Abstract
Recent advances in cancer immunotherapy suggest that manipulation of the immune system to enhance the anti-tumor response may be a highly effective treatment modality. One understudied aspect of immune surveillance is anti-angiogenic surveillance, the regulation of tumor angiogenesis by the immune system, independent of tumor cell lysis. CD4+ T cells can negatively regulate angiogenesis by secreting anti-angiogenic factors such as thrombospondin-1 (TSP-1). In tumor-bearing mice, we show that a Th1-directed viral infection that triggers upregulation of TSP-1 in CD4+ and CD8+ T cells can inhibit tumor angiogenesis and suppress tumor growth. Using bone marrow chimeras and adoptive T cell transfers, we demonstrated that TSP-1 expression in the T cell compartment was necessary and sufficient to inhibit tumor growth by suppressing tumor angiogenesis after the viral infection. Our results establish that tumorigenesis can be stanched by anti-angiogenic surveillance triggered by an acute viral infection, suggesting novel immunological approaches to achieve anti-angiogenic therapy.
Keywords: T-cells, Thrombospondin-1, Angiogenesis, Immune surveillance, Acute infection
Introduction
Recent clinical trials manipulating the immune system suggest that the latest iterations of immunotherapy may be highly efficacious for cancer treatment (1–3). Following successful manipulation of the immune system to achieve direct tumor cell lysis, other groups have targeted the tumor vasculature. One approach uses engineered T cells with chimeric antigen receptors (CARs) targeting antigens specifically expressed by tumor endothelial cells. (4, 5). Additionally, vaccines targeting tumor endothelium have been utilized in mouse models with moderate success (6–8). Immunotherapies targeting the tumor vasculature enhance the immune system’s ability to block tumor angiogenesis, referred to here as ‘anti-angiogenic surveillance’.
Anti-angiogenic surveillance is the negative regulation of the endothelium by the immune system during tumor growth. Lymphocyte subsets produce specific angiogenic regulators. For example, VEGF is highly expressed by T regulatory cells, while the anti-angiogenic protein IFN-γ is secreted by CD8+, CD4+, and NK cells (9, 10). Thus, T cell-secreted VEGF and IFN-γ play opposing roles regulating tumor angiogenesis and tumor growth (9, 11, 12). Anti-angiogenic surveillance has begun to be appreciated as a unique anti-tumor facet of traditional immune surveillance (12–14). However, the mechanisms by which the immune system regulates tumor vasculature are not understood. It is unclear whether the overall effect of an activated immune system is pro- or anti-angiogenic during tumor growth, and is likely to be context dependent.
Tumor cells pose a unique challenge for the immune system in that they require the recognition of subtle differences between normal and disease state. Cancer immunotherapy has focused on redirecting and enhancing the anti-tumor immune response through adoptively transferring ex vivo expanded cells or through vaccination strategies against tumor antigens. Different from tumor cell-directed approaches, there is evidence that acute activation of the immune system by a pathogen results in a bystander effect on tumor growth (15, 16). Studies using mouse models have revealed that acute infection with Toxoplasma gondii, listeria monocytogenes, and the Armstrong strain of lymphocytic choriomeningitis virus (LCMVa) have anti-tumor effects, whereas chronic infections such as Hepatitis C promote tumor growth, making the relationship between an active immune response and tumor growth unclear (16, 17). There have been several attempts to use controlled infection as a means of treating cancer, but have been met with limited success. Studies using acute infections investigated the mechanisms mediating the observed anti-tumor effect (15). Surprisingly, mice lacking key components of the cytolytic immune response, including inducible NO synthase (iNOS), perforin, tumor necrosis factor-alpha (TNF-α), and IL-12, still demonstrated a significant anti-tumor effect after acute infections that predominantly induced a Th1 directed immune response (16). Histological analysis of tumors isolated from infected mice revealed a significant suppression of tumor vascularization, presumably inhibiting expansion of tumor mass. The mechanism by which immune activation towards a non-tumor cell target could inhibit tumor angiogenesis remains unclear.
The endogenous angiogenesis inhibitor Thrombospondin-1 (TSP-1) is expressed by immune cells and could mediate anti-angiogenic surveillance (13). TSP-1 is a homotrimeric, extracellular matrix protein that is highly expressed in platelets, stromal cells and endothelial cells and is a potent negative regulator of endothelial cell proliferation and migration (18). TSP-1 expression negatively correlates with vascularity, tumor growth and disease progression in several cancer types (19–21). We have previously shown that TSP-1 is expressed by T cells and that splenocyte-derived TSP-1 is critical for tumor regression in an inducible Myc-regulated lymphoma model. Lymphomas relapsed in the absence of splenocyte-derived TSP-1, indicating the importance of TSP-1 in sustained tumor regression (13).
Here we show that T cell activation in response to a number of antigens including tumor antigen, bacterial toxin, virus and antibody treatment upregulates TSP-1 expression. We use the Armstrong strain of lymphocytic choriomeningitis virus (LCMVa), an acute Th1-inducing virus, to activate the immune system and upregulate TSP-1 expression. LCMVa infection causes a robust proliferation of T cells, particularly CD8+ cells, over an eight-day expansion phase followed by rapid contraction. It is easily cleared by healthy mice and produces no fever, lethargy, or loss of appetite that may confound effects on tumor growth. We demonstrate that in wild-type mice, LCMVa infection significantly suppresses tumor growth however this effect is lost is Tsp1−/− mice. Utilizing Tsp1−/− and Rag1−/− mice, we generated bone marrow chimeras and performed adoptive T cell transfers to confirm that TSP-1 in the T cell compartment is necessary and sufficient to suppress tumor growth after LCMVa infection by inhibiting tumor angiogenesis. Our studies demonstrate direct anti-angiogenic activity of T cells and identify TSP-1 as a link between acute infection, T cell activation, and anti-angiogenic surveillance, indicating that regulation of T cell expression of TSP-1 may be a novel approach to inhibit tumor angiogenesis.
Materials and Methods
Mice
C57Bl/6 mice, Rag1−/− mice were from The Jackson Laboratory. Balb/c SCID mice were from Massachusetts General Hospital animal breeding facility. Tsp1−/− mice were obtained from Dr. Jack Lawler (BIDMC/Harvard Medical School, described in (22)). Mice were males 5–8 weeks old. All animal experiments were performed according to protocols approved by the University of Pennsylvania IACUC.
In vitro stimulation of T cells and splenocytes
Spleens were filtered through 100uM filters in PBS, pelleted, and red blood cells lysed using ACK lysis buffer (Life Technologies). Splenocytes were plated at 2 ×106 cells per well in 24 well dishes in RPMI 1640-Glutamax containing 25 mM HEPES (Invitrogen), 10% fetal bovine serum and 0.05μM 2-mercaptoethanol. CD4+ or CD8+ cells were purified from spleens and lymph nodes by positive selection using microbeads and magnetic columns (Miltenyi Biotech) via manufacturer’s protocol. T cells were cultured in the same media and at the same numbers as splenocytes. Stimulated wells were pre-coated with 1 μg/mL anti-mouse CD3e antibody (BD Biosciences) in PBS and rinsed with PBS before plating cells in media containing 2 μg/mL anti-mouse CD28 antibody (eBioscience). 72 hours after plating, cells were lysed in RIPA buffer. For western blotting, membranes were incubated with TSP-1 antibody clones SPM-321 and A6.1 (1:500, Santa Cruz) or β-actin antibody (1:3000, Sigma) followed by HRP labeled secondary antibodies, and detected using ECL solution. Densitometry analysis was performed on minimally exposed blots using ImageJ software (http://rsbweb.nih.gov/ij/) to determine a TSP-1:actin ratio for each time point, and then this ratio for each time point compared to day 0 was calculated.
Tube formation assay
WT and Tsp1−/− T cells were isolated and cultured as described above for 72 hours ± CD3/CD28 antibody stimulation. Conditioned media was collected, centrifuged, and used immediately for endothelial cell tube formation assays. 12 well culture dishes were coated with a 1:1 mixture of ice cold RPMI:matrigel (BD Biosciences). Matrigel was hardened at 37°C for 45 minutes. Primary lung endothelial cells from WT mice were resuspended in the appropriate conditioned media at a final concentration of 1.25 × 105 cells per 2mL media then added on top of the matrigel and tube formation was documented over time. Assays were performed in 3 independent experiments with similar results.
Cell culture and tumor growth in mice
B16F10 cells were from ATCC (authenticated by STR fingerprinting, IDEXX RADIL at Univ. of Missourri, 2011). Cells were cultured in DMEM containing 10% FBS, 2mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. For injection, cells were resuspended at 3×106 cells/mL sterile PBS. Each mouse received 3×105 B16F10 cells intradermally on the left ventral side. Tumor growth was measured by caliper 3×/week. Mice were euthanized if moribund or tumors became ulcerated before the end of the experiment.
LCMVa
Mice were given 2 ×105 pfu of the Armstrong strain of Lymphocytic Choriomeningitis Virus (LCMVa) in 0.5 mL RPMI media, or RPMI alone control, by intraperitoneal injection. For tumor growth experiments, cells were allowed to form measurable tumors (≥ 35 mm3) or large tumors (> 150mm3), as indicated, before infecting with virus.
Microarray
Cell sorting and data analysis for mRNA microarray is described in (23). Briefly, LCMV-specific CD4+ or CD8+ T cells were sorted from LCMVa infected mice. RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA). RNA was processed and hybridized to Affymetrix GeneChip MoGene 1.0 microarrays (Santa Clara, CA) by the University of Pennsylvania Microarray facility. Affymetrix Power Tools were used to normalize fluorescent hybridization signals using Robust Multichip Averaging.
Bone Marrow Transplants and T cell transfers
Bone marrow (BM) was isolated from donor mice by flushing femurs with PBS. 1×106 BM cells were injected via tail vein into irradiated (9gy) recipient mice. Tumor cells were injected 5 weeks later.
For T cell transfer experiments, CD4+ and CD8+ T cells were isolated from the spleens of donor WT or Tsp1−/− mice using Pan T cell Isolation Kit II, CD4 positive selection, or CD8 positive selection kits (Miltenyi Biotech). 3×106 cells were transferred by tail vein injection to Rag1−/− recipient mice. Tumor cells were injected after 3 weeks.
Immunofluorescence and CD31 quantification
Tumors were harvested from mice, frozen in OCT (Tissue-Tek), or fixed in formalin, then sectioned for staining. Frozen slides were fixed and permeabilized in ice cold acetone for 10 minutes, blocked with 5% normal goat serum and then incubated O/N at 4° C with rat anti-mouse CD31 antibody (1:50, BD Biosciences) and rabbit anti-human/mouse NG2 antibody (1:500, Millipore). For TSP-1 and CD3 staining, sections were deparrafinized by xylene and EtOH, antigen retrieval was performed with Proteinase K at 50 degrees for 40 min, and sections were blocked with 10% mouse serum in PBS. Slides were then incubated O/N at room temp with anti-TSP-1 clones SPM-321 and A6.1 (1:500, Santa Cruz) and anti-CD3 (1:300, Dako). Secondary antibodies were Alexa 488 goat anti-rat, Alexa 594 goat anti-rabbit, Alexa 488 goat anti-rabbit, or Alexa 594 rat anti-mouse (1:1500, Invitrogen) and nuclei were identified with Hoechst33342 (1:1000, Invitrogen). Five random 10× magnification pictures were taken of each slide, the area of CD31+ structures, number of visible lumens, number of vessels, and number of vessels > 100 μm was counted, and an average for each tumor was found. Vessel structures for all tumors within a treatment group were averaged to determine average and standard deviation.
Flow cytometry
Flow cytometry was performed on cells from whole spleens or on tumors after digestion in DNAse/Collagenase for 40 min. at 37° C. Single cell suspensions were achieved by filtration through a 70 μm filter. Live/Dead Aqua Fixable Stain (Life Technologies, cat L34957) was used. Antibodies used: anti-CD4-APC (BioLegend cat 100412), anti-CD4-PE (Biolegend cat 100512), anti-CD8a-APCeFlour780 (eBioscience cat 47-0081-82), anti-IFNγ-PerCPCy5.5 (eBioscience, cat 45-7311-82), anti-IL4-APC (cat 17-7041-81). Debris, dead cells, and doublets were gated out before analysis. For IFN-γ, single cells were cultured at 2×106 cells per mL splenocyte media containing GP33 or GP61 LCMV peptides and Brefeldin A for 6 hours before staining. Flow cytometry was performed on a BD FACS Canto II (BD Biosciences).
CD45+ or CD8+ T cell isolation from tumors
To isolate T cells from tumors for cytospin preparation or western blot, WT mice bearing B16F10 tumors were infected with LCMVa or RPMI control. 8 days post-infection, tumors were harvested and homogenized by mechanical mincing and incubation in Type II collagenase (Worthington) in HBSS for one hour at 37 degrees C with shaking. Tumor homogenates were then passed through a 100 μm strainer to obtain a single cell suspension, and CD8 or CD45 cells were isolated using anti-CD8 or anti-CD45 beads for positive selection in magnetic columns per manufacturer’s protocol (Miltenyi). For western blots, CD8+ cells were then incubated for 4 hours with Brefeldin-A in splenocyte medium before cells were lysed using RIPA. For cytospins, 150,000 CD45+ cells per slide were immediately added to slides. Slides were then fixed in 4% paraformaldehyde and immunofluorescent staining was performed with TSP-1 and CD3 antibodies as described above.
Results
Thrombospondin-1 expression is increased in activated T cells
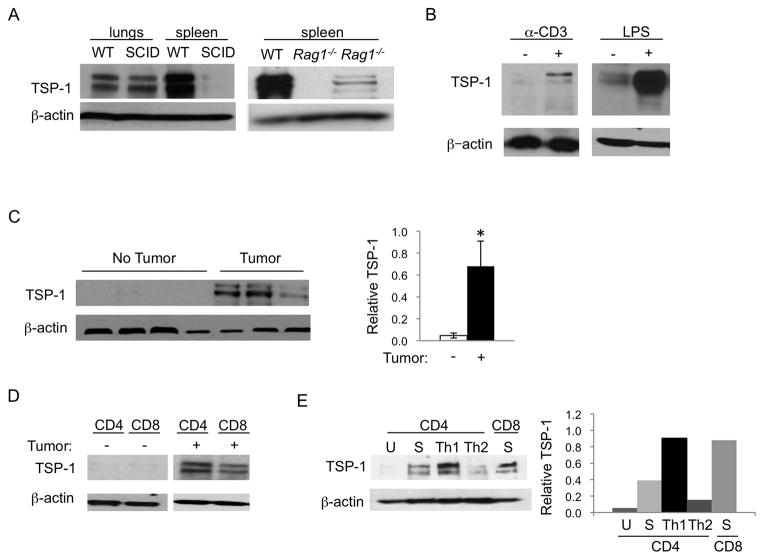
The growth of mouse tumor cells in immunocompromised SCID mice is accelerated in comparison to the growth of the same tumors in syngeneic immunocompetent wild-type (WT) mice (Fig. S1a). This difference has been attributed to the lack of immune surveillance in SCID mice due to the absence of T and B cells. Additionally, the spleen, a highly vascularized organ primarily comprised of lymphocytes, is only rarely the site of tumors despite its potential as a site for metastatic seeding. Of note, the highest expression of the endogenous angiogenesis inhibitor, thrombospondin-1 (TSP-1), outside of circulating platelets is in the spleen (Fig. 1a). While TSP-1 expression is similar between non-lymphoid organs in WT and immunocompromised mice, we found significantly decreased TSP-1 expression in the spleens of lymphocyte-deficient SCID and Rag1−/− mice as compared to WT mice, implicating lymphocytes as another source of TSP-1 (Fig. 1a). To understand the role of lymphocyte derived TSP-1, we examined TSP-1 expression after lymphocyte activation and found it to be dramatically upregulated after antibody stimulation of the TCR or by exposure to bacterial lipopolysaccharide (LPS) in vivo (Fig. 1b). We next examined TSP-1 expression upon immune system activation during tumorigenesis by isolating splenocytes, CD4+ and CD8+ T cells from tumor-bearing mice. TSP-1 expression was markedly upregulated as compared to non-tumor bearing controls (Fig. 1c, d). To narrow the relevant TSP-1 expressing lymphocyte population, we performed T cell skewing experiments. We found the highest TSP-1 expression in Th1-skewed CD4+ cells and TCR activated CD8+ lymphocytes (Fig. 1e).
Figure 1. Thrombospondin-1 protein expression is upregulated in T cells.
A) TSP-1 is expressed in spleens from WT but not immunocompromised mice. β-actin was probed as a loading control. B) TSP-1 is upregulated in lymphocytes activated by anti-CD3+anti-CD28 in vitro and in spleens after LPS treatment in vivo. C) TSP-1 is upregulated in the spleens of B16F10 tumor-bearing mice. Each lane is an individual mouse spleen, representative of multiple experiments. Quantification of TSP-1 expression relative to actin is on right, data are presented as mean+/− SEM, n=5 for no tumor and n=7 for tumor-bearing.*p<0.05. D) TSP-1 expression is upregulated in CD4+ or CD8+ T cells isolated from B16F10 tumor-bearing mice but not in non-tumor bearing mice. E) WT CD4+ T cells or CD8+ T cells were cultured in Th1 or Th2 skewing conditions and probed for TSP-1. U = unstimulated, media alone; S = stimulated with anti-CD3 and anti-CD28. Quantification of TSP-1 expression relative to actin is on right.
To investigate the consequence of lymphocyte-derived TSP-1 on tumor growth, we generated bone marrow chimeras whereby Tsp1−/− mice received either WT or Tsp1−/− BM. Lewis lung carcinoma tumor cells injected into the flanks of these mice grew significantly more rapidly in mice with Tsp1−/− BM compared to mice with WT BM, highlighting the tumor suppressive role of TSP-1 in BM-derived cells (Fig. S1b). However, TSP-1 in the immune compartment expressed at endogenous levels was not sufficient to effectively suppress tumor growth (Fig. S1b).
T cell derived TSP-1 inhibits tumor growth following LCMVa infection
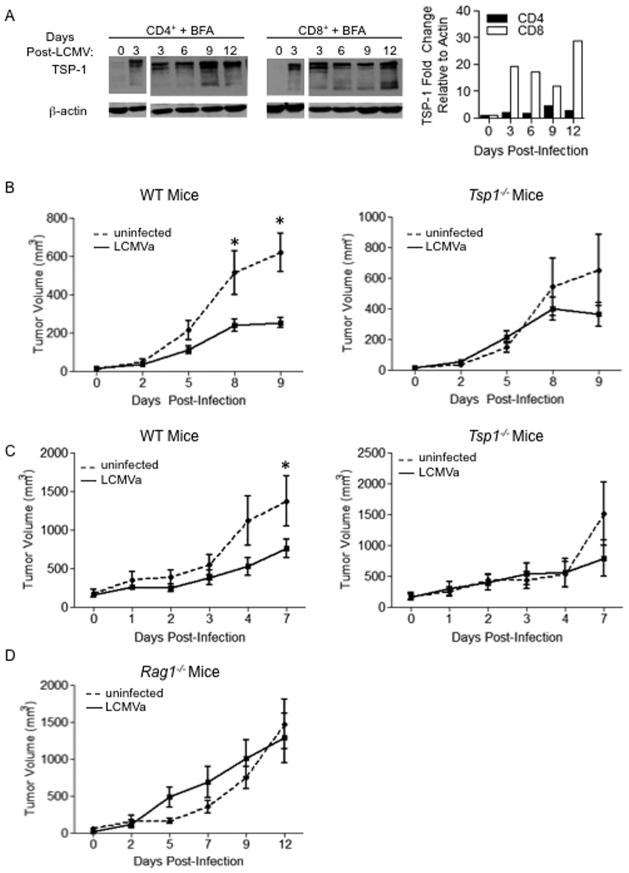
Since our in vitro studies indicate that Th1-skewed CD4+ and CD8+ cells produced significantly elevated levels of TSP-1, we utilized the Armstrong clone of lymphocytic choriomeningitis virus (LCMVa), an acute type I virus, to activate the immune system. Type I-inducing infections are known to inhibit tumor growth in a T cell-dependent manner (15). Interestingly, perforin and granzyme were dispensable for this inhibition, indicating that suppressing tumorigenesis was not due to tumor cell lysis (15, 16). Microarray analysis of LCMV-specific CD4+ or CD8+ cells collected from WT mice after LCMVa infection identified a spike in Tsp1 mRNA expression at days 6 and 8 post-LCMV infection in CD8+ and CD4+ T cells, respectively (Fig. S1c). Since Tsp1 is post-transcriptionally regulated and TSP-1 protein and RNA levels do not necessarily correlate, we examined TSP-1 protein expression in mice infected with LCMVa. We isolated lymphocytes and cultured them in the presence of brefeldin A to halt protein export, allowing us to examine total TSP-1 protein levels. We found an increase in TSP-1 protein expression at 9 and 12 days post-infection in CD4+ and CD8+ cells, respectively (Fig. 2a). LCMVa infection caused an approximately 100 kDa cleavage product of TSP-1 in T cells that was also present after LPS stimulation but was not seen during in vitro T cell stimulation. The identity of this 100kDa band as TSP-1 was confirmed by Western blot on Tsp1−/− splenocytes and mass spectrometry (Fig. S1d, e).
Figure 2. Infection induces TSP-1 expression in T cells and inhibits tumor growth.
A) LCMVa infection induces TSP-1 in CD4+ and CD8+ cells. WT mice were infected with LCMVa. On indicated days post-infection, T cells were isolated and cultured in the presence of Brefeldin-A (BFA) to inhibit protein secretion for 6 hours, then probed for TSP-1 expression. Each lane contains lymphocytes pooled from 3 mice. Graph shows densitometry analysis. Suppression of tumor growth after infection requires TSP-1. WT or Tsp1−/− mice were injected with B16F10 cells and infected with LCMVa or a media alone (RPMI) control B) when tumors were measurable or C) when tumors were > 150mm3. Graphs represent tumor volume. n=5 per group. D) Suppression of tumor growth requires adaptive immunity. Rag1−/− mice injected with B16F10 cells were infected with LCMVa or RPMI control when tumors were measurable. Graph represents tumor volume. n=5 per group.
To probe the functional significance of TSP-1 in T cells and to determine whether TSP-1 plays a role in abrogating tumor growth after immune stimulation, WT and Tsp1−/− mice were inoculated with B16F10 melanoma cells intradermally and when tumors were palpable, mice were infected with LCMVa to induce a T cell response. LCMVa infection significantly inhibited tumor growth in WT mice by day 8 (p = 0.02), but had no effect on tumorigenesis in Tsp1−/− mice (Fig. 2b). Importantly, immune activation by LCMVa infection also suppressed growth of well-established, highly vascularized B16F10 tumors in WT but not Tsp1−/− mice (Fig. 2c). To determine whether the tumor suppressive effects of infection persisted after the peak of the immune response to LCMVa, we infected B16F10 tumor-bearing WT or Tsp1−/− mice and followed tumor growth for 17 days post-infection (Fig S2a). Tumors in infected WT mice began to grow at 10 days post-infection, as LCMVa is cleared. In Tsp1−/− mice, tumor growth was not suppressed by LCMVa infection, even at day 14 post-infection. To confirm that the TSP-1 dependence of tumor suppression by infection was specific to lymphocytes and not dependent on innate immune populations such as macrophages and neutrophils, we used Rag1−/− mice with an intact innate immune system but no functional T or B cells. B16F10 tumor growth in Rag1−/− mice was similar in mice with or without LCMVa infection, implicating a specific role for T cell-derived TSP-1 in tumor suppression (Fig. 2d). Additionally, there was no difference in infiltration of F4/80+ macrophages or NIMP-R14+ neutrophils between uninfected or LCMVa infected tumors, regardless of Tsp1 status (Fig. S2b)
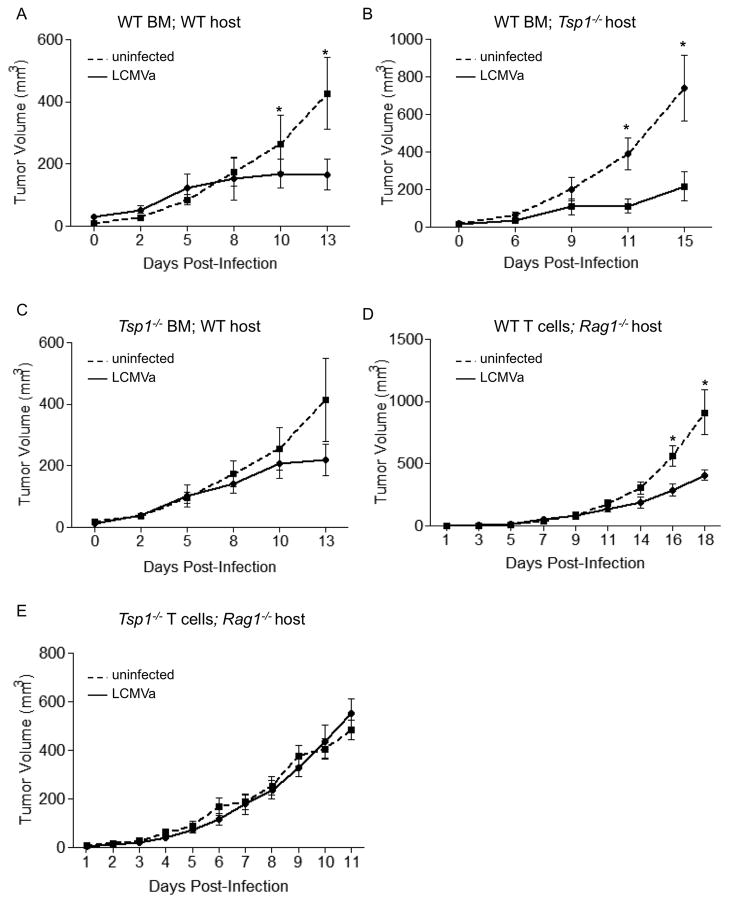
Endothelial and stromal cell-derived TSP-1 are thought to be the predominant source of TSP-1. To investigate whether the anti-tumor effect of TSP-1 was an immune-cell autonomous response or whether endothelial or stromal contribution of TSP-1 was necessary, we generated bone marrow (BM) chimeras with WT or Tsp1−/− mice receiving either syngeneic WT or Tsp1−/− BM followed by inoculation with B16F10 cells upon immune system reconstitution. Regardless of host status, in mice with WT BM, LCMVa infection significantly suppressed tumor growth (Fig. 3a, b). In contrast, tumor growth was unaffected after LCMVa infection in WT hosts with Tsp1−/− BM (Fig. 3c).
Figure 3. T cell-derived TSP-1 is necessary to suppress tumor growth by inhibition of angiogenesis after immune activation.
Host TSP-1 is dispensable for tumor inhibition. BM transplants were performed with A, B) WT or C) Tsp1−/− donors into Tsp1−/− or WT hosts, as indicated. After reconstitution, mice were injected with B16F10 cells then infected with either LCMVa or RPMI control when tumors were measurable. Graphs represent tumor volume. * p< 0.05. n ≥ 5 mice per group. D) WT or E) Tsp1−/− T cells were transferred into Rag1−/− mice. Mice were injected with B16F10 cells and infected with either LCMVa or RPMI control when tumors were measurable. Graphs indicate tumor volumes at indicated days after infection. * p< 0.05. n = 7 mice per group.
Lymphocytes isolated from either WT or Tsp1−/− mice were adoptively transferred into Rag1−/− hosts, and mice were inoculated with B16F10 melanoma cells. LCMVa infection suppressed tumor growth more than two-fold in mice with WT lymphocytes, but had no effect on tumor growth in Rag1−/− mice that received Tsp1−/− T cells (Fig. 3d, e). Adoptive transfer of either WT CD4+ or WT CD8+ T cells into Rag1−/− demonstrated that while CD4 cells partially inhibited tumor growth after infection, only CD8+ T cells significantly inhibit tumor growth after LCMVa infection (Fig. S3a).
LCMVa infection inhibits tumor angiogenesis in a TSP-1 dependent manner
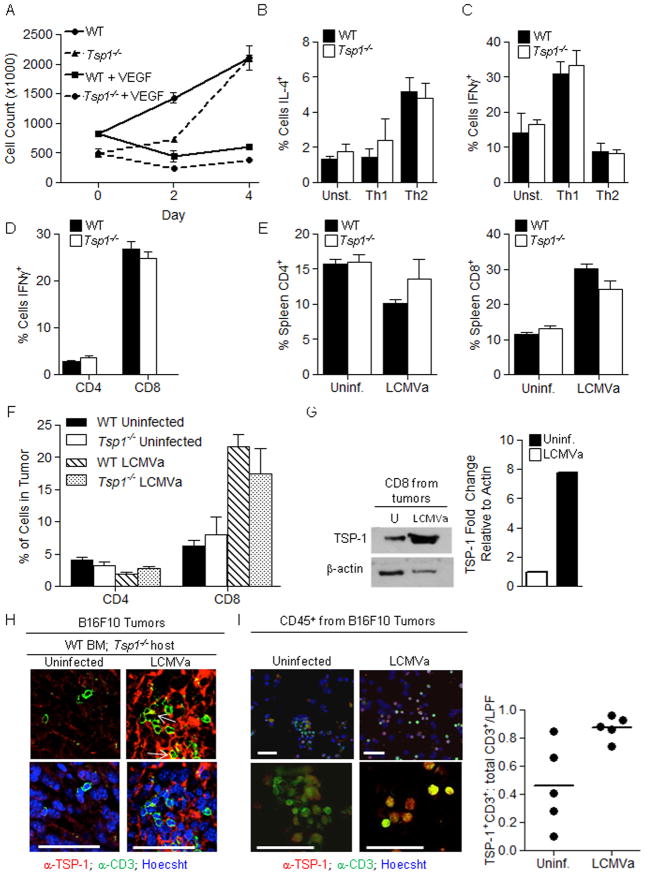
The upregulation of TSP-1 in activated lymphocytes implies a role for TSP-1 in immune cell function. Thus we examined both proliferation of Tsp1−/− T cells and inhibition by VEGF and found no differences in comparison to WT T cells (Fig. 4a). IL-4 and IFN-γ production were also unaffected by TSP-1 status (Fig. 4b, c). To ensure that TSP1 loss did not affect the anti-viral response to LCMVa, we examined the ratio of IFN-γ producing CD4+ and CD8+ cells and the proportions of CD4+ and CD8+ T cells in the spleens of naïve or LCMVa-infected WT or Tsp1−/− mice and found no differences (Fig. 4d, e). Both WT and Tsp1−/− mice clear LCMVa from the blood by 24 hours post-infection, indicating similar anti-viral responses (data not shown).
Figure 4. Tsp1−/− T cells are similar to Wild-type T cells and TSP-1 is upregulated in tumor infilitrating T cells after LCMVa infection.
A) Tsp1−/− splenocytes proliferate normally. Splenocytes were isolated from WT or Tsp1−/− mice and stimulated with anti-CD3, with or without VEGF. Tsp1−/− T cells produce cytokines at levels similar to WT. WT or Tsp1−/− T cells were skewed in vitro towards a Th1 or Th2 phenotype. The percent of cells producing B) IL-4 or C) IFN-γ were determined by flow cytometry. The number of CD4+ and CD8+ cells and percent of IFN-γ producing T cells after infection is similar in WT and Tsp1−/− mice. Splenocytes were harvested at 12 days post-LCMVa infection and restimulated in vitro with LCMV peptides GP33 or GP61. Flow cytometry for D) IFN-γ and E) CD4, CD8 was performed. n = 4 per group. F, G) B16F10 tumors from control or LCMVa infected mice were harvested: F) 10 days post-LCMVa infection, and quantified for CD4 and CD8 infiltration by flow cytometry. Differences between WT and Tsp1−/− groups were not significant. n ≥ 5 mice per group, or G) 8 days post-LCMVa infection, CD8+ cells were isolated by magnetic bead separation. CD8 cells were pooled from 4 tumors from uninfected mice and 3 tumors from LCMVa infected mice and immunoblotted for TSP-1. Graph shows densitometry quantification. H) Bone Marrow transplants were performed in which Tsp1−/− hosts received either WT or Tsp1−/− BM. After reconstitution, mice were injected with B16F10 tumors and infected with LCMVa or RPMI control. 13 days later, tumors were harvested and immunofluorescent staining was performed for TSP-1 (red) and CD3+ T cells (green). Arrows = co-localization. Bar = 50 μm. I) B16F10 tumor-bearing WT mice were infected with LCMVa or RPMI control. 8 days later, tumors were harvested, CD45+ cells isolated by magnetic bead separation, cytospin was performed, and samples were stained for TSP-1 (red) and CD3 (green). Bar = 50 μm. The percent of CD3+ cells that were TSP-1+ was determined by counting. Graph represents percentages for individual cytospins (n=5 or 6, p<0.05).
Since immune cell infiltration into tumors is a prerequisite for immune surveillance, we examined whether the lack of TSP-1 impacted T cell infiltration into tumors. Quantification of CD4+ and CD8+ cells in tumors harvested from WT or Tsp1−/− mice with or without LCMVa infection was assessed by flow cytometry and by immunofluorescent staining for CD3 (Fig. 4f and Fig. S3b). While there was no difference between the numbers or localization of WT and Tsp1−/− lymphocytes in tumors, the percent of CD8+ cells within the tumor increased more than two-fold after LCMVa infection in both WT and Tsp1−/− mice. TSP-1 expression was specifically and significantly upregulated in tumor infiltrating T cells in tumors harvested from LCMVa infected mice as indicated by co-immunofluorescence of TSP-1 and CD3 (Fig. 4h) and by Western blot analysis (Fig. 4g). CD8+ T cells were purified from tumors harvested from LCMVa infected and non-infected mice and lysates probed for TSP-1 expression, showing a dramatic increase in TSP-1 in CD8+ T cells from tumors of LCMVa infected mice (Fig. 4g). Cytospin preparations of CD45+ cells isolated from tumors harvested from LCMVa infected or non-infected mice and co-stained for TSP-1 and CD3 further indicated that TSP-1 is produced specifically by T cells within the tumor, and that the portion of TSP-1+ T cells increases 1.9-fold (p=0.01) in tumors from infected mice (Fig. 4i).
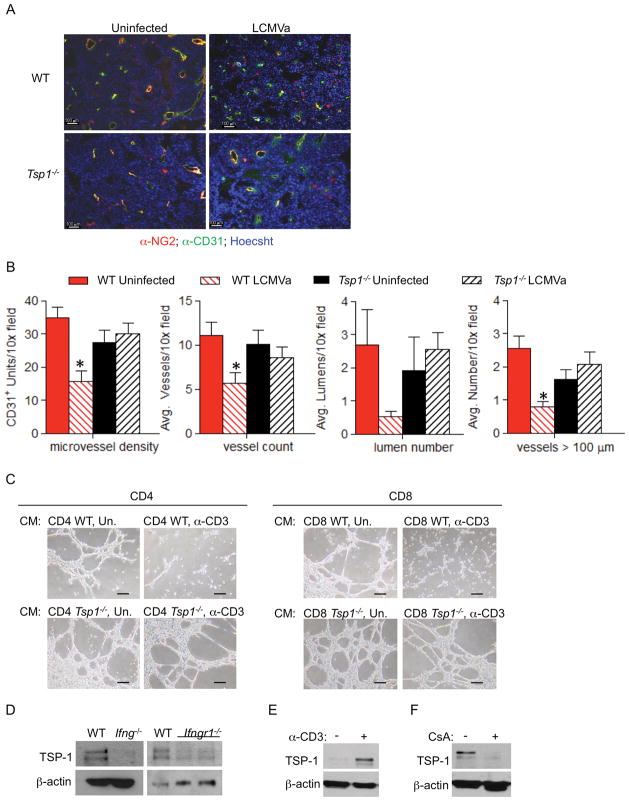
Similar to previous reports showing that acute infections suppress tumor angiogenesis, we find a significant decrease in the microvessel density of tumors harvested from WT mice infected by LCMVa as compared to tumors from uninfected mice. To more fully characterize tumor angiogenesis after LCMVa infection, tumor sections were analyzed for the average total vessel number, number of visible lumens, and number of vessels >100μm per field. All of these parameters of angiogenesis were significantly decreased in tumors from WT mice after LCMVa infection (Fig. 5a, b). Further, the number of NG2+ pericytes was decreased in tumors from LCMVa-infected WT mice indicating less stable vessels (Fig. 5a). In contrast, no difference in angiogenesis was observed in tumors harvested from Tsp1−/− mice, regardless of infection, implicating a requirement for TSP-1 to suppress tumor angiogenesis (Fig. 5a, b). Supporting a direct anti-angiogenic role for T cell-secreted TSP-1, conditioned media collected from in vitro stimulated WT but not Tsp1−/− CD4+ or CD8+ T cells dramatically inhibited endothelial cell tube formation on Matrigel (Fig. 5c).
Figure 5. T cell-derived TSP-1 inhibits tumor growth by suppressing tumor angiogenesis.
WT or Tsp1−/− mice were injected with B16F10 cells and infected with LCMVa or RPMI control. 9 days after infection, A) tumor vascularity was analyzed by immunofluoresence with the endothelial specific marker CD31 (green), the pericyte marker NG2 (red) and Hoechst (blue). Bar = 100 μM and B) Microvessel density, average number of vessels, average number of lumens, and average number of vessels >100 μm per 10× field was determined. *p<0.05; n =5 tumors per group. Average tumor volumes were 620.45mm3 for WT uninfected, 253.7mm3 for WT LCMVa, 652.5mm3 for Tsp1−/− uninfected, and 363.8mm3 for Tsp1−/− LCMVa. C) Representative images of endothelial tube formation on Matrigel in the presence of conditioned media collected from WT or Tsp1−/− CD4+ or CD8+ cells stimulated with anti-CD3 and anti-CD28 (stimulated: α-CD3, unstimulated:Un). Bar = 50 μM. D) TSP-1 expression is reduced in the absence of IFN-γ. Splenocytes were isolated from WT, Ifng−/−, or Ifngr−/− mice, stimulated in vitro with anti-CD3 and anti-CD28 and probed by Western blot for TSP-1. TSP-1 expression was probed by Western blot in T cells isolated from (E) p53−/− mice or (F) WT mice and stimulated in vitro with anti-CD3 and anti-CD28 followed by treatment with cyclosporin A (CsA) or vehicle.
Many cytokines also have anti-angiogenic effects. For example, IFN-γ suppresses tumor angiogenesis after acute infection by an unknown mechanism (14). Thus to test whether TSP-1 functions downstream of IFN-γ, we probed for TSP-1 expression in Ifnγ−/− or Ifnγr−/− splenocytes after in vitro stimulation and found that TSP-1 expression was diminished in the absence of either IFN-γ or IFNγR, even though these cells had similar growth rates as WT splenocytes (Fig. 5d). Regulation of TSP-1 expression is not fully understood, although it has been shown to be a direct transcriptional target of the tumor suppressor p53. Thus we examined TSP-1 upregulation in p53−/− CD4+ cells after TCR ligation and found that TSP-1 expression was still induced, obviating a need for p53 in TSP-1 upregulation in lymphocytes (Fig. 5e).
We have reported that calcineurin-NFAT signaling could be regulating TSP-1 expression in lymphocytes (13) due to the presence of NFAT consensus sequences in the Tsp1 promoter. Addition of the calcineurin inhibitor, cyclosporin A (CsA), to activated T cells attenuated TSP-1 upregulation confirming our previous study implicating TSP-1 regulation by the calcineurin-NFAT signaling pathway in lymphocytes (13) (Fig. 5f). Collectively, these data support an important role for TSP-1 in anti-angiogenic surveillance after immune cell activation and may be a critical component of tumor suppression by the immune system.
Discussion
Published studies have demonstrated that T cells, particularly the Th1 subset of CD4+ cells, have anti-angiogenic properties (16). However, the immune specific factors that function as angiogenesis inhibitors are not well studied. Here we show that TSP-1, a potent endogenous angiogenesis inhibitor, is expressed by CD4+ and CD8+ T lymphocytes and is upregulated after in vitro or in vivo immune cell activation and in particular after acute infection. Through the use of BM chimeras and adoptive T cell transfers, our data illustrates that CD8+-derived TSP-1 is necessary for LCMVa-mediated tumor suppression. Our work shows that decreased tumor angiogenesis observed in tumors of WT mice after LCMVa infection is due in part to T cell-derived TSP-1 directly suppressing endothelial cell activation. Collectively, our data demonstrate that anti-angiogenic surveillance is a consequence of immune system activation in response to acute infection and that T cell-derived TSP-1 plays a key role inhibiting tumor angiogenesis.
Regulation of angiogenesis by the immune system under physiologic circumstances, such as wound healing, has long been appreciated. The positive association between angiogenesis, inflammation, and immune suppression in pathologic disease states has also been well-studied with innate immune populations promoting tumor growth in part by promoting tumor angiogenesis. The polarization of these cells towards a pro-angiogenic, pro-tumor state appears to be enhanced by chronic viral infection and sustained inflammation. For example, the inflammatory state created by hepatitis B or C infection is estimated to account for 80% of hepatocellular carcinomas, and 70% of cervical cancers are attributed to human papilloma virus infection (17, 24). Chronic infection perpetuates the presence of T regulatory cells, which express high levels of pro-angiogenic VEGF (25). In contrast, our studies support a role for suppression of angiogenesis and tumor growth by acute infection, particularly by engagement of an adaptive Th1 CD4+ and CD8+ T cell response. Infection with LCMVa significantly inhibited tumor growth in WT but not Tsp1−/− mice, indicating a critical role for TSP-1 in infection-mediated tumor suppression.
Previous studies have demonstrated that infection with the agents T. gondii, listeria monocytogenes or LCMVa, but not the chronic strain of LCMV (clone 13), can suppress tumor growth (15, 16). Perforin, iNOS, TNF-α, IL-12 were unnecessary for tumor suppression demonstrating anti-tumor activity separate from cytolytic immune cell function. In these reports, decreased angiogenesis was observed in tumors from infected mice with limited mechanistic insights (15, 16). In our studies, we identify TSP-1 as a key factor for LCMVa-mediated blockade of tumor angiogenesis and subsequent tumor inhibition. Identification of TSP-1 as a key regulator of tumor suppression after acute infection provides a mechanism to explain this phenomenon. Transfer of WT but not Tsp1−/− CD8+ T cells alone suppressed tumor growth and confirmed that TSP-1 derived from CD8+ T cells is necessary for maximal tumor growth suppression. T cells rapidly expand after transfer, leading to a partially activated state. Transfer of WT but not Tsp1−/− T cells delayed tumor growth even in uninfected mice, further supporting a role for TSP-1 secreted by activated T cells in tumor growth suppression. While our data demonstrate that TSP-1 derived specifically from T cells is necessary for maximal tumor growth suppression, immunofluorescent staining of tumor sections and of CD45+ cells isolated directly from tumors indicated that several other cell types in addition to T cells secrete TSP-1. It is unclear why the TSP-1 produced by these other immune cells is not sufficient for tumor growth suppression. One possibility is that TSP-1 production by innate immune populations does not increase after infection, and thus a threshold of TSP-1 in the microenvironment that is needed for suppression of tumor angiogenesis is not reached without the contribution of increased T cell-derived TSP-1. Indeed, the total number of T cells in the tumor as well as the portion of TSP-1+ T cells increases after LCMVa infection.
The role of TSP-1 in the immune system is not well understood. TSP-1 has been suggested to be important for T cell motility and an important negative feedback regulator of T cell proliferation and cytokine production during Th1 skewed immune responses (26–29). TSP-1 also activates the immunomodulatory protein TGF-β, suggesting that TSP-1 is likely to have pleiotropic effects on immune cells (30). Despite this, we confirmed that TSP-1 did not affect the immune response to LCMVa with regards to lymphocyte expansion or cytokine production. WT and Tsp1−/− LCMVa infected mice had comparable T cell numbers in the tumor bed but tumor growth was not inhibited in Tsp1−/− mice, suggesting that T cell infiltration in this tumor model may be more important for anti-angiogenesis rather than tumor cell lysis. While we demonstrated equal numbers of T cells, macrophages, and neutrophils in tumors regardless of Tsp1 status, it is possible that changes in the microenvironment created by decreased angiogenesis cause secondary effects in tumor-infiltrating immune cells. For example, hypoxia was recently demonstrated to suppress Treg differentiation, which could shift the balance of intratumoral T cell activity towards tumor cell lysis (31). Additionally, hypoxia regulates polarization of both macrophages and neutrophils, and can promote a pro-tumor phenotype in innate immune cells (32, 33). The relationship between angiogenic suppression by T cell-derived TSP-1, the tumor microenvironment, and secondary skewing of infiltrating immune cells is complex and merits further study.
One cytokine that is hypoxia responsive and has been previously identified as important for infection-induced suppression of tumor growth is IFN-γ. In prior studies using T. gondii infection, circulating IFN-γ levels inversely correlated with tumor growth. Additionally, tumors grown in Ifnγ−/− mice were more vascular than those in WT mice, suggesting that IFN-γ inhibits angiogenesis after infection. Our data demonstrate that TSP-1 expression is significantly abrogated in splenocytes from ifnγ−/− or ifnγr−/− mice after TCR ligation in vitro, while Tsp1−/− T cells produce similar levels of IFN-γ as WT lymphocytes after LCMVa infection. This suggests that TSP-1 may be an effector of IFN-γ on endothelial cells. Our data also indicate that TSP-1 expression may be regulated by calcineurin-NFAT signaling. NFAT is a well known regulator of cytokines in T cells, and our finding that NFAT also regulates an anti-angiogenic protein in the context of immune activation lends insight into the development of novel immunotherapies which commandeer angiogenic regulatory functions by the immune system.
In addition to traditional approaches to immunotherapy for the treatment of cancer, this work suggests that immune activation to suppress angiogenesis warrants further study. While anti-angiogenic therapies have had moderate clinical success, the concept of inhibition of tumor growth by preventing the formation of blood vessels is still viable. In clinical trials using a TSP-1 peptidomimetic had limited efficacy, due in part to the instability of the peptide in the blood and the decreased functionality of a TSP-1 peptide versus full-length, appropriately modified TSP-1 (34, 35). In our studies, since TSP-1 is endogenously produced by T cells, it is appropriately modified and processed circumventing the shortcomings of TSP-1 peptides. Activating a host response to inhibit tumor angiogenesis via the immune system represents an attractive therapeutic mechanism that would bypass the obstacles of delivering recombinant anti-angiogenic proteins in vivo. Our demonstration of TSP-1 induction in response to multiple immune stimuli suggests that TSP-1 may be a key modulator of angiogenesis by T cells in physiologic and pathologic situations. Identification of TSP-1 as the mechanism by which T cells negatively regulate tumor angiogenesis may lead to therapies co-opting a patient’s immune system for effective anti-angiogenic therapy during cancer treatment.
Supplementary Material
Acknowledgments
We gratefully acknowledge Jonathan Johniddis and Sheena Baratano for their input.
Grant Support
This work was supported by T32 CA009140 (K.S.), AI082630 (E.J.W.), AI083022 (E.J.W.), AI078897 (E.J.W.), HHSN266200500030C (E.J.W.), the Dana Foundation (E.J.W), R01 CA118374 (S.R.), P30 CA147859 (S.R.) and the Garrett B. Smith Foundation (S.R.).
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. New England Journal of Medicine. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA. Raising the Bar: The Curative Potential of Human Cancer Immunotherapy. Science Translational Medicine. 2012;4:127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali R, Morgan R, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. The Journal of Clinical Investigation. 2010;120:3953–68. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan R, Restifo N, et al. Local Delivery of lnterleukin-12 Using T Cells Targeting VEGF Receptor-2 Eradicates Multiple Vascularized Tumors in Mice. Clinical Cancer Research. 2012;18:1672–83. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matejuk A, Leng Q, Chou S-T, Mixson A. Vaccines targeting the neovasculature of tumors. Vascular Cell. 2011;3:7. doi: 10.1186/2045-824X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J, Jia R, Song H, Liu Y, Zhang L, Zhang W, et al. A promising new approach of VEGFR2-based DNA vaccine for tumor immunotherapy. Immunology Letters. 2009;126:60–6. doi: 10.1016/j.imlet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Ruan ZY, Zhao, Wang Y, Wang H, Chen Y, Shang X, et al. DNA Vaccine Against Tumor Endothelial Marker 8 Inhibits Tumor Angiogenesis and Growth. Journal of Immunotherapy. 2009;32:486–91. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 9.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 10.Bansal R, Tomar T, Ostman A, Poelstra K, Prakash J. Selective targeting of interferon gamma to stromal fibroblasts and pericytes as a novel therapeutic approach to inhibit angiogenesis and tumor growth. Molecular Cancer Therapeutics. 2012;11:2419–2428. doi: 10.1158/1535-7163.MCT-11-0758. [DOI] [PubMed] [Google Scholar]
- 11.Beatty GL, Paterson Y. IFN-γ Can Promote Tumor Evasion of the Immune System In Vivo by Down-Regulating Cellular Levels of an Endogenous Tumor Antigen. The Journal of Immunology. 2000;165:5502–8. doi: 10.4049/jimmunol.165.10.5502. [DOI] [PubMed] [Google Scholar]
- 12.Briesemeister D, Sommermeyer D, Loddenkemper C, Loew R, Uckert W, Blankenstein T, et al. Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. International Journal of Cancer. 2010;128:371–8. doi: 10.1002/ijc.25350. [DOI] [PubMed] [Google Scholar]
- 13.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4+ T Cells Contribute to the Remodeling of the Microenvironment Required for Sustained Tumor Regression upon Oncogene Inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Z, Schwartzkopff J, Pradera F, Kammertans T, Seliger B, Pircher H, et al. A Critical Requirement of Interferon gamma-mediated Angiostasis for Tumor Rejection by CD8+ T Cells. Cancer Research. 2003;63:4095–100. [PubMed] [Google Scholar]
- 15.Hunter CA, Yu D, Gee M, Ngo C, Sevignani C, Goldschmidt M, et al. Cutting Edge: Systemic Inhibition of Angiogenesis Underlies Resistance to Tumors During Acute Toxoplasmosis. The Journal of Immunology. 2001;166:5878–81. doi: 10.4049/jimmunol.166.10.5878. [DOI] [PubMed] [Google Scholar]
- 16.Rankin EB, Yu D, Jiang J, Shen H, Pierce E, Goldschmidt M, et al. An Essential Role of Th1 Responses and Interferon-gamma in Infection-Mediated Suppression of Neoplastic Growth. Cancer Biology & Therapy. 2003;2:685–91. [PubMed] [Google Scholar]
- 17.Harford JB. Viral infections and human cancers: the legacy of Denis Burkitt. British Journal of Haematology. 2012;156:709–18. doi: 10.1111/j.1365-2141.2011.09017.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawler PR, Lawler J. Molecular Basis for the Regulation of Angiogenesis by Thrombospondin-1 and -2. Cold Spring Harbor Perspectives in Medicine. 2012:2. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. Journal of Cellular and Molecular Medicine. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossfeld GD, Ginsberg DA, Stein JP, Bochner B, Esrig D, Nichols P, et al. Thrombospondin-1 Expression in Bladder Cancer: Association With p53 Alterations, Tumor Angiogenesis, and Tumor Progression. Journal of the National Cancer Institute. 1997;89:219–27. doi: 10.1093/jnci/89.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Ahuja N, Burger PC, Issa JP. Methylation and silencing of the Thrombospondin-1 promoter in human cancer. Oncogene. 1999;18:3284–9. doi: 10.1038/sj.onc.1202663. [DOI] [PubMed] [Google Scholar]
- 22.Lawler J, Sunday M, Thibert V, Duquette M, George E, Rayburn H, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. The Journal of Clinical Investigation. 1998;101:982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doering T, Crawford A, Angelosanto J, Paley M, Ziegler C, Wherry EJ. Network Analysis Reveals Centrally Connected Genes and Pathways Involved in CD8+ T Cell Exhaustion versus Memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone A, De Paoli P. Cancers related to viral agents that have a direct role in carcinogenesis: pathological and diagnostic techniques. Journal of Clinical Pathology. 2012;65:680–6. doi: 10.1136/jclinpath-2012-200717. [DOI] [PubMed] [Google Scholar]
- 25.Losikoff PT, Self AA, Gregory SH. Dendritic cells, regulatory T cells and the pathogenesis of chronic hepatitis C. Virulence. 2012;3:0–1. doi: 10.4161/viru.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Christensson M, Forslaw A, De Meester I, Sundqvist K-G. A CD26-Controlled Cell Surface Cascade for Regulation of T Cell Motility and Chemokine Signals. The Journal of Immunology. 2009;183:3616–24. doi: 10.4049/jimmunol.0804336. [DOI] [PubMed] [Google Scholar]
- 27.Kaur S, Kuznetsova SA, Pendrak ML, Sipes J, Romeo M, Li Z, et al. Heparan Sulfate Modification of the Transmembrane Receptor CD47 Is Necessary for Inhibition of T Cell Receptor Signaling by Thrombospondin-1. Journal of Biological Chemistry. 2011;286:14991–5002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van VQ, Baba N, Rubio M, Wakahara K, Panzini B, Richard C, et al. CD47Low Status on CD4 Effectors Is Necessary for the Contraction/Resolution of the Immune Response in Humans and Mice. PLoS ONE. 2012;7:e41972. doi: 10.1371/journal.pone.0041972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krispin A, Bledi Y, Atallah M, Trahtemberg U, Verbovetski I, Nahari E, et al. Apoptotic cell thrombospondin-1 and heparin-binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood. 2006;108:3580–9. doi: 10.1182/blood-2006-03-013334. [DOI] [PubMed] [Google Scholar]
- 30.Venkatraman L, Chia S-M, Narmada Balakrishnan C, White J, Bhowmick S, Forbes D, et al. Plasmin Triggers a Switch-Like Decrease in Thrombospondin-Dependent Activation of TGFbeta1. Biophysical Journal. 2012;103:1060–8. doi: 10.1016/j.bpj.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang E, Barbi J, Yang H, Jinasena D, Yu H, Zheng Y, et al. Control of Th17/Treg Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdoch C, Muthana M, Lewis CE. Hypoxia Regulates Macrophage Functions in Inflammation. The Journal of Immunology. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 33.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J. Angiogenesis and Immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer and Metastasis Reviews. 2011;1:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 34.Nabors Lb, Fiveash JB, Markert JM, Kekan MS, Gillespie JY, Huang Z, et al. A phase 1 trial of abt-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Archives of Neurology. 2010;67:313–9. doi: 10.1001/archneurol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahora AI, Rusk AW, Henkin J, McKeegan EM, Shi Y, Khanna C. Prospective Study of Thrombospondin-1 Mimetic Peptides, ABT-510 and ABT-898, in Dogs with Soft Tissue Sarcoma. Journal of Veterinary Internal Medicine. 2012;26:1169–76. doi: 10.1111/j.1939-1676.2012.00966.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.