Abstract
Background:
We evaluated the role of hyperhomocysteinemia as a risk factor for retinal vein occlusion (RVO) in Indian patients.
Type of Study:
Matched case control type of longitudinal study was conducted in 2006–2007.
Materials and Methods:
Two medical retina specialists examined the eyes having an event of RVO in the last 15 days. A similar number of eyes without RVO were also examined. The serum and urine homocysteine levels of these persons were tested. Matched pair analysis was carried out to determine the risk of RVO among those with hyperhomocysteinemia.
Results:
We included 20 cases of RVO and 20 age- and sex-matched persons without RVO. The risk of RVO was significantly higher in persons with hyperhomocysteinemia [difference of mean 31.62 μmol/L (95% Confidence Interval 16.60–47. 86), P = 2.1 × 10−13]. The mean urine homocysteine level among cases and controls was not statistically significant. There were 12 persons with hypertension in both cases and control groups.
Conclusion:
Hyperhomocysteinemia is a risk factor for RVO. Any list of investigations for a case of RVO should include total plasma homocysteine (tHcy) levels.
Keywords: Homocysteine, retinal vein occlusion, visual impairment
Homocysteine is a sulfur amino acid. In the human body, it is derived from methionine by a chemical process called demethylation. The thrombo-embolismic effect of a high total plasma homocysteine (tHcy) (an amino acid) level has been documented since as early as 1968.[1] A meta-analysis to review a number of studies proved that the risk of a retinal vascular accident is higher in cases with hyperhomocysteinemia.[2] However, Di Crecchio et al. and McGimpsey et al. did not find it as an independent risk factor of retinal vein occlusion (RVO), but a proxy marker of atherosclerosis.[3,4] Genes also play a large role in the body's metabolism of homocysteine.[5,6] Thus, information on the association between tHcy and RVO in different races, exhibiting different genetic profiles, will be useful. Limited information on this issue is available in the Indian subcontinent. Therefore, our study reviewed the role of hyperhomocysteinemia in the occurrence of RVO in the absence of other known risk factors like diabetes, age, and sex.
Materials and Methods
The administrators gave written permission to undertake this study. This study was conducted between 2006 and 2009. Verbal consent of participants was obtained for using the collected data for research purpose.
This was a matched pair type of case control study. To calculate the sample size in a matched case control study, we assumed that the difference in the mean levels of tHcy among cases and controls to be 15 μmol/L. The standard deviation in cases could be as high as 25 μmol/L and in controls it could be 5 μmol/L. To have 95% confidence interval and 80% precision in our study, with 1:1 ratio of age- ad sex-matched cases and controls, we need at least 20 patients with RVO and 20 cases without RVO.
Patients aged 15 years and older, diagnosed at our clinic between 2006 and 2009, and having had an event of RVO in the previous fortnight were included as cases. Age- and sex-matched eye patients without RVO were included as controls. Patients with diabetes or hyperthyroidism, with a history of consuming tobacco products or alcohol, having undergone eye surgery in the last 1 year, and currently pregnant or with a history of taking diuretic or oral contraceptives were excluded from the study. If the principal cause of visual impairment was anterior segment pathology, the case was excluded from the study.
Two ophthalmologists undergoing training for a fellowship in vitreo-retina subspecialty were our field staff. Visual acuity for distance was noted using Snellen's literate chart held at a 6-m distance from the patient. The best visual acuity of the involved eye was noted using a pinhole and with refractive correction. If the person could not correctly recognize the top letter of the chart, visual acuity was noted using the finger counting method at a 3-m distance. If both the eyes had RVO, the findings of the eye with the worse visual acuity were taken for the analysis. The ocular pressure was measured using a non-contact tonometer (Medtronic, Minneapolis, USA). The details of the anterior segment were evaluated using a slit-lamp bio-microscope (Topcon, Oakland, USA). The posterior segment was evaluated after dilation of the pupil using 10% phenylephrine eye drops. A binocular indirect ophthalmoscope (Keeler, Windsor, UK) and + 30 D Volk lens were used for this purpose. In selected cases, we performed fluorescein angiography and Optical Coherent Tomography (OCT) to confirm the diagnosis of posterior segment pathologies. The RVO was further grouped as central retinal vein occlusion (CRVO), branch retinal vein occlusion (BRVO), macular branch vein occlusion (MBRVO), and venous stasis.
A fasting blood sample was obtained for the tHcy level. A urine sample was also collected and assessed in the laboratory. The tHcy levels were determined by high-performance liquid chromatography with electrochemical detection. Hyperhomocysteinemia was defined as having a serum level greater than 15 μmol/L. It was further categorized as moderate, intermediate, and severe if the level was 16–30, 31–100, and more than 100 μmol/L, respectively. A homocysteine level of 5 μmol/L or less in the urine was considered as normal.
The cardiologist assessed the status of hypertension in each participant. A manual mercury sphygmomanometer was used to measure systolic and diastolic blood pressures. Those known to have hypertension and already on medication were labeled as hypertensive even though their pressure readings were within the normal range. In persons ≥50 years of age, a systolic pressure of more than 140 mmHg and diastolic pressure more than 90 mmHg was considered as high. In patients <50 years of age, systolic and diastolic pressure of more than 120 and 80 mmHg, respectively, were considered as high. The patients underwent investigations like electrocardiogram to rule out pathologies in the cardiovascular system.
We used Microsoft XL spreadsheets for data entry. We transferred the data onto spreadsheets of Statistical Package for Social Studies (SPSS 12; IBM, Boston, MA, USA). As the distribution of homocysteine level was not normal, we calculated their log values. They had normal distribution. Hence, we used the parametric method of univariate analysis. We calculated the difference in the mean of log values of the serum homocysteine level of each matched pair and calculated its variance. Then, we obtained the difference in the mean of serum homocysteine levels and its 95% confidence interval using antilog calculations. For understanding the influence of other risk factors like hypertension and cardiovascular pathologies, we studied the association of RVO and homocysteine levels using stratification by these risk factors.
Those with higher homocysteine levels were treated in our hospital free of cost. The ophthalmologists followed though on cases of RVO and, if needed, provided active treatment free of cost.
Results
The characteristics of persons with RVO and those without RVO were compared. In each group, there were 12 males and 8 females. In the age group 20–39 years, 40–59 years, and more than 60 years, there were 3, 11, and 6 persons, respectively.
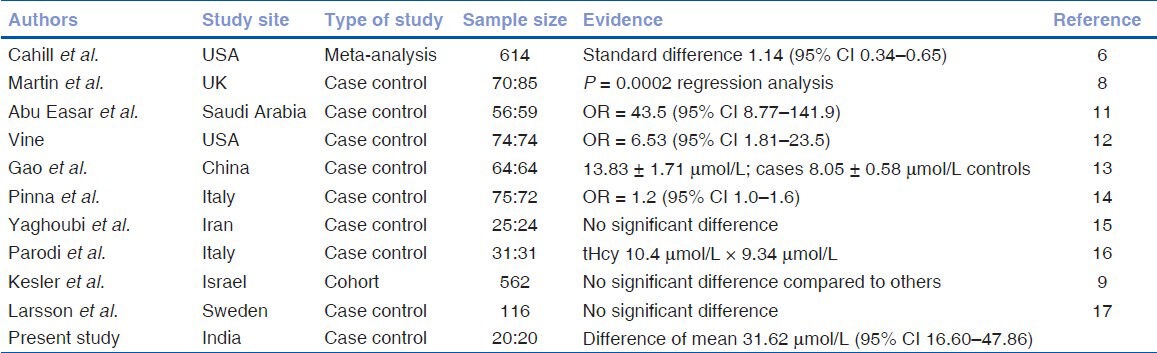
The serum homocysteine levels of patients with RVO did not show normal distribution. Therefore, we calculated the log values of serum levels of both cases and controls, and they showed normal distribution [Figs. 1 and 2]. The risk of RVO was significantly higher in persons with hyperhomocysteinemia [difference of mean 31.62 μmol/L (95% Confidence Interval or CI 16.60-47.86), P = 2.1 × 10−13]. We compared the results of our study with those of different case control studies [Table 1]. Hyperhomocysteinemia seems to be risk factor for RVO.
Figure 1.
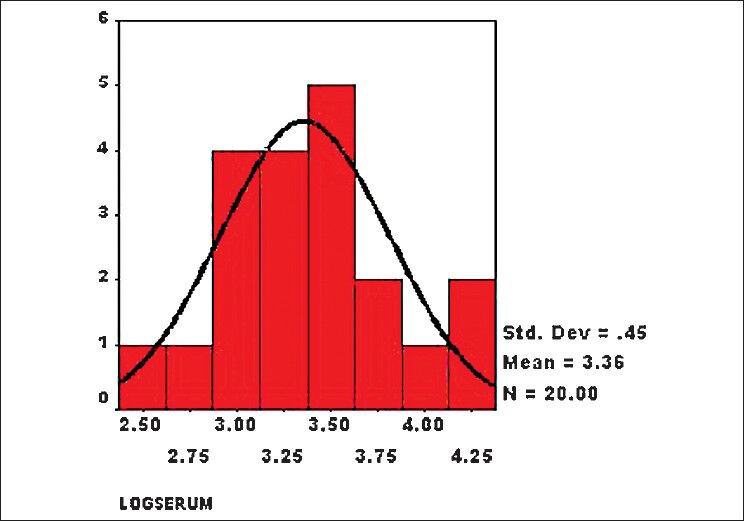
Histogram showing distribution of log values of serum homocysteine level in patients with retinal vein occlusion (cases) (n = 20, mean = 3.36, standard deviation = 0.45)
Figure 2.
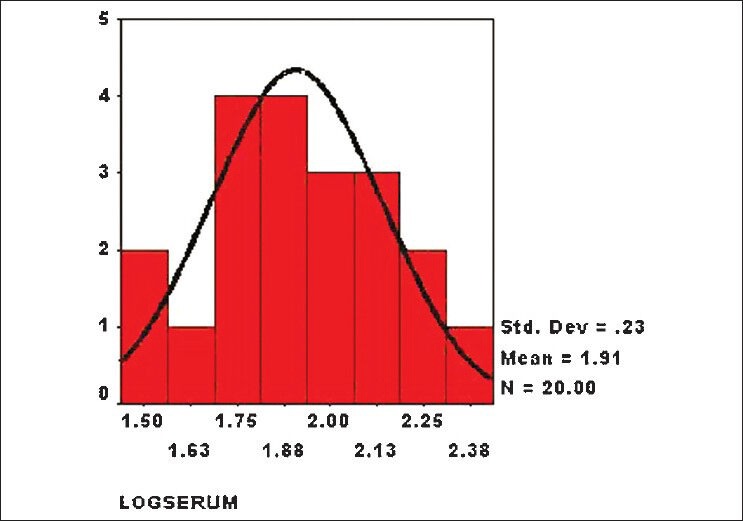
Histogram showing distribution of log values of serum homocysteine level in patients without retinal vein occlusion (controls)(n = 20, mean = 1.91, standard deviation = 0.23)
Table 1.
Evidence for and against hyperhomocysteinemia as a risk factor for retinal vein occlusion
The mean urine homocysteine level was 12.38 μmol/L (95% CI 2.68-28.88) among 20 persons with RVO. It was 4.52 μmol/L (95% CI 3.18–5.78) among controls.
We assessed the status of hyperhomocysteinemia in eyes and compared them with different types of RVO [Table 2]. We did not find any statistical correlation between the type of RVO and severity of hyperhomocysteinemia.
Table 2.
Hyperhomocysteinemia and type of retinal vein occlusion

Among cases with RVO, 12 persons had hypertension and 8 persons were having normal systolic and diastolic pressures. In control group also, 12 persons had hypertension and 8 persons showed normal blood pressure. We also did not find any significant association of hypertension with the severity of hyperhomocysteinemia among cases with RVO [odds ratio (OR) = 2.33 (95% CI 0.37–14.6)].
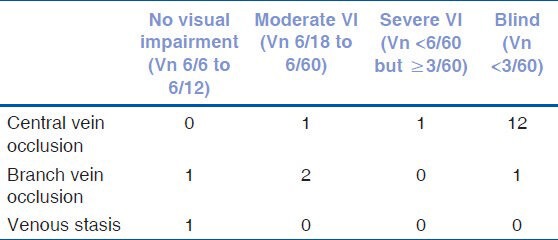
We analyzed the visual acuity of eyes with hyperho-mocysteinemia [Table 3]. It seems that those with BRVO and venous stasis had less severe grades of visual disabilities.
Table 3.
Best corrected visual acuity in eyes with different types of retinal vein occlusion
Discussion
To our knowledge, this was the first study to review the role of hyperhomocysteinemia as a risk for RVO in the Indian subcontinent. By undertaking a matched pair type of case control study, we could address the confounding effect of age and gender that was noted in different studies in the past.[7,8,9,10] Hyperhomocysteinemia was a significant independent risk factor for RVO. In view of strict exclusion criteria, our study also ruled out other causes of CRVO affecting the association. Hypertension was not a confounding factor for the association of tHcy and RVO.
Homocysteine is a sulfur amino acid. Its metabolism through two different pathways is affected by genetic enzyme defects or deficiency of vitamins that are needed as precursors of these enzymes.[11] The former can cause severe hyperhomocysteinemia, while folic acid and vitamin B12 deficiency could cause mild hyperhomocysteinemia. A diet rich in vegetables, fruits, and dairy products usually prevents vitamin deficiency related hyperhomocysteinemia.[12,13,14] In the diet of the Indian subcontinent, these food items are routinely consumed. This is in contrast to the residents of urban communities where fast food, which includes meat and carbohydrates in large proportions, is being adopted rapidly. In the rural population of India, observance to regular consumption of dairy product and fresh vegetables could vary depending on the income and attitude among poor communities. Elevated homocysteine plasma levels have been associated with ischemic heart disease, deep vein thrombosis and pulmonary embolism, stroke, and abdominal aortic aneurysm.[15,16,17,18]
Although hyperhomocysteinemia was a risk factor for RVO, severity of tHcy levels and types of RVO had no “dose response” relationship. In literature, we were able to find one meta-analysis of large sample specifically covering all the case control studies on tHcy and RVO.[2] Our findings matched with the findings of other researches.[8,19,20,21] Before and subsequent to this confirmed association, a number of studies did not show positive association of tHcy levels to RVO.[4,22,23,24] The researchers had therefore suggested the collection of more evidence for this association.[25]
We did not find hypertension as a confounding factor for the association of tHcy and RVO. Hypertension is known to cause atherosclerosis and is linked to the age of patients. Due to matching cases and controls by age group, this difference in tHcy levels among hypertensives and normotensives could be less. Our observations differ from those of Di Crecchio et al., who noted that atherosclerosis (age, hypertension) and not tHcy may be the main culprit for RVO.[3]
The cases with central vein occlusion had poorer vision compared to those with branch vein occlusion. However, we did not find any association of tHcy levels with the type of RVO.
Visual outcomes of patients with RVO have been documented by other researchers as not very encouraging.[20,26] This matched with our study outcomes.
Our study had a few limitations. The calculation of sample size was based on the assumption of difference of 15 μmol/L. This could be argued to be too high, resulting in reduced precision within the study. The strict exclusion criteria based on systematic conditions and selecting controls among eye patients and not healthy people could have introduced a selection bias. Hence, the study outcomes of cases should be compared with caution.
If clinicians know about the presence of risk factors like hyperhomocysteinemia, which is amenable to treatment, prompt preventive actions could be taken to halt the progress and avoid severe visual disabilities.[27,28,29,30] It would also be interesting to study the predictive value of CRVO in the development of systemic complications of hyperhomocysteinemia, like stroke and coronary ischemic heart diseases.
Acknowledgment
we thank Dr. Nagpal for giving consent to undertake this study and to guide us during different stages of field part of the study. We also thank all the staff of Retina foundation, Ahmedabad, India and cardiac hospital for the support extended in maintaining high quality of the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ratnoff OD. Activation of Hageman factor by L-homocystine. Science. 1968;162:1007–9. doi: 10.1126/science.162.3857.1007-a. [DOI] [PubMed] [Google Scholar]
- 2.Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol. 2003;136:1136–50. doi: 10.1016/s0002-9394(03)00571-3. [DOI] [PubMed] [Google Scholar]
- 3.Di Crecchio L, Parodi MB, Sanguinetti G, Iacono P, Ravalico G. Hyperhomocysteinemia and the methylenetetrahydrofolate reductase 677C-T mutation in patients under 50 years of age affected by central retinal vein occlusion. Ophthalmology. 2004;111:940–5. doi: 10.1016/j.ophtha.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 4.McGimpsey SJ, Woodside JV, Bamford L, Gilchrist SE, Graydon R, McKeeman GC, et al. Retinal vein occlusion, homocysteine, and methylene tetrahydrofolate reductase genotype. Invest Ophthalmol Vis Sci. 2005;46:4712–6. doi: 10.1167/iovs.04-1229. [DOI] [PubMed] [Google Scholar]
- 5.De Bree A, Verschuren WM, Kromhout D, Kluijtmans LA, Blom HJ. Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev. 2002;54:599–618. doi: 10.1124/pr.54.4.599. [DOI] [PubMed] [Google Scholar]
- 6.Cahill M, Karabatzaki M, Donoghue C, Meleady R, Mynett-Johnson LA, Mooney D, et al. genotype and retinal vascular occlusive disease. Br J Ophthalmol. 2001;85:88–90. doi: 10.1136/bjo.85.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua B, Kifley A, Wong TY, Mitchell P. Homocysteine and retinal vein occlusion: A population-based study. Am J Ophthalmol. 2005;139:181–2. doi: 10.1016/j.ajo.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 8.Martin SC, Rauz S, Marr JE, Martin N, Jones AF, Dodson PM. Plasma total homocysteine and retinal vascular disease. Eye (Lond) 2000;14(Pt 4):590–3. doi: 10.1038/eye.2000.148. [DOI] [PubMed] [Google Scholar]
- 9.Kesler A, Shalev V, Rogowski O, Shimron O, Shainberg B, Sela BA, Berliner S, et al. Comparative analysis of homocysteine concentrations in patients with retinal vein occlusion versus thrombotic and atherosclerotic disorders. Blood Coagul Fibrinolysis. 2008;19:259–62. doi: 10.1097/MBC.0b013e3282f2b60e. [DOI] [PubMed] [Google Scholar]
- 10.Moghimi S, Najmi Z, Faghihi H, Karkhaneh R, Farahvash MS, Maghsoudipour M. Hyperhomocysteinemia and central retinal vein occlusion in Iranian population. Int Ophthalmol. 2008;28:23–8. doi: 10.1007/s10792-007-9103-4. [DOI] [PubMed] [Google Scholar]
- 11.Selhub J. Homocystiene metabolism. [last accessed on 2011 Oct 7];Annu Rev Nutr. 1999 19:217–49. doi: 10.1146/annurev.nutr.19.1.217. http://www.annualreviews.org/doi/pdf/10.1146/annurev.nutr.19.1.217 . [DOI] [PubMed] [Google Scholar]
- 12.4th ed. Washington DC: US Government Printing Office; 1995. US Department of Health and Human Services. Nutrition and Your Health: Dietary Guidelines for Americans. [Google Scholar]
- 13.Riddell LJ, Chisholm A, Williams S, Mann JI. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr. 2000;71:1448–54. doi: 10.1093/ajcn/71.6.1448. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Miller ER, 3rd, Jee SH, Stolzenberg-Solomon R, Lin PH, Erlinger T, et al. Effect of dietary patterns on serum homocysteine: Results of a randomized, controlled feeding study. Circulation. 2000;22(102):852–7. doi: 10.1161/01.cir.102.8.852. [DOI] [PubMed] [Google Scholar]
- 15.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 16.Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: Evidence on a causal link from mendelian randomisation. Lancet. 2005;365:224–32. doi: 10.1016/S0140-6736(05)17742-3. [DOI] [PubMed] [Google Scholar]
- 17.Takagi H, Umemoto T. Homocysteinemia is a risk factor for aortic dissection. Med Hypotheses. 2005;64:1007–10. doi: 10.1016/j.mehy.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Giusti B, Marcucci R, Lapini I, Sestini I, Lenti M, Yacoub M, et al. Role of hyperhomocysteinemia in aortic disease. Cell Mol Biol (Noisy-le-grand) 2004;50:945–52. [PubMed] [Google Scholar]
- 19.Abu El-Asrar AM, Abdel Gader AG, Al-Amro SA, Al-Attas OS. Hyperhomocysteinemia and retinal vascular occlusive disease. Eur J Ophthalmol. 2002;12:495–500. doi: 10.1177/112067210201200608. [DOI] [PubMed] [Google Scholar]
- 20.Vine AK. Hyperhomocysteinemia: A new risk factor for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2000;98:493–503. [PMC free article] [PubMed] [Google Scholar]
- 21.Gao W, Wang YS, Zhang P, Wang HY. Hyperhomocysteinemia and low plasma folate as risk factors for central retinal vein occlusion: a case-control study in a Chinese population. Graefes Arch Clin Exp Ophthalmol. 2006;244:1246–9. doi: 10.1007/s00417-005-0191-4. [DOI] [PubMed] [Google Scholar]
- 22.Pinna A, Carru C, Zinellu A, Dore S, Deiana L, Carta F. Plasma homocysteine and cysteine levels in retinal vein occlusion. Invest Ophthalmol Vis Sci. 2006;47:4067–71. doi: 10.1167/iovs.06-0290. [DOI] [PubMed] [Google Scholar]
- 23.Yaghoubi GH, Madarshahian F, Mosavi M. Hyperhomocysteinaemia: Risk of retinal vascular occlusion. East Mediterr Health J. 2004;10:633–9. [PubMed] [Google Scholar]
- 24.Parodi MB, Di Crecchio L. Hyperhomocysteinemia in central retinal vein occlusion in young adults. Semin Ophthalmol. 2003;18:154–9. doi: 10.1076/soph.18.3.154.29809. [DOI] [PubMed] [Google Scholar]
- 25.Larsson J, Hultberg B, Hillarp A. Hyperhomocysteinemia and the MTHFR C677T mutation in central retinal vein occlusion. Acta Ophthalmol Scand. 2000;78:340–3. doi: 10.1034/j.1600-0420.2000.078003340.x. [DOI] [PubMed] [Google Scholar]
- 26.Sodi A, Giambene B, Marcucci R, Sofi F, Bolli P, Abbate R, et al. Atherosclerotic and thrombophilic risk factors in patients with recurrent central retinal vein occlusion. Eur J Ophthalmol. 2008;18:233–8. doi: 10.1177/112067210801800211. [DOI] [PubMed] [Google Scholar]
- 27.Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, et al. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology. 2010;117:1094–1101.e5. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 28.Marcucci R, Sofi F, Grifoni E, Sodi A, Prisco D. Retinal vein occlusions: A review for the internist. Intern Emerg Med. 2011;6:307–14. doi: 10.1007/s11739-010-0478-2. [DOI] [PubMed] [Google Scholar]
- 29.Wright AD, Martin N, Dodson PM. Homocysteine, folates, and the eye. Eye (Lond) 2008;22:989–93. doi: 10.1038/sj.eye.6703061. [DOI] [PubMed] [Google Scholar]
- 30.Prisco D, Marcucci R. Retinal vein thrombosis: Risk factors, pathogenesis and therapeutic approach. Pathophysiol Haemost Thromb. 2002;32:308–11. doi: 10.1159/000073587. [DOI] [PubMed] [Google Scholar]


