012). Based on this earlier report, we conducted the western blot analysis of 5310 and 4910 non-GICs and GICs, which revealed a decrease in the protein expression levels of Ras, p-PI3K, Rac-1 and p-Pak-1 in the cells treated with pUC alone or in combination with radiation when compared to that of their controls (Supplementary Fig. 4). Irradiated non-GICs and GICs displayed an increase in the protein levels of the above mentioned Ras-pathway molecules against their non-irradiated counterparts. An increase in the expression of MEKK-1 was observed in the non-GICs and GICs treated with pUC, radiation and their combination when compared to the controls.
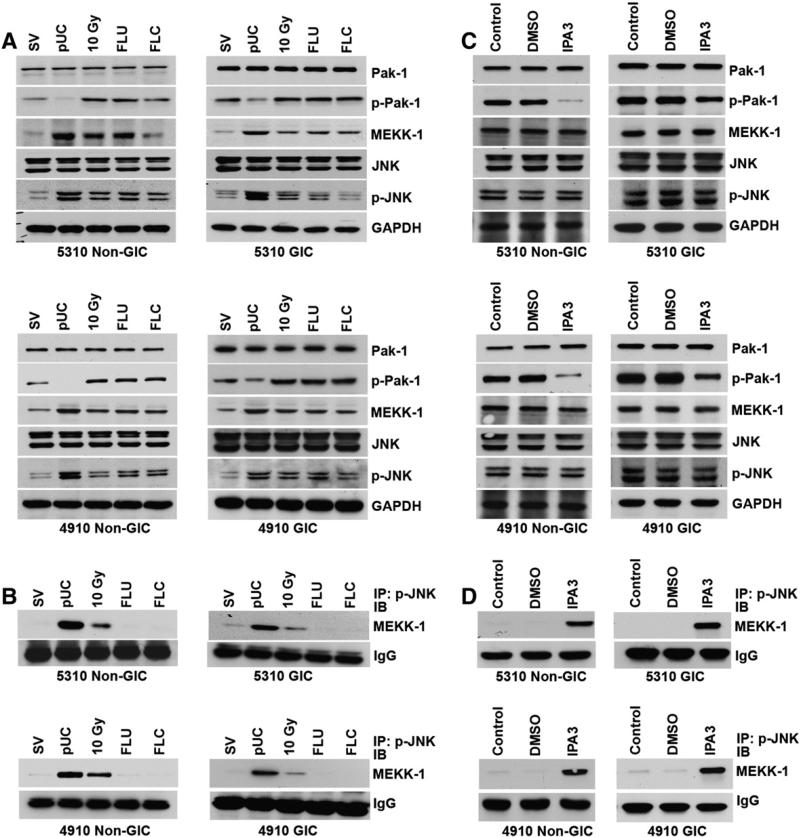
A bulk of p-JNK co-immunoprecipitated with MEKK-1 in the pUC-treated 5310 and 4910 xenograft cells (Fig. 6A). Some amount of interaction between p-JNK and MEKK-1 was also observed in the cells treated with irradiation whereas the control cells and the cells treated with FLU and FLC did not display any interaction between p-JNK and MEKK-1 (Fig. 6B). It was also observed that the Pak-1 inhibitor IPA3 did not induce any effect on the expression of p-JNK and MEKK-1 (Fig. 6C). However, treating 5310 and 4910 non-GICs and GICs with Pak-1 inhibitor (IPA3) induced the co-immunoprecipitation of p-JNK with MEKK-1 while the control cells displayed no interaction between these molecules (Fig. 6D). This indicates that pUC treatment inhibits the function of p-Pak-1 and thereby induces the formation MEKK-1–p-JNK complex formation and the translocation of this complex into the nucleus of the pUC-treated cells.
Figure 6.
uPAR and cathepsin B regulated the translocation of p-JNK through Ras signaling. A) Cell lysates from 5310 and 4910 xenograft cells treated with SV, pUC, 10 Gy, FLU and FLC were isolated. The cell lysates were used for SDS-PAGE and immunoblotted for Pak-1, p-Pak-1, MEKK-1, JNK and p-JNK. GAPDH served as a loading control. B) Cell lysates of 5310 and 4910 non-GICs and GICs treated with SV, pUC, 10 Gy, FLU and FLC were used for immunoprecipitation analysis with p-JNK antibody and then western blotted for MEKK-1. IgG served as a loading control. C) 5310 and 4910 glioma cells were treated with DMSO and Pak-1 inhibitor (10 μM IPA3), and their cell lysates were extracted. Their lysates were western blotted for Pak-1, p-Pak-1, MEKK-1, JNK and p-JNK. GAPDH served as a loading control. D) Cell lysates of the glioma xenograft cells treated with DMSO and IPA3 were immunoprecipitated with p-JNK antibody and then western blotted for MEKK-1.
Nuclear translocation of MEKK-1–p-JNK complex nullifies the effect of PI3K inhibitor
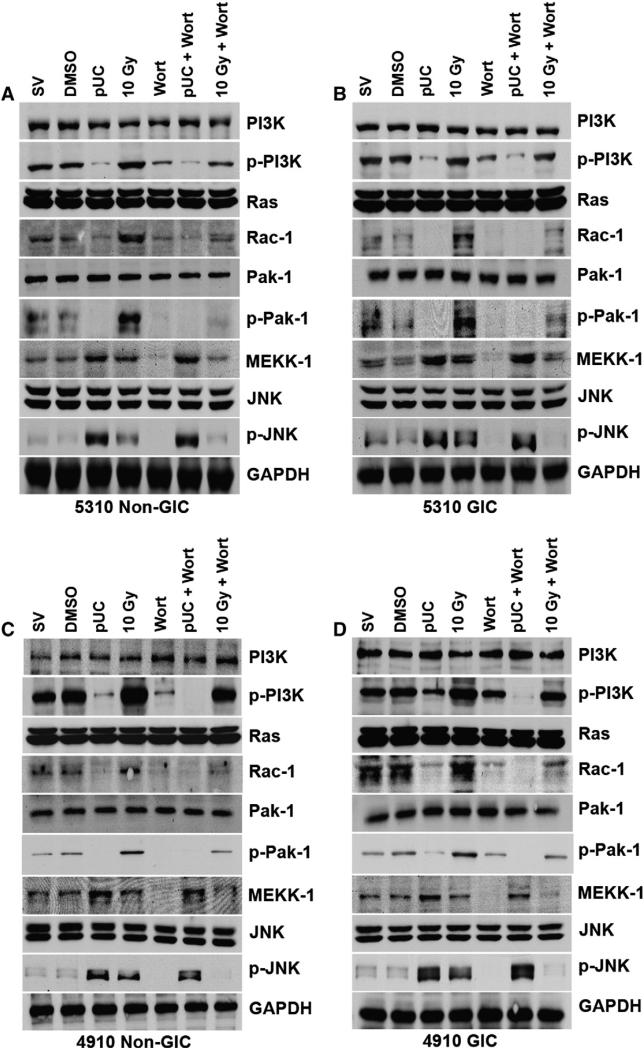
To further confirm the regulation of p-JNK by uPAR and cathepsin B through the Ras–Pak-1 pathway, we treated the glioma xenograft cells with PI3K inhibitor Wortmannin (Wort). 5310 and 4910 non-GICs and GICs treated with Wort and 10 Gy + Wort showed a decrease in the protein levels of p-PI3K, Rac-1, p-Pak-1, MEKK-1 and p-JNK when compared to that of the SV/DMSO treated cells and 10 Gy irradiated cells, respectively (Fig. 7). A decrease in the expression of p-PI3K, Rac-1 and p-Pak-1 was observed in pUC + Wort treated 5310 and 4910 cells while there was no effect on the expression of p-JNK and MEKK-1 when compared to pUC-treated cells.
Figure 7.
Wortmannin was ineffective on pUC-induced expression of p-JNK and MEKK-1. A) 5310 non-GIC, B) 5310 GIC, C) 4910 non-GIC, and D) 4910 GIC cells were treated with pUC, 10 Gy, and PI3K inhibitor (10 μM Wortmannin represented as Wort) alone or in combination. Cell lysates were isolated and western blotted for PI3K, p-PI3K, Ras, Rac-1, Pak-1, p-Pak-1, MEKK-1, JNK and p-JNK. GAPDH served as a loading control.
Discussion
Malignant gliomas are extremely lethal and have a 5-year survival rate of less than 3%. Despite aggressive clinical treatment including surgical resection, radiation and chemo-therapy, tumor recurrence is essentially universal (Lathia et al., 2011; Stupp et al., 2005). Failure of these regimens might be attributed to the highly infiltrative nature of the glioma cells that reside in the normal brain at distant locations from the origin of the tumor. Also, the existence of highly resistant, self-replicating glioma-initiating cells (GICs) decreases the success of existing treatment strategies. Approaches that target the invasive capacity of glioma cells as well as the proliferative nature of GICs may significantly improve therapeutic outcomes.
We have previously demonstrated the isolation and characterization of GICs from established cell lines (Alapati et al., 2012; Malla et al., 2012a); we used 5310 and 4910 GICs for the present study. Radiotherapy is a key treatment modality for treating patients with intracranial tumors, but its efficacy is limited by radioresistance and by the promotion of malignant behavior of the cancer cells (Kang et al., 2012; Kil et al., 2012). In our present study, radiation treatment increased the migration of the glioma cells as well as the expression of uPAR and cathepsin B. Elevated levels of uPAR and cathepsin B have been strongly correlated with tumor invasiveness (Besch et al., 2007; Levicar et al., 2003; Rao, 2003; Sevenich et al., 2011), indicating that the cells treated with radiation were adapting towards an aggressive invasive phenotype.
The three core protein kinases of the MAPK family are capable of responding to a number of stimuli to produce specific cellular outcomes. In particular, the precise nature of the extracellular stimuli and the repertoire of molecules available in each cell type can determine the localization, timing, intensity and duration of the activation of each member of the MAPK family (Raman et al., 2007; Turjanski et al., 2007). Several reports indicate the involvement of MAPKs in gene expression, proliferation, motility, metabolism and apoptosis (Cuevas et al., 2007; Dhillon et al., 2007; Huang et al., 2004b; Qi and Elion, 2005). Here, we studied the regulation of glioma cell migration by uPAR and cathepsin B via the MAPK pathway.
In our study, ERK and JNK inhibitors effectively inhibited the migration of glioma cells while the effect of the p38 inhibitor was not as significant. Hence, we further evaluated the effect of downregulation of uPAR and cathepsin B on the expression of ERK and JNK. Reduction in the expression levels of p-ERK with pUC and pUC + 10 Gy and the inhibition of migration of the cells with ERK inhibitor indicated that the activation of ERK is necessary for cell migration (Lind et al., 2006; Nguyen et al., 1999). shRNA treatment and the inhibitor treatment with respect to the activation of JNK and migration of glioma cells seemed to be contradictory. These phenomena prompted us to further concentrate on the involvement of JNK in the migration of glioma cells.
Based on the stimuli, compartment-specific localization and activation of JNK have been demonstrated in several earlier reports (Bjorkblom et al., 2008; Coffey et al., 2000). In our study, pUC treatment alone and in combination with radiation induced the activation and translocation of JNK into the nucleus of non-GICs and GICs. Even though a minor pool of nuclear activity existed in the irradiated cells, the majority of p-JNK remained in their cytoplasmic compartments. This means that the pUC-induced translocation of p-JNK into the nucleus might impair its ability to influence the migration of cells. Overexpressing uPAR and cathepsin B by transfecting the cells with FLU and FLC induced an increase in the accumulation of p-JNK in the cytoplasm of the cells. Overexpression of uPAR and cathepsin B did not show any effect on the nuclear pools of p-JNK. Also an increase in the migration of the glioma non-GICs and GICs was observed after FLU and FLC treatments. Taken together, these results further provide evidence that uPAR and cathepsin B-mediated activation of cytoplasmic JNK is required for the migration of the cells whereas the nuclear pool of active JNK cannot impact migration. Previously, it was reported that the activation of cytoplasmic pool of JNK was required for the migration of NRK cells (Rosse et al., 2009) and dendritic cells (Bjorkblom et al., 2005).
The dynamic assembly and disassembly of focal adhesions play central roles in cell migration. Adapter proteins such as Paxillin, Vinculin, α-Actinin and Talin are very important for the formation of these focal adhesion complexes at the leading edge of the migrating cell (Huttenlocher and Horwitz, 2011; Vicente-Manzanares et al., 2009). In our present study, the non-GICs and GICs treated with pUC and pUC + 10 Gy showed a significant decrease in the expression of the adhesion machinery molecules p-Paxillin, Vinculin, α-Actinin, Talin, Integrin αvβ3 and Integrin β1. Along with various well-known transcription factors and apoptosis-related proteins that are substrates for JNK, several cytoskeleton-associated proteins and signaling molecules as well as adaptor proteins have recently been identified (Bogoyevitch and Kobe, 2006; Huang et al., 2004b). Radiation, FLU and FLC treatments increased the expression of the above mentioned adhesion molecules, which were inhibited by treating the cells with the JNK inhibitor. This result further confirms that uPAR and cathepsin B regulate the migration and adhesion of glioma cells through the activation of cytoplasmic JNK.
Localized activation of JNK at the leading edge of migrating NRK cells (Rosse et al., 2009) and the localization of JNK to the actin dense membrane ruffles of the migrating fibroblast cells (Amagasaki et al., 2006) were observed earlier. In our present study, p-JNK interacted with p-Paxillin at the leading edge of the migrating glioma cells. Along with p-Paxillin, p-JNK directly interacted with Vinculin as well as α-Actinin. The direct interaction between these molecules was inhibited by treating the cells with pUC alone or in combination with radiation. This further provides evidence for uPAR and cathepsin B-mediated regulation of p-JNK and its interaction with the focal adhesion molecules required for glioma cell migration. Inhibition of co-localization of p-JNK and p-Paxillin was also evident in the in vivo sections treated with pUC alone or in combination with radiation and led to the regression of tumor growth.
Ras–MAPK signaling is often deregulated and is constitutively active in many types of cancers including pancreatic, colon, lung, melanoma and breast (Dunn et al., 2005). Activation of PI3K, Rac-1 and JNK was necessary for bFGF-induced fibroblast migration and blocking the activation of PI3K, Rac-1 or JNK has been shown to significantly downregulate the wound healing capacity of these cells (Kanazawa et al., 2010). PI3K–Rac-1– JNK signaling was for collagen I-induced fibroblastic transformation and scattering of NMuMG mammary epithelial cells (Shintani et al., 2006). Further, the cytoplasmic accumulation of JNK activated by constitutively active Rac-1 has been demonstrated in intestinal epithelial cells (Stappenbeck and Gordon, 2001). Taken together, these reports suggest the involvement of Ras–PI3K signaling in regulating the activation of JNK. In our present study, the upstream signal required for the activation and accumulation of cytoplasmic JNK appears to involve Ras–PI3K–Rac-1–Pak-1 pathway as evidenced by the upregulation of these molecules in the cells treated with radiation. Further, cells treated with pUC and pUC + 10 Gy showed a decrease in the expression of these molecules, indicating that uPAR and cathepsin B regulate the Ras–Pak-1 pathway. Inhibition of Ras–Pak-1 pathway by pUC treatment, alone or in combination with radiation, led to an increase in the expression of MEKK-1.
MEKK-1 interacted with p-JNK in the cells treated with pUC and to some extent in the cells treated with 10 Gy. In contrast, there was no interaction between these molecules in the cells treated with SV, FLU or FLC. We have previously reported that apoptosis was induced in glioma cells treated with pUC (Malla et al., 2010, 2012b) and to some extent in glioma cells treated with radiation (Malla et al., 2012a). Hence, it is possible that these molecules are interacting only in the cells that are undergoing apoptosis. An increase in the interaction between MEKK-1 and p-JNK was also observed in the cells treated with the Pak-1 inhibitor (IPA3). Further, we observed that the expression of MEKK-1 and p-JNK remained unaltered with the Pak-1 inhibitor. As per the above findings, it can be considered that Pak-1 negatively regulates the binding of MEKK-1 with p-JNK. A similar kind of Pak-1-mediated negative regulation of MEKK-1-dependent JNK pathway was observed in 293 human embryonic kidney cells (Gallagher et al., 2002). Pak-1 constitutively phosphorylates MEKK-1 on serine 67 in resting 293 cells, but its dephosphorylation following exposure to anisomycin allows the binding of JNK to MEKK-1.
To further confirm that uPAR and cathepsin B were regulating the activation of JNK through the Ras–Pak-1 pathway, cells were treated with Wortmannin, a PI3K inhibitor. It is noteworthy that Wortmannin did not show any effect on the expression of MEKK-1 and p-JNK in pUC-treated cells while a decrease in the expression of these molecules was observed in the non-irradiated and irradiated control cells treated with Wortmannin. This might be due to the translocation of the MEKK-1–p-JNK complex into the nucleus of pUC-treated non-GICs and GICs. These results further confirm that the translocation of activated JNK is regulated through the Ras– PI3K pathway.
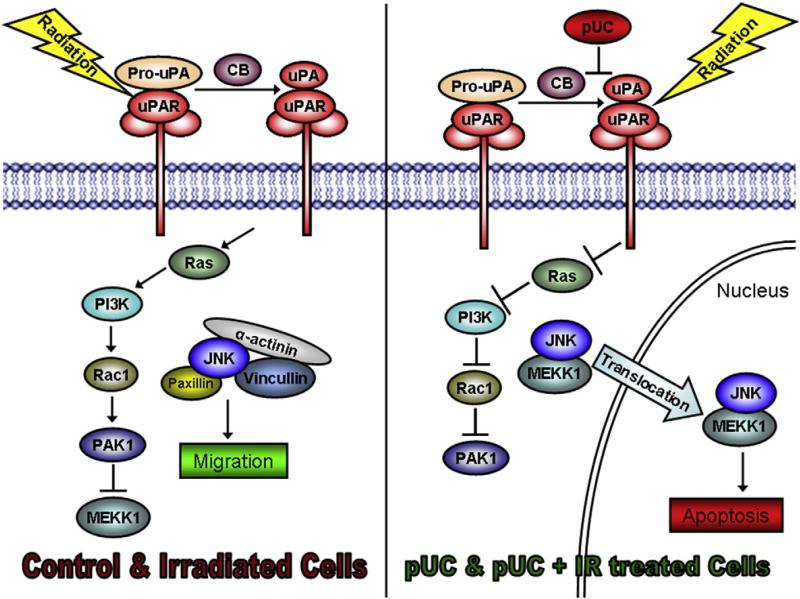
In conclusion, uPAR and cathepsin B mediate the migration of glioma cells by increasing the localization of cytoplasmic JNK at the focal complexes of the leading edge of glioma cells as depicted in Fig. 8. uPAR and cathepsin B regulate the Ras–Pak-1 pathway by controlling the activation and translocation of JNK. shRNA treatment against uPAR and cathepsin B inhibits the Ras–Pak-1 pathway, thereby inducing the activation and interaction of MEKK-1 with p-JNK. The MEKK-1 and p-JNK complex further translocates into the nucleus, reducing the availability of the cytoplasmic pool of JNK required for the migration of the glioma cells. Taken together, it can be concluded that the cytosolic activity of JNK induces the migration of cells and radiation further enhances this phenomenon, thereby driving these glioma cells towards a more malignant and resistant phenotype. pUC treatment induces nuclear translocation of p-JNK and thus reduces the migration of 5310 and 4910 non-GICs and GICs. Finally, it can be concluded that the regulation of JNK–MAPK through the simultaneous suppression of uPAR and cathepsin B proves to be a potential therapeutic target for inhibiting the migration of glioma cells.
Figure 8.
uPAR and Cathepsin B mediated migration of glioma cells.
Supplementary Material
Acknowledgments
The authors wish to thank Debbie McCollum for manuscript preparation and Diana Meister and Sushma Jasti for manuscript review.
Footnotes
Funding: This research was supported by award number CA116708 (to JSR) from the National Cancer Institute. Contents are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scr.2014.02.008.
References
- Ahmed N, Oliva K, Wang Y, Quinn M, Rice G. Downregulation of urokinase plasminogen activator receptor expression inhibits Erk signalling with concomitant suppression of invasiveness due to loss of uPAR-beta1 integrin complex in colon cancer cells. Br. J. Cancer. 2003;89:374–384. doi: 10.1038/sj.bjc.6601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapati K, Gopinath S, Malla RR, Dasari VR, Rao JS. uPAR and cathepsin B knockdown inhibits radiation-induced PKC integrated integrin signaling to the cytoskeleton of gliomainitiating cells. Int. J. Oncol. 2012;41:599–610. doi: 10.3892/ijo.2012.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagasaki K, Kaneto H, Heldin CH, Lennartsson J. c-Jun N-terminal kinase is necessary for platelet-derived growth factor-mediated chemotaxis in primary fibroblasts. J. Biol. Chem. 2006;281:22173–22179. doi: 10.1074/jbc.M513307200. [DOI] [PubMed] [Google Scholar]
- Besch R, Berking C, Kammerbauer C, Degitz K. Inhibition of urokinase-type plasminogen activator receptor induces apoptosis in melanoma cells by activation of p53. Cell Death Differ. 2007;14:818–829. doi: 10.1038/sj.cdd.4402065. [DOI] [PubMed] [Google Scholar]
- Bjorkblom B, Ostman N, Hongisto V, Komarovski V, Filen JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 2005;25:6350–6361. doi: 10.1523/JNEUROSCI.1517-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET. All JNKs can kill, but nuclear localization is critical for neuronal death. J. Biol. Chem. 2008;283:19704–19713. doi: 10.1074/jbc.M707744200. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan ZK, Liu K, Huang S. Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression. Cancer Res. 2009;69:9228–9235. doi: 10.1158/0008-5472.CAN-09-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J. Neurosci. 2000;20:7602–7613. doi: 10.1523/JNEUROSCI.20-20-07602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras–MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Cancer, Murphy G. Proteases–invasion and more. Nature. 1998;394:527–528. doi: 10.1038/28961. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Gallagher ED, Xu S, Moomaw C, Slaughter CA, Cobb MH. Binding of JNK/SAPK to MEKK1 is regulated by phosphorylation. J. Biol. Chem. 2002;277:45785–45792. doi: 10.1074/jbc.M207702200. [DOI] [PubMed] [Google Scholar]
- Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J. Cell. Biochem. 2009;108:1031–1038. doi: 10.1002/jcb.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossage L, Eisen T. Targeting multiple kinase pathways: a change in paradigm. Clin. Cancer Res. 2010;16:1973–1978. doi: 10.1158/1078-0432.CCR-09-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011 May;18(5):829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. A role for JNK–paxillin signaling in cell migration. Cell Cycle. 2004a;3:4–6. [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J. Cell Sci. 2004b;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Huang Z, Yan DP, Ge BX. JNK regulates cell migration through promotion of tyrosine phosphorylation of paxillin. Cell. Signal. 2008;20:2002–2012. doi: 10.1016/j.cellsig.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Takahashi H, Harada H, Kohno S, Ohue S, Kobayashi K, Yano H, Tanaka J, Ohnishi T. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int. J. Oncol. 2010;37:1121–1131. doi: 10.3892/ijo_00000764. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M, Miyata S, Tohyama M, Sakai Y, Yano K, Hosokawa K, et al. bFGF regulates PI3-kinase–Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS One. 2010;5:e12228. doi: 10.1371/journal.pone.0012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KB, Zhu C, Wong YL, Gao Q, Ty A, Wong MC. Gefitinib radiosensitizes stem-like glioma cells: inhibition of epidermal growth factor receptor-Akt–DNA-PK signaling, accompanied by inhibition of DNA double-strand break repair. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e43–e52. doi: 10.1016/j.ijrobp.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Kil WJ, Tofilon PJ, Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat. Oncol. 2012;7:25–27. doi: 10.1186/1748-717X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011;2:e200. doi: 10.1038/cddis.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, Osborne J, McGlynn K, Stippec S, Earnest S, et al. The roles of MAPKs in disease. Cell Res. 2008;18:436–442. doi: 10.1038/cr.2008.37. [DOI] [PubMed] [Google Scholar]
- Levicar N, Nuttall RK, Lah TT. Proteases in brain tumour progression. Acta Neurochir. (Wien) 2003;145:825–838. doi: 10.1007/s00701-003-0097-z. [DOI] [PubMed] [Google Scholar]
- Lind CR, Gray CW, Pearson AG, Cameron RE, O'Carroll SJ, Narayan PJ, Lim J, Dragunow M. The mitogen-activated/extracellular signal-regulated kinase kinase 1/2 inhibitor U0126 induces glial fibrillary acidic protein expression and reduces the proliferation and migration of C6 glioma cells. Neuroscience. 2006;141:1925–1933. doi: 10.1016/j.neuroscience.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Malla RR, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, Mohanam S, Rao JS. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One. 2010;5:e13731. doi: 10.1371/journal.pone.0013731. (PMCID: PMC2966405) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Malla RR, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS. uPAR and cathepsin B inhibition enhanced radiation-induced apoptosis in glioma initiating cells. Neuro Oncol. 2012a;14:745–760. doi: 10.1093/neuonc/nos088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla RR, Gopinath S, Gondi CS, Alapati K, Dinh DH, Tsung AJ, Rao JS. uPAR and cathepsin B downregulation induces apoptosis by targeting calcineurin A to BAD via Bcl-2 in glioma. J. Neuro Oncol. 2012b;107:69–80. doi: 10.1007/s11060-011-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matarrese P, Ascione B, Ciarlo L, Vona R, Leonetti C, Scarsella M, Mileo AM, Catricala C, Paggi MG, Malorni W. Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study. Mol. Cancer. 2010;9:207. doi: 10.1186/1476-4598-9-207. http://dx.doi.org/10.1186/1476-4598-9-207:207-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multi-functional enzymes in cancer. Nat. Rev. Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010;17:599–613. doi: 10.1038/cgt.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J. Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Qi M, Elion EA. MAP kinase pathways. J. Cell Sci. 2005;118:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- Rabbani SA, Ateeq B, Arakelian A, Valentino ML, Shaw DE, Dauffenbach LM, Kerfoot CA, Mazar AP. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12:778–788. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev. Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 2009;7:e1000235. doi: 10.1371/journal.pbio.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, Muller S, Follo M, Peters C, Reinheckel T. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene. 2011;30:54–64. doi: 10.1038/onc.2010.387. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Wheelock MJ, Johnson KR. Phosphoinositide-3 kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol. Biol. Cell. 2006;17:2963–2975. doi: 10.1091/mbc.E05-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Gordon JI. Extranuclear sequestration of phospho-Jun N-terminal kinase and distorted villi produced by activated Rac1 in the intestinal epithelium of chimeric mice. Development. 2001;128:2603–2614. doi: 10.1242/dev.128.13.2603. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Veeravalli KK, Chetty C, Ponnala S, Gondi CS, Lakka SS, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. MMP-9, uPAR and cathepsin B silencing downregulate integrins in human glioma xenograft cells in vitro and in vivo in nude mice. PLoS One. 2010;5:e11583. doi: 10.1371/journal.pone.0011583. (PMCID: PMC2904700) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—the actin connection. J. Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor BC, Anbalagan A, Mohamed MM, Sloane BF, Cavallo-Medved D. Inhibition of cathepsin B activity attenuates extracellular matrix degradation and inflammatory breast cancer invasion. Breast Cancer Res. 2011;13:R115. doi: 10.1186/bcr3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kuiatse I, Lee AV, Pan J, Giuliano A, Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelialmesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol. Cancer Res. 2010;8:266–277. doi: 10.1158/1541-7786.MCR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Jiborn T, Abrahamson M, Helczynski L, Otterbein L, Persson JL, Bjartell A. Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways. PLoS One. 2009;4:e7953. doi: 10.1371/journal.pone.0007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WS, Wu JR, Hu CT. Signal cross talks for sustained MAPK activation and cell migration: the potential role of reactive oxygen species. Cancer Metastasis Rev. 2008;27:303–314. doi: 10.1007/s10555-008-9112-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.