Abstract
Aim:
To compare the effects of intravitrealbevacizumab (IVB) and intravitreal triamcinolone acetonide (IVT) in the treatment of macular edema (ME) secondary to central retinal vein occlusion (CRVO).
Materials and Methods:
There were 20 patients treated with IVB (1.25 mg/0.05 mL) and 16 treated with IVT (4 mg/0.1 mL). The two groups were compared with regard to best-corrected visual acuity (BCVA), central macular thickness (CMT) on optical coherence tomography (OCT), slit-lamp biomicroscopy and fundus fluorescein angiography results, intraocular pressure (IOP), numbers of injections, and adverse events.
Results:
The mean follow-up times in the IVB and IVT groups were 17.45±8.1 months (range: 8–33 months) and 19.94±10.59 months (range: 6–40 months), respectively (P = 0.431). Visual acuity increased and CMT decreased significantly within both groups, but no differences were observed between the groups (P = 0.718). The percentages of patients with increased IOP and iatrogenic cataracts were significantly higher in the IVT group than in the IVB group.
Conclusions:
Treatment with IVB and IVT both resulted in significant improvement in visual acuity and a decrease in CMT in patients with ME secondary to non-ischemic CRVO, with no difference between the two treatments. The incidence of adverse events, however, was significantly greater in the IVT group than in the IVB group. IVB may be preferred over IVT for the treatment of ME in patients with non-ischemic CRVO.
Keywords: Central retinal vein occlusion, intravitrealbevacizumab, intravitrealtriamcinolone acetonide, macular edema
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy. Branch retinal vein occlusions (BRVOs) are approximately 12 times more common than central retinal vein occlusions (CRVOs), and the non-ischemic type of RVO is roughly 9 times more common than the ischemic type.
Macular edema (ME) occurring secondary to CRVO can be treated with intravitreal injections of triamcinolone acetonide (IVT) or bevacizumab (IVB). In this study, we aimed to compare the long-term changes in visual acuity, macular thickness on optical coherence tomography (OCT), and adverse events in patients who received IVT or IVB for ME secondary to non-ischemic CRVO.
Materials and Methods
This comparative, retrospective, non-randomized clinical study was carried out at SisliEtfal Training and Research Hospital's ophthalmology clinic between June 2008 and April 2011. The study protocol was in accordance with the Declaration of Helsinki and was approved by our Institutional Research Board.
The patients were recruited into the study if they had significant ME (>320 μm) as measured by OCT (RTVue-100 Model, Optovue Inc., Fremont, CA, USA), loss of visual acuity, and macular vessel leakage on fluorescein angiography. The diagnosis of each patient was confirmed by fluorescein angiography and by OCT showing significant cystoid ME without marked retinal ischemia, as defined by the Central Retinal Vein Occlusion Study Group.[1]
The exclusion criteria were the existence of other retinal vascular diseases (e.g. diabetic retinopathy, vasculitis), age-related macular degeneration, glaucoma, previous treatment for CRVO (e.g.intravitreal injection, sub-Tenon injection, or laser photocoagulation), iris neovascularization,and >10 disc retinal ischemia as detected by fluorescein angiography.
At baseline and during follow-up, all the patients underwent ophthalmologic examinations, including measurements of best-corrected visual acuity (BCVA; ETDRS chart at 4 m), intraocular pressure (IOP; GoldmannApplanation Tonometer, Model AT 900; Haag-Streit, Bern, Switzerland), slit-lamp examination of the anterior segment, dilated fundus examination with indirect ophthalmoscopy, fluorescein angiography (VX-10i, Kowa Co.,Ltd.,Tokyo, Japan), and OCT for the measurement of macular thickness.
Thirty-six patients with non-ischemic CRVO were recruited into the study. Informed consent was obtained from all patients. One group received IVB (n=20) and the other received IVT (n=16). The same drug was used during the whole study period for each eye. Under sterile conditions, the patients in the IVT group received intravitreal injections of 4 mg/0.1 mL triamcinolone acetonide (Kenocort A®, Bristol Myers Squib Co., Princeton, NJ, USA) and the patients in the IVB group received intravitreal injections of 1.25 mg/0.05 mL bevacizumab (Avastinâ, Genentech Inc., San Francisco, CA, USA).
Eyes were treated with one initial bevacizumab injection in the IVB group and with one initial intravitreal triamcinolone injection in the IVT group, and then as needed in both groups. The patients were followed up at day 1 and 3, at weeks 1, 2, and 4, and monthly thereafter. When required, based on macular thickness, IVB was injected at 4-week intervals and IVT at 3-month intervals.
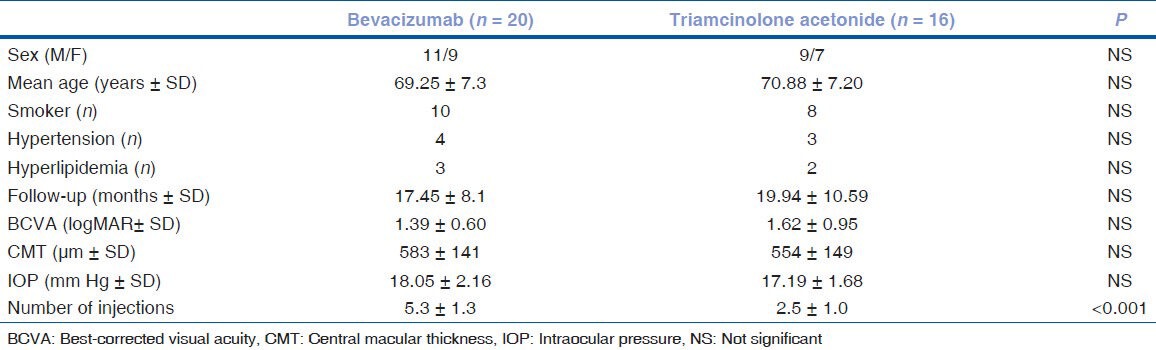
The characteristics of the patients are summarized in Table 1. Arterial hypertension was diagnosed in seven subjects (four in the IVB group and three in the IVT group). Five patients had hyperlipidemia (three in the IVB group and two in the IVT group). Ten patients were cigarette smokers in the IVB group, whereas there were eight smokers in the IVT group.
Table 1.
Baseline parameters in patients with central retinal vein occlusion
Recurrence of ME was defined as a decrease in visual acuity of one line or more or increases in intraretinal or subretinal fluid, as detected by OCT in patients with a macular thickness >320 μm or by fluorescein angiography. Cataract surgery was performed in four patients in the IVT group at 18 months. In these patients, preoperative visual acuity was accepted as final visual acuity for this study. The primary outcomes were BCVA, central macular thickness (CMT; at 1 mm) on OCT, IOP, and percentage of patients with cataracts.
Statistical analyses were performed using a commercially available statistical software package (SPSS for Windows, Version 17.0, SPSS, Chicago, IL, USA). Visual acuity was converted into the logarithm of the minimum angle of resolution (logMAR) for statistical calculations. Univariate categorical analyses were performed using Student's t-tests and Pearson's Chi-square tests, and a P-value of <0.05 was considered statistically significant.
Results
Of the 36 patients, 20 (11 men, 9 women) received IVB and 16 (9 men, 7 women) received IVT for ME secondary to CRVO. The sex distribution was similar in the two groups (P=0.502), as was the mean patient age (69.25±7.3 years vs. 70.88±7.20 years; P=0,509). The mean follow-up times were 17.45±8.1 months (range: 8–33 months) in the IVB group and 19.94±10.59 months (range: 6–40 months) in the IVT group (P=0.431).
The mean baseline visual acuity (logMAR) was 1.39±0.60 versus 1.62±0.95, and the mean baseline CMT (583±141 μm vs. 554±150 μm) and IOP (18.05±2.6 mm Hg vs. 17.9±1.68 mm Hg) were higher in the IVB group than in the IVT group; however, these differences were not statistically significant. All of the subjects in the IVB group required three IVB injections during the first 3 months of therapy. The mean number of injections was 5.3±1.3 in the IVB group (range: 4–8 injections) and 2.5±1.0 in the IVT group (range: 1–4 injections) [Table 1].
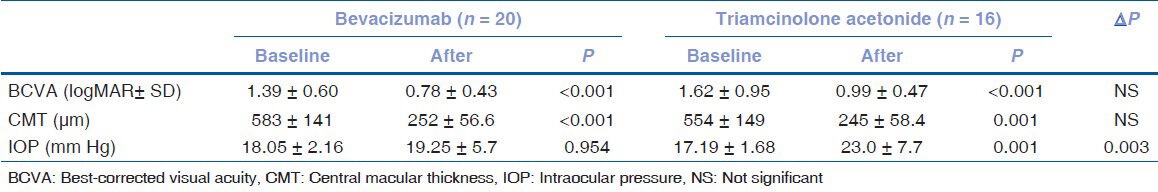
The mean visual acuity improved from 1.39±0.60 to 0.78±0.43 (P<0.001) in the IVB group and from 1.62±0.95 to 0.99±0.47 (P<0.001) in the IVT group. The mean visual acuity at baseline (P=0.368) and at the end of follow-up (P=0.176), however, were similar, as was the gain in visual acuity in the two groups. CMT decreased significantly in both groups: from 583±141.6 μm to 252±56.6 μm in the IVB group (P<0.001) and from 554±149.5 μm to 245±58.4 μm in the IVT group (P=0.001). At the final follow-up, however, CMT was similar in both groups (P=0.718). A significant increase in IOP was observed only in the IVT group [Table 2].
Table 2.
Comparison of baseline and end-of-follow-up values in patients with central retinal vein occlusion
In the IVT group, four eyes (25%) required cataract surgery at the end of follow-up, four eyes developed glaucoma, and one developed iris neovascularization. In contrast, none of the eyes in the IVB group developed cataracts, glaucoma, or iris neovascularization. Of the four eyes in the IVT group that developed glaucoma, three received anti-glaucomatous medication. No other complications were observed in the two groups, including endophthalmitis, vitreous hemorrhage, or retinal detachment.
Discussion
Vascular obstruction can result in decreased tissue perfusion and increased hydrostatic pressure within the involved segments, leading to intraretinal hemorrhages in all four quadrants, exudation of fluid, tortuous and dilated veins, and varying levels of tissue ischemia.[2] Pathological findings have suggested that in patients with CRVO, the site of obstruction is located in the lamina cribrosa.[3] CRVO causes loss of vision resulting from ME and/or retinal ischemia.[4] Moreover, neovascular complications, such as rubeosisiridis and neovascular glaucoma, may occur. The treatment modalities for CRVO include laser photocoagulation, IVT, and anti-vascular endothelial growth factor (anti-VEGF) therapy. Grid laser photocoagulation failed to demonstrate a statistically significant benefit in visual acuity in patients with ME.[5]
More recently, however, a change in paradigm has occurred in which the vitreous cavity is now considered a reservoir for drugs used to treat retinal disorders, such as diabetic retinopathy and retinal vein occlusions.[6] In recent years, IVT has been widely used to treat intraocular proliferative, edematous, and neovascular disorders, including CRVO.[7,8,9,10,11] Although the mechanism of action of corticosteroids in the treatment of ME secondary to CRVO has not yet been determined, these agents are thought to act primarily by suppressing inflammation and permeability and by down-regulating VEGF.[12,13,14]
VEGF has been shown to play an important role in increased vascular permeability.[15] Clinical and experimental studies have demonstrated that IVT and IVB are nontoxic to the retina.[16,17,18] Previous studies have compared the effects of IVT and IVB for up to 8–13 months.[19,20,21,22] This study compared the effects of IVT and IVB over 18–19 months in patients with ME secondary to CRVO.
Both IVT and IVB have been shown to reduce ME markedly, accompanied by an improvement in visual acuity.[23,24,25,26,27] We observed significant improvement in visual acuity and reduced CMT in both groups.
The mean number of intravitreal injections was higher in the IVB group than in the IVT group. The two major side effects associated with IVT are increased IOP and the development of cataracts.[28,29,30] Development of glaucoma and cataracts were the main side effects of IVT in this study.
In contrast, bevacizumab, a recombinant human monoclonal anti-VEGF antibody, reduces vascular permeability by neutralizing VEGF. IVB was first used to treat ME related to CRVO in 2005,[31] and several subsequent studies have evaluated its efficacy and safety.[32,33,34,35,36,37,38] IVB has been shown to improve visual acuity, decrease macular thickness, and cause only minor complications in patients with CRVO.[39,40,41] In this retrospective study, side effects of IVB were not observed during the follow-up period. A retrospective comparison of the outcomes at 17–19 months of IVB (1.25 mg/0.05 mL) and IVT (4 mg/0.1 mL) in patients with ME secondary to CRVO showed that 4 of 16 IVT-treated patients had steroid-induced elevated IOP; of these, 3 patients were controlled with topical anti-glaucomatous medications, but 1 needed filtering glaucoma surgery. In contrast, IOP was normal in all of the IVB-treated patients. Moreover, the incidence of cataract formation was higher in the IVT group than in the IVB group.
Thus, although intravitreal injections of triamcinolone or bevacizumab can lead to similarly significant improvements in visual acuity and to resolution of ME in patients with CRVO, their effects are not permanent, and IVT has been associated with a higher incidence of side effects.[19,20,21]
We found that the rates of cataract and glaucoma were higher in the IVT group than in the IVB group, similar to the findings detected in previous studies.[19,21,42,43] These findings indicate that the initial treatment for ME secondary to CRVO should be IVB. Similar to previous studies,[19,20,21,44,45] bevacizumab was well tolerated by our patients, and both agents were associated with significant improvements in visual acuity and reductions in ME secondary to CRVO.
We did not observe any of the previously reported systemic or injection-related complications, such as conjunctival ulcerations, vitreous hemorrhages, retinal detachments, or infectious endophthalmitis.[46] However, sterile endophthalmitis after IVT was observed in one patient, who was treated with topical corticosteroids afterward.
This study can be differentiated from previous studies in two ways: 1) the study had a longer follow-up period than previous studies and 2) all the patients in the IVB group needed three IVB injections during the first 3 months of the follow-up period. This study had several limitations, including its retrospective design and inclusion of a small numbers of patients. Moreover, the progression of cataracts in the IVT group could have masked improvements in visual acuity. In conclusion, both IVT and IVB were associated with similar gains in visual acuity and a reduction in CMT in patients with long-standing non-ischemic CRVO. IVT has side effects, including the development of cataracts and increased IOP. Considering the side effects of IVT, IVB can be considered as a first-line therapy for the treatment of ME secondary to non-ischemic CRVO.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.The Central Vein Occlusion Study group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 3.Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983;90:458–74. doi: 10.1016/s0161-6420(83)34530-9. [DOI] [PubMed] [Google Scholar]
- 4.A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434–44. [PubMed] [Google Scholar]
- 5.Evaluation of grid pattern photocoagulation for macular edema in central retinal vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995;102:1425–33. doi: 10.1016/s0161-6420(95)30849-4. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB. Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmol Scand. 2005;83:645–63. doi: 10.1111/j.1600-0420.2005.00592.x. [DOI] [PubMed] [Google Scholar]
- 7.Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR, et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina. 2006;26:896–901. doi: 10.1097/01.iae.0000231543.45699.e1. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:782–3. doi: 10.1007/s00417-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PB, Martidis A, Rogers AH, Duker JS, Reichel E. Intravitreal triamcinolone acetonide for macular edema due to central retinal vein occlusion. Br J Ophthalmol. 2002;86:247–8. doi: 10.1136/bjo.86.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth DB, Cukars C, Radhakrishnan R, Feuer WJ, Yarian DL, Green SN, et al. Intravitreal triamcinolone acetonide injections in the treatment of retinal vein occlusions. Ophthalmic Surg Lasers Imaging. 2008;39:446–54. doi: 10.3928/15428877-20081101-16. [DOI] [PubMed] [Google Scholar]
- 11.Gregori NZ, Rosenfeld PJ, Puliafito CA, Flynn HW, Jr, Lee JE, Mavrofrides EC, et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the managment of macular edema secondary to central retinal vein occlusion. Retina. 2006;26:889–95. doi: 10.1097/01.iae.0000237111.82357.30. [DOI] [PubMed] [Google Scholar]
- 12.Vinores SA, Youssri AI, Luna JD, Chen YS, Bhargave S, Vinores MA, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12:99–109. [PubMed] [Google Scholar]
- 13.Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105:412–6. doi: 10.1016/S0161-6420(98)93020-2. [DOI] [PubMed] [Google Scholar]
- 14.Fisher S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular growth factor. Eur J Pharmacol. 2001;411:231–43. doi: 10.1016/s0014-2999(00)00915-8. [DOI] [PubMed] [Google Scholar]
- 15.Ogino K, Tsujivaka A, Murakami T, Muraoka Y, Kurashige Y, Yoshimura N. Grid photocoagulation combined with intravitreal bevacizumab for recurrent macular edema associated with retinal vein occlusion. Clin Ophthalmol. 2011;5:1031–6. doi: 10.2147/OPTH.S22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCuen BWII, Bessler M, Tano Y, Chandler D, Machemer R. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981;91:785–8. doi: 10.1016/0002-9394(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 17.Hida T, Chandler D, Arena JE, Machemer R. Experimental and clincal observations of the intraocular toxicity of commerical corticosterid preparations. Am J Ophthalmol. 1986;101:190–5. doi: 10.1016/0002-9394(86)90593-3. [DOI] [PubMed] [Google Scholar]
- 18.Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:270–4. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Wu WC, Cheng KC, Wu HJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema duo to central retinal vein occlusion. Eye (Lond) 2009;23:2215–22. doi: 10.1038/eye.2008.429. [DOI] [PubMed] [Google Scholar]
- 20.Tao Y, Hou J, Jiang Y-R, Li X-X, Jonas JB. Intravitreval bevacizumab vs triamcinolone acetonideformacular oedema for due to central retinal vein occlusion. Eye (Lond) 2010;24:810–5. doi: 10.1038/eye.2009.220. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Li J, Hu X, Yu S, Pan J, Tang S. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina. 2011;31:838–45. doi: 10.1097/IAE.0b013e3181f4420d. [DOI] [PubMed] [Google Scholar]
- 22.Guthoff R, Meigen T, Hennemann K, Schrader W. Comparison of bevacizumab and triamcinolone for treatment of macular edema secondary to central retinal vein occlusion--a matched-pairs analysis. Ophthalmologica. 2010;224:126–32. doi: 10.1159/000235995. [DOI] [PubMed] [Google Scholar]
- 23.Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101–14. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular oedema from central retinal vein occlusion. Arch Ophthalmol. 2002;120:1217–9. [PubMed] [Google Scholar]
- 25.Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004;122:1131–6. doi: 10.1001/archopht.122.8.1131. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007;144:864–71. doi: 10.1016/j.ajo.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 27.Hsu J, Kaiser RS, Sivalingam A, Abraham P, Fineman MS, Samuel MA, et al. Intravitreal bevacizumab (avastin) in central retinal vein occlusion. Retina. 2007;27:1013–9. doi: 10.1097/IAE.0b013e318050ca7c. [DOI] [PubMed] [Google Scholar]
- 28.Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24–7. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitareal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–3. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone -induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112:139–43. doi: 10.1016/j.ophtha.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld PJ, Fung AE, Puliafito CA. Optical cohorence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular oedema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- 32.Nqhiem-Buffet S, Cohen SY. Retinal vein occlusion: Anti- VEGF treatments. J Fr Ophtalmol. 2009;32:679–86. doi: 10.1016/j.jfo.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadi AA, Chuo JY, Banashkevich A, Ma PE, Maberley DA. The effects of intravitreal bevacizumab on patients with macular edema secondary to branch retinal vein occlusion. Can J Ophthalmol. 2009;44:154–9. doi: 10.3129/i09-040. [DOI] [PubMed] [Google Scholar]
- 34.Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic branch retinal vein occlusion. Ophthalmologica. 2009;223:124–7. doi: 10.1159/000183844. [DOI] [PubMed] [Google Scholar]
- 35.Kim YJ, Park SP. Comparison between intravitreal bevacizumab and triamcinolone for macular edema secondary to branch retinal vein occlusion. Korean J Ophthalmol. 2009;23:259–65. doi: 10.3341/kjo.2009.23.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, Arevalo JF, Berrocal MH, Maia M, Roca JA, Morales-Canton V, et al. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to branch retinal vein occlusions: Resulsts of the Pan American Collaborative Study Group at 24 months. Retina. 2009;29:1396–403. doi: 10.1097/IAE.0b013e3181bcef53. [DOI] [PubMed] [Google Scholar]
- 37.Priglinger SG, Wolf AH, Kreutzer TC, Kook D, Hofer A, Strauss RW, et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: Six month results of a prospective trial. Retina. 2007;27:1004–12. doi: 10.1097/IAE.0b013e3180ed458d. [DOI] [PubMed] [Google Scholar]
- 38.Jonas JB, Spandau UH, Schlichtenbrede F. Short term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond) 2008;22:590–1. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 39.Kriechbum K, Michels S, Prager F, Georgopoulos M, Funk M, Geitzenauer W, et al. Intravitreal avastin for macular oedema secondary to retinal vein occlusion: A prospective study. Br J Ophthalmol. 2008;92:518–22. doi: 10.1136/bjo.2007.127282. [DOI] [PubMed] [Google Scholar]
- 40.Moschos MM, Moschos M. Intraocular bevacizumab for macular edema due to CRVO. A multifocal-ERG and OCT study. Doc Ophthalmol. 2008;116:147–52. doi: 10.1007/s10633-007-9110-9. [DOI] [PubMed] [Google Scholar]
- 41.Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2009;87:77–81. doi: 10.1111/j.1755-3768.2008.01313.x. [DOI] [PubMed] [Google Scholar]
- 42.Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular oedemaassociated with central retinal vein occlusion. Am J Ophthalmol. 2003;136:419–25. doi: 10.1016/s0002-9394(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 43.Williamson TH, O’Donnel A. Intravitreal triamcinolone acetonide for cystoid macular oedema in nonischaemic central retinal vein occlusion. Am J Ophthalmol. 2005;139:860–6. doi: 10.1016/j.ajo.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Cheng KC, Wu WC, Chen KJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment macular oedema secondary to branch retinal vein occlusion. Eye (Lond) 2009;23:2023–33. doi: 10.1038/eye.2009.230. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal S, Agrawal J, Agrawal TP. Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol. 2003;136:539–40. doi: 10.1016/s0002-9394(03)00320-9. [DOI] [PubMed] [Google Scholar]
- 46.Nelson ML, Tennant MT, Sivalingam A, Regillo CD, Belmont JB, Martidis A. Infectious and presumed noninfectious endopthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003;23:686–91. doi: 10.1097/00006982-200310000-00014. [DOI] [PubMed] [Google Scholar]


