Abstract
Background:
During the menopausal period, sexual desire may decrease. Therefore, restoring the sexual desire may help to improve sexual functioning in this group of women. The aim of this study was to examine the effect of Ginkgo biloba extract (GBE) on sexual desire in postmenopausal women.
Materials and Methods:
In this triple-blind, randomized, placebo-controlled trial, 80 healthy female volunteers attending three healthcare centers of Tehran University of Medical Sciences (TUMS) were enrolled. The instrument of this study had two main parts. The first part covered the personal characteristics of the volunteers and the second part used the Sabbatsberg Sexual Rating Scale (SSRS) to subjectively evaluate sexual desire before and after intervention. The participants received GBE at a dose of 120-240 mg (n = 40) or received placebo (n = 40) daily for 30 days. The results were analyzed using Mann–Whitney test. All analyses were performed using SPSS software.
Results:
The sexual desire was significantly improved in the GBE group compared to the placebo group (P = 0.02).
Conclusions:
In this study, we found that GBE had a positive effect on sexual desire of menopausal women; thus, these findings support the positive effect of GBE on the sexual function of menopausal women.
Keywords: Ginkgo biloba extract, menopause, sabbatsberg sexual rating scale, sexual desire
INTRODUCTION
Sexual health is an important aspect of women's health[1] that affects the well-being and quality of life, both for the women themselves and for their spouses. During the menopausal transition, a reduction in estradiol and dehydroepiandrosterone sulfate levels may interfere negatively with sexual function.[2,3]
Sexual desire is an essential domain of sexual function because low desire can be caused in low sexual relations. Dennerstein et al. (2006) reported that there was significant correlation of desire with other domains of female sexual functioning. Women with low desire are also highly likely to experience low arousal, pleasure, or orgasmic difficulties. Overall, the results show that women with low desire have, on average, a very different sexual experience than women without low sexual desire. These findings indicate that restoring a woman's sexual desire may help to improve her satisfaction with her own sexual functioning and with the intimate relationship between her and her sexual partner.[4]
Hormone replacement therapy (HRT)[5] is the most effective method for relieving sexual function,[6] but some studies mentioned concerns about its side effects.[1,7,8,9] Therefore, investigators are looking for both complementary and alternative medicine (CAM) that has the same benefit but with minimized risks.[6,10] Although some alternatives are not as effective as HRT, a reduction in symptoms may be sufficient.[11] Ginkgo biloba extract (GBE) is a phytoestrogen.[6,10] It facilitates blood flow,[12,13,14] influences nitric oxide systems, and has a relaxant effect on smooth muscles, all of which are important in the female sexual response,[13] but controversy remains concerning its effects on sexual desire.[1,13,15,16]
GBE leaves were used as early as 1505 in Chinese herbal text. GBE is said to be effective for a wide range of medical conditions. Most of the illnesses relieved by ginkgo are associated with old age, a time of life when the blood flow deteriorates.[14]
Although some studies show a positive effect of GBE alone or in combination with other supplements on sexual desire,[12,17,18] other studies have not reported any significant effect.[13,19] Therefore, the goal of this study was to assess the effect of G. biloba extract on sexual desire in women aged 50-60 years.
MATERIALS AND METHODS
In this study approved by the Research Ethics Committee of Tehran University of Medical Sciences (TUMS), 80 healthy volunteers attending three healthcare centers of TUMS in the west of Tehran were enrolled in the study during summer and autumn 2010.
All the study participants were women aged 50-60 years, who had begun experiencing menopausal symptoms at least a year back. None of them was receiving HRT, and neither they nor their spouses had any acute or chronic physical or psychological diseases. All the participants were literate and had sex at least twice during the study period of 30 days. Any participant who had consumed other drugs during the study was excluded. The other exclusion criteria included consuming G. biloba capsules for less than 3 weeks, any serious medical conditions, and not having sex at least once during the study period.
This triple-blind, placebo-controlled trial investigated the effects of GBE at a dose of 120-240 mg on sexual desire, as assessed by the Sabbatsberg Sexual Rating Scale (SSRS), which is a valid and reliable questionnaire;[20] its Persian version has previously been used in various studies in Iran.[21]
The assessment of this study had two main parts. The first part covered personal characteristics, including age, time of last menstruation, number of coitus, number of children, education, occupation, and economic status of the volunteers, and age, education, and occupation status of their spouses. The second part of the assessment used the SSRS, which measured sexual function before initiation of intervention and after 1 month of therapy. The part of SSRS measured sexual desire which assess sexual desire in the last month and in comparison to the previous years. The response options differed between the questions, but they were based on a 5-point Likert scale rating system. The SSRS has six domains (sexual desire, activity, satisfaction, arousal or pleasure, orgasm, and importance of sex) and each domain has two items, six of which assess different aspects of sexual function in the last month, while six others assess different aspects of sexual function in comparison to the previous years. The response options differed between the questions, but they were based on a 5-point Likert scale rating system. The responses to each question were summed and converted to a percentage, producing a “sexual rating” between 0 and 100. After a detailed explanation of the procedure and general objective of the study was given to the participants, they provided informed consent, and were randomly assigned to the GBE or placebo group (A and B bottles). Since the study was triple-blind, only the pharmacist knew the identity of each type of capsule, which means that apart from the participants and investigator being blind to the treatment, the statistician was also blinded until the analysis was completed. The two bottles were completely same; therefore, investigator and participants were not aware of the content of the bottles.
Each G. biloba capsule contained 60 mg of GBE, while the placebo capsules contained pea powder and wheat blossom. Both capsules appeared identical.
The treatment group received GBE capsules (60 mg), twice daily for 1 week, and the control group received placebo capsules, following the same regimen as the GBE group. Dosage continued at this level in case of gastric irritation. Participants who tolerated the capsules received them at a dose of 60 mg four times a day for the remainder of the study.
All the participants were followed up weekly by telephone. At the end of each week, the research group called the participants and asked them if they had experienced any complications during the test period, such as nausea, vomiting, or epigastric pain. No complications were reported.
Of the 80 participants who were enrolled in this study, 63 women successfully followed and completed the study protocol. We excluded 21.2% of the participants. This reduction in sample size was attributed to the following reasons: Not having sex at least twice during the study period (n = 1), diagnosis of diabetes (n = 3), diagnosis of hypertension (n = 2), use of aspirin (n = 2), and irregular consumption of the capsules (n = 6).
After a month, all the participants visited the healthcare centers again and completed SSRS assessment. The scores were measured, compared, and analyzed for the two groups before and after the intervention.
The results were analyzed by using Mann–Whitney test. A level of 0.05 was considered significant in the tests. All analyses were performed using SPSS software. All ethical considerations considered and approved by Research Chancellor of Tehran University of Medical Sciences.
RESULTS
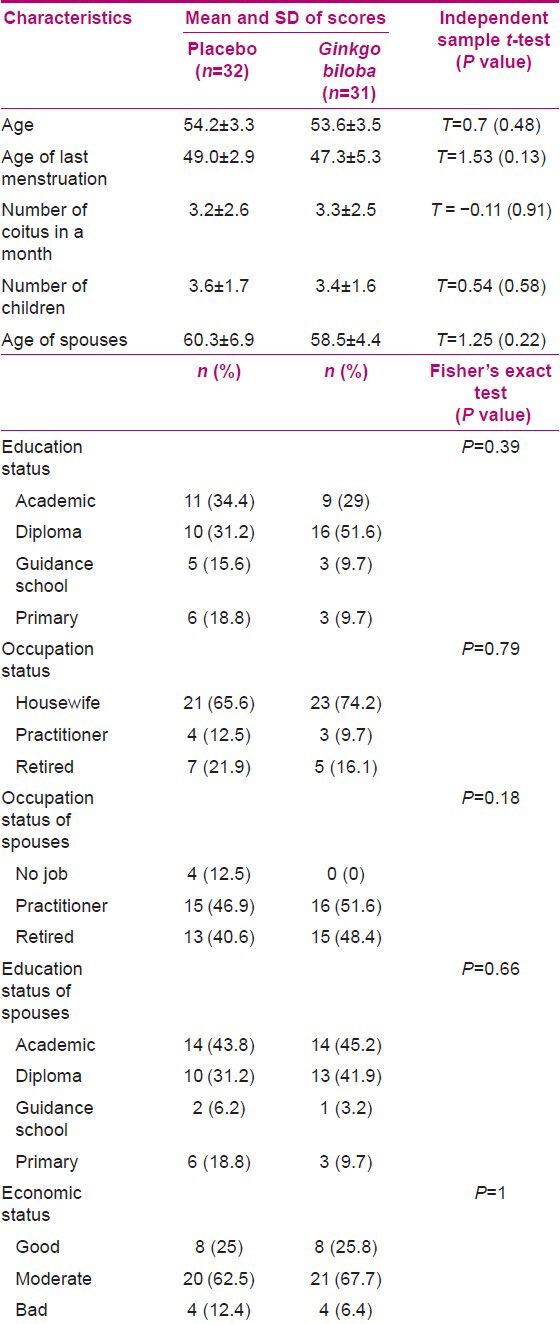
All 63 participants completed the SSRS for the second time following the intervention. To assess the equivalence of the two groups, personal characteristics were analyzed. No significant differences were noted in the personal characteristics of the two groups regarding age, time of last menstruation, number of coitus, number of children, education, occupation, and economic status of the volunteers, and age, education, and occupation status of the spouses [Table 1].
Table 1.
Personal characteristics of women in placebo and Ginkgo biloba groups
The results of Mann–Whitney test showed equality of sexual desire of the SSRS before intervention in the two groups and no significant difference between the mean scores in sexual satisfaction [Tables 2 and 3].
Table 2.
Sexual desire during last month in the two groups before and after intervention
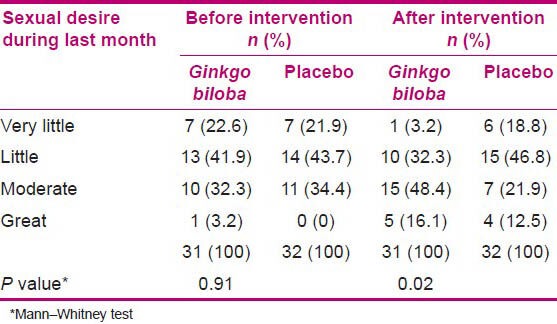
Table 3.
Sexual desire in comparison to previous years in the two groups before and after intervention
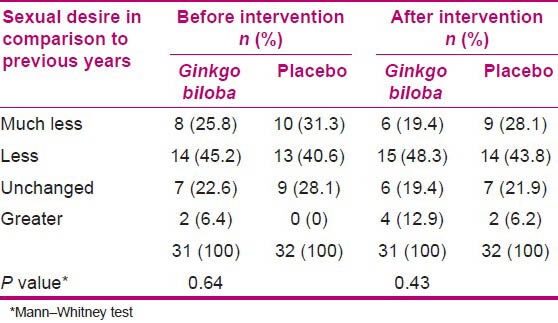
After intervention, significant difference was found between the groups in the sexual desire (P = 0.02) during the previous month [Table 2], whereas no difference was found between the sexual desire in comparison to the previous years [Table 3].
DISCUSSION
In spite of the high prevalence of sexual dysfunction in women, only few clinical trials have been conducted for potential treatments.[6,14,22] The aim of this study was to investigate the effect of GBE on sexual desire in menopausal women, as some previous studies had reported a significant improvement in sexual function after using GBE.
Cohen and Bartlik in an open clinical trial showed that GBE at a dose of 120-240 mg was 84% effective in treating sexual dysfunction due to antidepressant drugs. GBE generally had a positive effect on all four phases of the sexual response cycle (desire, excitement, orgasm, and resolution).[17]
Ito et al. in a double-blind placebo-controlled study of ArginMax (a nutritional compound consisting of the extracts of ginseng, ginkgo, and damiana, and L-arginine, multivitamins, and minerals) showed that after 4 weeks, the ArginMax-treated group reported an improvement in sexual desire.[12] The same researchers in another study reported the effects of the same supplement on sexual function in pre-, peri-, and postmenopausal women. In pre-menopausal women, this supplement had positive effects on sexual desire, sexual satisfaction, frequency of sexual desire, and frequency of intercourse compared to the placebo group. In the peri-menopausal women, this supplement had positive effects on the frequency of intercourse, sexual satisfaction, and vaginal dryness compared to the placebo group, while vaginal dryness, sexual activity, and clitoral sensation had not been improved. Postmenopausal women showed a slight but significant improvement in sexual desire.[18]
In this study, GBE improved sexual desire and the result was consistent with previous findings. However, we found that GBE was not effective in increasing sexual desire in comparison to previous years.[12,18]
In contrast to the result of this study, Wheatley (2004) did not report any significant difference in sexual function after GBE therapy. The method of consumption was the same, but the participants were also consuming antidepressant drugs.[18] In another study, Meston et al. showed that a single dose of 300 mg of GBE had not a small but significant effect on sexual desire compared to the effect of placebo in women.[13]
Since GBE is considered a safe supplement[14,21] with weak estrogenic activities, it may be a suitable method of replacement therapy for treating menopausal symptoms.[6]
In this study, we found that GBE had a positive effect on the sexual desire of menopausal women; therefore, the investigators suggest that it may have effects on improving the sexual function in menopausal women.
CONCLUSIONS
This study found that GBE had a positive effect on the sexual desire of menopausal women; therefore, the investigators suggest that GBE may improve sexual function in menopausal women.
ACKNOWLEDGMENTS
The authors would like to thank the Research Chancellor of TUMS for support and grants for this study. They would also like to thank Andrea Garratt for providing valuable information about the use of SSRS. Controlled Trial #: IRCT N5 201010192172.
Footnotes
Source of Support: The Research Chancellor of Tehran University of Medical Sciences
Conflict of Interest: Nil.
REFERENCES
- 1.Warren MP. Use of Alternatives therapies in menopause. Best Pract Res Clin Obstet Gynaecol. 2002;16:411–48. doi: 10.1053/beog.2002.0290. [DOI] [PubMed] [Google Scholar]
- 2.Al-Azzawi F, Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–7. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Valadares AL, Pinto-Neto AM, Conde DM, Osis MJ, Sousa MH, Costa-Paiva L. The sexuality of middle-aged women with a sexual partner: A population-based study. Menopause. 2008;15:706–13. doi: 10.1097/gme.0b013e31815cd3fb. [DOI] [PubMed] [Google Scholar]
- 4.Dennerstein L, Choochaki P, Geraziottin A. Hypoactive sexual desire disorder in menopausal women: A survey of western European women. J Sex Med. 2006;3:212–22. doi: 10.1111/j.1743-6109.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 5.Gollschewski S, Anderson D, Skerman H, Lyons-Wall P. The Use of Complementary and Alternative Medications by Menopausal Women in South East Queensland. Womens Health Issues. 2004;14:165–71. doi: 10.1016/j.whi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Oh SM, Chung KH. Estrogenic activities of Ginkgo biloba extracts. Life Sci. 2004;74:1325–35. doi: 10.1016/j.lfs.2003.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Huang K, Xu L, Nasri N, Jaisamrarn U. The Asian Menopause Survey: Knowledge, perceptions, hormone treatment and sexual function. Maturitas. 2010;65:276–83. doi: 10.1016/j.maturitas.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Rees M. Alternative treatments for the menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:151–61. doi: 10.1016/j.bpobgyn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Chen LC, Wang BR, Chen IC, Shao CH. Use of Chinese herbal medicine among menopausal women in Taiwan. Inte J Gynecol Obstet. 2010;109:63–6. doi: 10.1016/j.ijgo.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Panay N, Rees M. Alternatives to hormone replacement therapy for management of menopause symptoms. Curr Obstet Gynaecol. 2005;15:259–66. [Google Scholar]
- 11.Brockie J. Alternative approaches to the menopause. Rev Gynaecol Pract. 2005;5:1–7. [Google Scholar]
- 12.Ito TY, Trant AS, Polan M. A Double-Blind Placebo-Controlled study of ArginMax, a Nutritional supplement for enhancement of female sexual function. J Sex Marital Ther. 2001;27:541–9. doi: 10.1080/713846828. [DOI] [PubMed] [Google Scholar]
- 13.Meston CM, Rellini AH, Telch MJ. Short- and Long-term Effects of Ginkgo Biloba Extract on Sexual Dysfunction in Women. Arch Sex Behav. 2008;37:530–47. doi: 10.1007/s10508-008-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaby AR. Ginkgo Biloba Extract: A Review. Altern Med Rev. 1996;1:236–42. [Google Scholar]
- 15.Dennerstein L. Sexuality, midlife, and menopause. Menopause. 2008;15:880–90. doi: 10.1097/gme.0b013e31815f98c5. [DOI] [PubMed] [Google Scholar]
- 16.Geller SE, Studee L. Contemporary alternatives to plant estrogens for menopause. Maturitas. 2006;55:3–13. doi: 10.1016/j.maturitas.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JA, Bartlik B. Ginkgo Biloba for Antidepressant Induced Sexual Dysfunction. J Sex Marital Ther. 1998;24:139–43. doi: 10.1080/00926239808404927. [DOI] [PubMed] [Google Scholar]
- 18.Ito TY, Polan M, Whipple B, Trant AS. The Enhancement of Female Sexual Function with ArginMax, a Nutritional Supplement, Among Women Differing in Menopausal Status. J Sex Marital Ther. 2006;32:369–78. doi: 10.1080/00926230600834901. [DOI] [PubMed] [Google Scholar]
- 19.Wheatley D. Triple-blind, placebo-controlled trial of Ginkgo biloba in sexual dysfunction due to antidepressant drugs. Hum Psychopharmacol Clin Exp. 2004;19:545–8. doi: 10.1002/hup.627. [DOI] [PubMed] [Google Scholar]
- 20.Garratt AM, Torgerson DJ, Wyness J, Hall MH, Reid DM. Measuring sexual functioning in premenopausal women. Br J Obstet Gynaecol. 1995;102:311–6. doi: 10.1111/j.1471-0528.1995.tb09138.x. [DOI] [PubMed] [Google Scholar]
- 21.Taavoni S, Unesie Kafshgiry M, Shahpoorian F, Mahmoudie M. Hormon replacement therapy: Post-menopausal sex life and attitudes towards sex. Psychogeriatrics. 2005;5:9–14. [Google Scholar]
- 22.Rowland DL, Tai W. A review of plant-derived and herbal approaches to the treatment of sexual dysfunctions. J Sex Marital Ther. 2003;29:185–205. doi: 10.1080/00926230390155096. [DOI] [PubMed] [Google Scholar]


