Abstract
Purpose:
To investigate the effect of optic neuritis (ON), ischemic optic neuropathy (ION) and compressive optic neuropathy (CON) on multifocal visual evoked potential (mfVEP) amplitudes and latencies, and to compare the parameters among three optic nerve disorders.
Materials and Methods:
mfVEP was recorded for 71 eyes of controls and 48 eyes of optic nerve disorders with subgroups of optic neuritis (ON, n = 21 eyes), ischemic optic neuropathy (ION, n = 14 eyes), and compressive optic neuropathy (CON, n = 13 eyes). The size of defect in mfVEP amplitude probability plots and relative latency plots were analyzed. The pattern of the defect in amplitude probability plot was classified according to the visual field profile of optic neuritis treatment trail (ONTT).
Results:
Median of mfVEP amplitude (log SNR) averaged across 60 sectors were reduced in ON (0.17 (0.13-0.33)), ION (0.14 (0.12-0.21)) and CON (0.21 (0.14-0.30)) when compared to controls. The median mfVEP relative latencies compared to controls were significantly prolonged in ON and CON group of 10.53 (2.62-15.50) ms and 5.73 (2.67-14.14) ms respectively compared to ION group (2.06 (-4.09-13.02)). The common mfVEP amplitude defects observed in probability plots were diffuse pattern in ON, inferior altitudinal defect in ION and temporal hemianopia in CON eyes.
Conclusions:
Optic nerve disorders cause reduction in mfVEP amplitudes. The extent of delayed latency noted in ischemic optic neuropathy was significantly lesser compared to subjects with optic neuritis and compressive optic neuropathy. mfVEP amplitudes can be used to objectively assess the topography of the visual field defect.
Keywords: Compressive optic neuropathy, humphrey visual field, ischemic optic neuropathy, multifocal visual evoked potential, optic neuritis
Optic nerve disorder is a functional impairment of the optic nerve, which may be a manifestation of most common pathological processes such as inflammation, ischemia or compressive lesions that can be life threatening.[1] The study of incidence of neuro-ophthalmology diseases in Singapore reported that optic nerve disorders, such as ischemic optic neuropathy (ION) and optic neuritis (ON), were found to be the one of the most common conditions that affects 1.08 per 100,000 and 0.83 per 100,000 population, respectively.[2] The classic clinical signs of optic neuropathy are acute or chronic visual loss with visual field defect, dyschromatopsia, and presence of relative afferent pupillary defect (RAPD).[3,4,5,6,7] Visual field testing is an important component of neuro-ophthalmic examination in diagnosing and monitoring the progression of optic nerve diseases. There is a considerable overlap of clinical features between various entities of optic neuropathy. Evaluation with imaging studies,[8] electrophysiological,[9,10] and serological and genetic testings[6] are essential for establishing the correct diagnosis. The functional visual disturbances may manifest without neuro-radiological change.[11,12] Both visual field and electrophysiological test can provide evidence of optic nerve/ganglion cell functional disturbances. Though automated perimetry testing remains the reliable means of assessing the visual field, it requires the full co-operation of the subject because of its subjective nature.[13,14]
Sutter (1991)[15] and Baseler et al., (1994)[16] designed multifocal visual evoked potential (mfVEP) that evokes multiple locations of V1 area in response to pattern reversal stimulation. This explores an electrophysiological topographical map of the V1 area of the visual cortex.[17,18] Amplitude decrease and prolonged latency have been reported in many disorders involving the impairment of conductivity along the optic nerve.[9,10] To the best of our knowledge, there is limited research that has focused on the comparison of mfVEP parameters among different optic nerve disorders.
This study aims at investigating the effect of optic neuritis, ischemic optic neuropathy and compressive optic neuropathy on amplitudes and latencies of mfVEP and to compare them among these three optic nerve disorders.
Materials and Methods
Subjects
A total of 119 eyes (48 eyes of optic nerve disorders and 71 eyes of age matched controls) were recruited. Right eye of all controls and subjects with bilateral diseases were used for mfVEP analysis. Subjects, who reported to the clinic from the time of onset of symptoms, were found to be median (17.5 (13.15-51.85) days). Subjects who were diagnosed based on acute clinical signs and symptoms with 21 eyes of optic neuritis (ON), 14 eyes of non-arteritic anterior ischemic optic neuropathy (NAAION), and 13 eyes of compressive optic neuropathy (CON) were included as cases. Among CON subjects, there were six eyes of meningioma and seven eyes of pituitary adenoma[19] based on MRI imaging were recruited as CON subjects. Patients with a known history of glaucomatous optic neuropathy, other optic nerve disorders and retinal diseases, which are known to affect the mfVEP responses, were not included for the study. All the subjects had BCVA better than 20/600 in the affected eyes to allow a stable fixation throughout the multifocal visual evoked potential testing. Subjects, who had best corrected visual acuity of 20/20 or better and were found to have no ocular disease after comprehensive eye examination, were included as control group. All the subjects underwent a complete eye examination which included detailed medical and ocular history, best corrected visual acuity (BCVA) assessment, color vision assessment using Ishihara pseudoisochromatic plates, pupillary examination, slit lamp biomicroscopy, applanation tonometry, and dilated fundus evaluation. All subjects underwent Swedish Interactive Threshold Algorithm (SITA) FAST 30-2 or 24-2 protocol in Humphrey visual field (HVF) analyzer. mfVEP and HVF were performed within a week from the time of diagnosis. The study follows the principles of the Declaration of Helsinki and was done after consent from institutional review board was obtained. An informed consent was obtained from subjects prior to mfVEP testing.
mfVEP recording
The mfVEP stimulus was elicited using cortically scaled dartboard pattern with 60 sectors stimulus subtends an angle of 44.5o which was available in VERIS 5.2.2X software as shown in Fig. 1. Subjects viewed the stimulus on the CRT monitor (Nortech Imaging Technologies) monocularly without pupillary dilatation at 53 cm. Each sector contained eight black and eight white checks. Each sector underwent pattern reversal stimulation independently and simultaneously by a binary m sequence at the frame rate of 75 Hz. A band pass filter was set between 3 and 100 Hz and was sampled at 1200 Hz.
Figure 1.
mfVEP stimulus
The mfVEP three channels recording model of Hood and Greenstein[17] with Gold Disc electrodes (Grass Model F-E5GH, Astro-Med, Inc., West Warwick, RI) was followed. Three active electrodes were placed at 4 cm above the inion and at 4 cm lateral to and 1 cm above the inion on either side. The common reference electrode was placed on the inion and ground electrode on the forehead.
Data analysis
The first slice of second order kernel responses with highest signal-to-noise ratio (log SNR) was extracted from three recorded and three additional derived channels using mfVEP processing program from Professor Don Hood's Laboratory in MATLAB 7 software (The Mathworks, Inc, Natick, MA, USA).[17,20] Signal-to-noise ratios for each sector (log SNR) were calculated by dividing the root mean square (RMS) of the amplitude for each mfVEP response over a time interval of 45 to 150 ms by the average of the 60 RMS values of the noise-only window.[21,22] The monocular amplitudes were represented in terms of signal-to-noise ratio (SNR).[20,21,22] The monocular latency represented the relative delay in latency in comparison with the controls using cross-correlation function built in software.[23,24]
The amplitude and latency probability plots of 60 sectors were obtained for each subject in comparison with normative database[21] built in Multifocal VEP processing program. The monocular amplitude probability plots[21,22] and latency probability plots[23,24] were coded in square and circle respectively. The normal responses (P > 0.05) were marked in black. The depth of the defects for deviation from control with P < 0.01 and P < 0.05 was coded as saturated color and desaturated color, respectively. Blue and red color were used for right eye and left eye, respectively. The sectors with signal-to-noise ratio too small (i.e., <1.7) in the monocular latency probability was indicated in grey circle.
mfVEP parameters analysis
The log SNR and relative latency of averaged over the 60 sectors of each monocular plots were compared among three optic nerve disorders and age matched controls. The number of adjacent abnormal points with P < 0.05 or P < 0.01 in amplitude and latency probability plots were counted to allow the topographical distribution of amplitude and latency defect in optic neuritis, ischemic optic neuropathy, and compressive optic neuropathy groups.
The pattern of the amplitude and latency defect in probability plots were classified based on the visual field profile of Optic Neuritis Treatment Trial (ONTT).[25,26] The significant defects involving central 2 mfVEP rings have been classified as central scotoma, defect involving central four rings as cecocentral scotoma, defect in upper or lower field of 6 mfVEP rings as altitudinal defect, temporal or nasal field defect of 6 mfVEP rings as hemianopia defect and defect involving all the quadrants in mfVEP six rings as diffuse or generalized defect.
Statistical analysis
The statistical analysis was performed using PASW (Predictive Analytics Software) Statistics version 18.0 for windows. Right eye of all the controls was used for analysis. Right eye of optic nerve disorders in cases of bilateral disease were recruited. Mann-Whitney U test was performed to assess the difference in mfVEP parameters between optic nerve disorders and age matched controls.
Results
A sample of 119 eyes (48 eyes of optic nerve disorders and 71 eyes of controls) was enrolled for the study during the period of April 2009-March 2010 after fulfilling the inclusion criteria. The demographic details of the cases with optic nerve disorders and age matched controls are summarized in Table 1. The median age of the three subgroups showed a significant difference (Kruskal-Wallis test, P = 0.013) and, hence, different sets of age matched controls were used for each optic nerve disorder.
Table 1.
Demographic details of the subjects
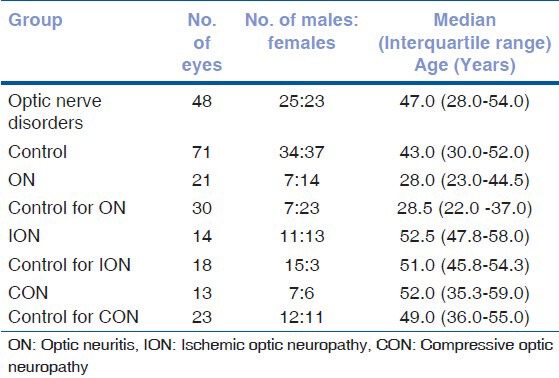
Subjects with optic nerve disorder presented with unilateral or bilateral progressive visual acuity loss, color vision impairment and visual field defect. The median logMAR (logarithm of minimum angle of resolution) visual acuity in subjects with optic nerve disorders was 0.20 (interquartile range, 0.025-1.00. Subjects who were unable to identify the plates in color vision Ishihara Pseudisochromatic chart was observed in 45 out of 48 eyes. The median of HVF MD (mean deviation) was-14.73 dB, P < 0.05% (interquartile range, -8.82 to -19.56 dB).
Comparison of multifocal VEP findings in optic nerve disorders
Mean mfVEP responses
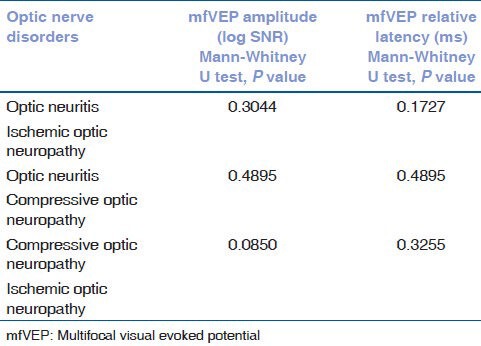
The median amplitude (log SNR) and relative latency (ms) in optic neuritis, ischemic optic neuropathy and compressive optic neuropathy compared with age matched control are summarized in Table 2. This shows that mfVEP amplitude was significantly reduced among optic nerve disorder group. The relative latency was significantly prolonged in optic neuritis and compressive optic neuropathy group. However, the comparison of log SNR amplitude reduction and relative latency delay between optic neuritis and ischemic optic neuropathy, between optic neuritis and compressive optic neuropathy, and between ischemic optic neuropathy and compressive optic neuropathy was not significant as summarized in Table 3.
Table 2.
Multifocal VEP amplitudes among optic nerve disorders and controls
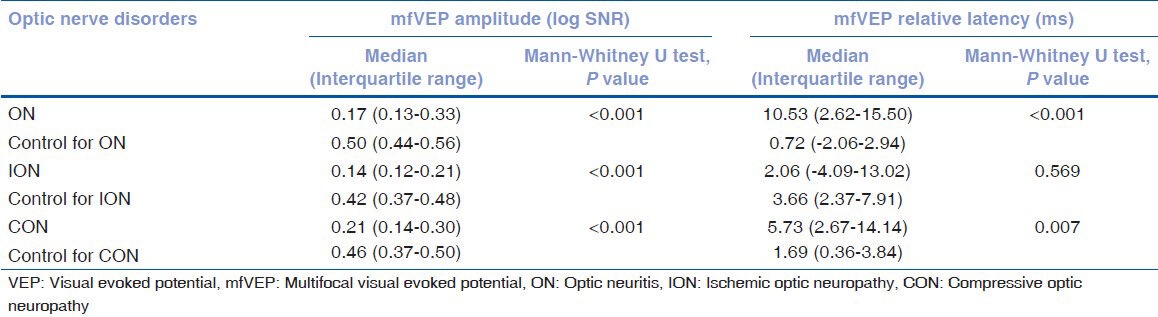
Table 3.
Comparison of mfVEP log SNR and relative latency among optic nerve disorders
Topographical probability map
Amplitude probability plot
The size and the pattern of the amplitude defects in optic nerve disorders are shown in Table 4 and Fig. 2.
Table 4.
Pattern of amplitude defect among optic nerve disorder eyes
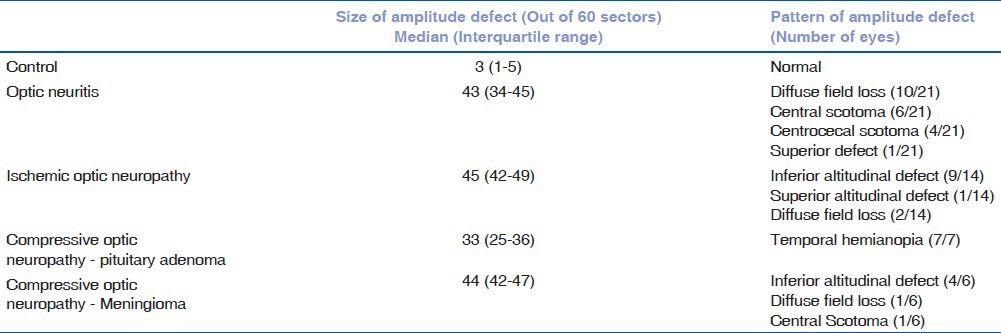
Figure 2.
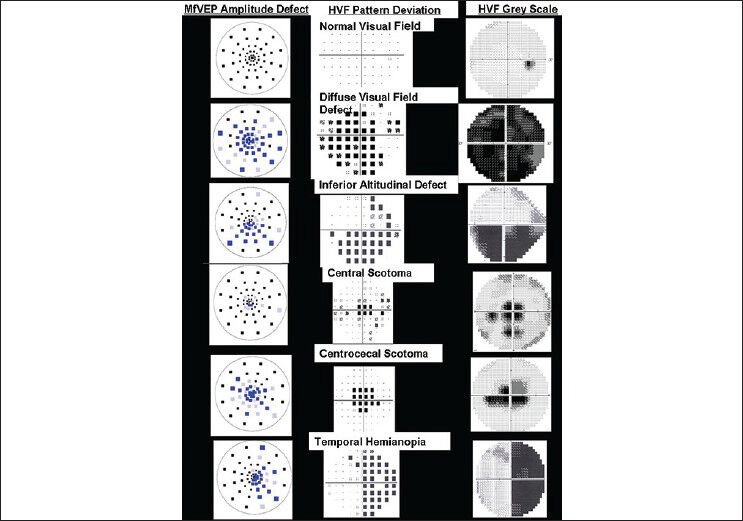
Monocular amplitude defect as per optic neuritis treatment trial visual field classification
Latency probability plot
The number of contiguous abnormal latency points in probability was counted for optic nerve disorder group. The latency probability did not follow any specific pattern of prolonged latency. In the case of optic neuritis and compressive optic neuropathy, 3 out of 9 measurable points and 3 out of 11 points (SNR >0.23) showed delayed latency respectively. One out of six measurable locations showed a delayed latency in ischemic optic neuropathy eyes.
Discussion
The value of mfVEP parameters in objective assessment of the visual function in optic nerve disorder is illustrated in this study. To the best of our knowledge, limited numbers of studies have compared the PVEP[9,10,27,28,29,30] and mfVEP findings[31,32,33,34] in optic nerve disorders. In our study, the mean amplitude (log SNR) of 60 mfVEP responses was significantly reduced in all optic nerve disorder groups compared to the age matched controls. Although the mfVEP latency was prolonged in all optic nerve disorder groups, the optic neuritis and compressive optic neuropathy group showed a more prolonged latency than ischemic optic neuropathy group. However, comparison of mfVEP amplitude (log SNR) and relative latency between optic neuritis and ischemic optic neuropathy, ischemic optic neuropathy and compressive optic neuropathy, compressive optic neuropathy and optic neuritis was not significant in our study. This could be due to significant difference in median age of three groups in our study.[21]
These results were similar to PVEP findings in optic neuritis and ischemic optic neuropathy subjects reported by Wilson,[27] Thompson et al.,[28] Veselinovic and Duric,[29] Mukartihal et al.,[30] Holder GE,[9] and Holder et al.,[10] They reported that the extent of delayed latency in PVEP responses for ischemic optic neuropathy was significantly small compared to optic neuritis. Our findings of amplitude reduction and delayed latency are consistent with those of Sanders et al.,[35] and Wildberger et al.,[36] findings on pathophysiology of disease mechanisms and its effect on VEP parameters. They have shown that amplitude reduction is because of the axonal loss in optic nerve diseases and delayed latency indicates the extent of demyelination. The edema in ON,[28,29] ischemia in ION[28,29] and demyelination in CON[36] explains the noted amplitude reduction in mfVEP responses. The demyelination of axons in ON and CON eyes contributes to the delayed latency.[28,29,36]
Several studies compared the mfVEP amplitude and HVF thresholds and reported that amplitude probability plots are similar to HVF total deviation map.[34,37,38,39,40,41] In this study, we have analyzed the size of the amplitude reduction and its pattern in mfVEP topographical amplitude deviation map among optic nerve disorders using the visual field profile classification of ONTT as shown in Fig. 1. In our analysis in optic neuritis group, a diffuse pattern of amplitude loss was noted in 10/21 (47.62%) eyes and central or centrocecal defect in 10/21 (47.62%) of the optic neuritis eyes. Rinnaldussi et al.,[42] performed PVEP stimulations of central, temporal and nasal regions and concluded that central fibers were more affected by demyelination in optic neuritis than peripheral fibers. Pakrou et al.,[43] reported that only 8.3% of the 16 optic neuritis subjects showed a central or centrocecal mfVEP defect using cluster criteria. Laron et al.,[37] analyzed the mfVEP amplitude and latency abnormality using cluster criteria in MS-ON eyes. They reported that although abnormal sectors were diffusely distributed, the central 10° was more affected than periphery.
In this study, we have illustrated that the mfVEP amplitude can be used to objectively assess the topography of the visual field defect similar to that obtained in subjective HVF testing. Several studies in optic neuritis,[37,38,39,40,41,43,44,45,46] compressive optic neuropathy,[34,47] and ischemic optic neuropathy[48] have reported that mfVEP amplitude provides a valuable diagnostic tool in detecting visual field loss. Distribution of delayed latencies in latency probability plot did not follow any specific pattern, although delayed latencies were noted in optic neuritis and compressive optic neuropathy. This could be due to the acute stage of disease which showed large regions with SNR <0.23. Hence latencies could not be measured in those locations in our study.
We emphasize that pattern of amplitude defect in combination with delayed latency can be used to differentiate optic neuritis and compressive optic neuropathy from ischemic optic neuropathy. These results were consistent with the findings of Gih et al.,[31] Odel et al.,[32] and Wenick et al.,[33] They reported the findings in ION and ON subjects that delayed latency combined with abnormal points in cluster criteria can help to discriminate the ON from ION eyes. Semela et al.,[34] also reported delayed latency in series of meningioma subjects and this delayed latency was similar to that seen in studies of optic neuritis subjects and larger than ischemic optic neuropathy and glaucoma subjects.
The two limitations of the study are the findings have been reported in small sample size and the reproducibility of the mfVEP parameters has not been assessed.
In conclusion, optic neuritis, ischemic optic neuropathy and compressive optic neuropathy cause the reduction in mfVEP amplitude. The extent of delayed latency noted in ischemic optic neuropathy was significantly lesser compared to subjects with optic neuritis and compressive optic neuropathy. Thus, mfVEP amplitudes can be used to objectively assess the topography of the visual field defect. However, it is not possible to differentiate the ischemic optic neuropathy, optic neuritis, and compressive optic neuropathy based on our study. The limitation of the study is point to point comparison of mfVEP amplitude with HVF is not analyzed. There is a scope of future studies on mfVEP responses in differentiating the subgroups of optic nerve disorders.
Acknowledgments
The authors thank Dr. Don Hood and Dr. Xian Zhang for assistance in mfVEP analysis. Authors acknowledge authors of multifocal visual evoked potential recordings in compressive optic neuropathy secondary to pituitary adenoma. Doc Ophthalmol 2010;121:197-204 for part of CON subjects have been included in current paper.
Footnotes
Source of Support: Instuituion review board of medical research foundation.
Conflict of Interest: None declared.
References
- 1.Riordan-Eva P. Clinical assessment of optic nerve disorders. Eye (Lond) 2004;18:1161–8. doi: 10.1038/sj.eye.6701575. [DOI] [PubMed] [Google Scholar]
- 2.Lim SA, Wong WL, Fu E, Goh KY, Seah A, Tan C, et al. The incidence of neuro-ophthalmic diseases in Singapore: A prospective study in public hospitals. Ophthalmic Epidemiol. 2009;16:65–73. doi: 10.1080/09286580902737516. [DOI] [PubMed] [Google Scholar]
- 3.Prasad S, Volpe NJ, Balcer LJ. Approach to optic neuropathies: Clinical update. Neurologist. 2010;16:23–34. doi: 10.1097/NRL.0b013e3181be6fad. [DOI] [PubMed] [Google Scholar]
- 4.Behbehani R. Clinical approach to optic neuropathies. Clin Ophthalmol. 2007;3:233–46. [PMC free article] [PubMed] [Google Scholar]
- 5.Van Stavern GP, Newman NJ. Optic neuropathies. An overview. Ophthalmol Clin North Am. 2001;14:61–71. [PubMed] [Google Scholar]
- 6.Purvin VA. Optic neuropathies for the neurologist. Semin Neurol. 2000;20:97–110. doi: 10.1055/s-2000-6836. [DOI] [PubMed] [Google Scholar]
- 7.Hornyak M, Digre K, Couldwell WT. Neuro-ophthalmologic manifestations of benign anterior skull base lesions. Postgrad Med. 2009;121:103–14. doi: 10.3810/pgm.2009.07.2036. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JF, 3rd, Andreoli C, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic ischemic optic neuropathy. Ophthalmology. 2002;109:1679–84. doi: 10.1016/s0161-6420(02)01148-x. [DOI] [PubMed] [Google Scholar]
- 9.Holder GE. Electrophysiological assessment of optic nerve disease. Eye (Lond) 2004;18:1133–43. doi: 10.1038/sj.eye.6701573. [DOI] [PubMed] [Google Scholar]
- 10.Holder GE, Gale RP, Acheson JF, Robson AG. Electrodiagnostic assessment in optic nerve disease. Curr Opin Neurol. 2009;22:3–10. doi: 10.1097/WCO.0b013e328320264c. [DOI] [PubMed] [Google Scholar]
- 11.Wolsey DH, Larson SA, Creel D, Hoffman R. Can screening for optic nerve gliomas in patients with neurofibromatosis type I be performed with visual-evoked potential testing? J AAPOS. 2006;10:307–11. doi: 10.1016/j.jaapos.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Moradi P, Robson AG, Rose GE, Holder GE. Electrophysiological monitoring in a patient with an optic nerve glioma. Doc Ophthalmol. 2008;117:171–4. doi: 10.1007/s10633-008-9118-9. [DOI] [PubMed] [Google Scholar]
- 13.Beck RW, Bergstrom TJ, Lichter PR. A clinical comparison of visual field testing with a new automated perimeter, the Humphrey Field Analyzer, and the Goldmann perimeter. Ophthalmology. 1985;92:77–82. doi: 10.1016/s0161-6420(85)34065-4. [DOI] [PubMed] [Google Scholar]
- 14.Kelly JP, Weiss AH. Comparison of pattern visual-evoked potentials to perimetry in the detection of visual loss in children with optic pathway gliomas. J AAPOS. 2006;10:298–306. doi: 10.1016/j.jaapos.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Sutter EE. The fast m-transform: A fast computation of cross-correlations with binary m-sequences. Society for Industrial and Applied Mathematics Journal on Computing. 1991;20:686–94. [Google Scholar]
- 16.Baseler HA, Sutter EE, Klein SA, Carney T. The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysiol. 1994;90:65–81. doi: 10.1016/0013-4694(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 17.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: Applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–51. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Hood DC. A principal component analysis of multifocal pattern reversal VEP. J Vis. 2004;4:32–43. doi: 10.1167/4.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Jayaraman M, Ambika S, Gandhi RA, Bassi SR, Ravi P, Sen P. Multifocal visual evoked potential recordings in compressive optic neuropathy secondary to pituitary adenoma. Doc Ophthalmol. 2010;121:197–204. doi: 10.1007/s10633-010-9246-x. [DOI] [PubMed] [Google Scholar]
- 20.Hood DC, Zhang X, Hong JE, Chen CS. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol. 2002;104:303–20. doi: 10.1023/a:1015235617673. [DOI] [PubMed] [Google Scholar]
- 21.Fortune B, Zhang X, Hood DC, Demirel S, Johnson CA. Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol. 2004;109:87–100. doi: 10.1007/s10633-004-3300-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Hood DC, Chen CS, Hong JE. A signal-to-noise analysis of multifocal VEP responses: An objective definition for poor records. Doc Ophthalmol. 2002;104:287–302. doi: 10.1023/a:1015220501743. [DOI] [PubMed] [Google Scholar]
- 23.Hood DC, Ohri N, Yang EB, Rodarte C, Zhang X, Fortune B, et al. Determining abnormal latencies of multifocal visual evoked potentials: A monocular analysis. Doc Ophthalmol. 2004;109:189–99. doi: 10.1007/s10633-004-5512-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM. Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci. 2007;48:692–8. doi: 10.1167/iovs.06-0475. [DOI] [PubMed] [Google Scholar]
- 25.Keltner JL, Johnson CA, Spurr JO, Beck RW. Baseline visual field profile of optic neuritis. The experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Arch Ophthalmol. 1993;111:231–4. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 26.Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis. One-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1994;112:946–53. doi: 10.1001/archopht.1994.01090190094027. [DOI] [PubMed] [Google Scholar]
- 27.Wilson WB. Visual evoked response differentiation of ischemic optic neuritis from the optic neuritis of multiple sclerosis. Am J Ophthalmol. 1978;86:530–5. doi: 10.1016/0002-9394(78)90302-1. [DOI] [PubMed] [Google Scholar]
- 28.Thompson PD, Mastaglia FL, Carroll WM. Anterior ischaemic optic neuropathy. A correlative clinical and visual evoked potential study of 18 patients. J Neurol Neurosurg Psychiatry. 1986;49:128–35. doi: 10.1136/jnnp.49.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veselinovic D, Duric S. Differentiation of posterior ischemic optic neuropathy from Retrobulbar neuritis with pattern visual evoked potential response. Medicine and Biology. 2004;11:127–30. [Google Scholar]
- 30.Mukartihal G, Radhakrishnan S, Ramasubba Reddy M, K Ayyar S. Statistical analysis of visual evoked potentials in optic neuritis and ischemic optic neuropathy subjects. Conf Proc IEEE Eng Med Biol Soc. 2005;2:1193–5. doi: 10.1109/IEMBS.2005.1616637. [DOI] [PubMed] [Google Scholar]
- 31.Gih DE, Ringger C, Woodward K, Doyle C, Allen J, Wall M. Separating demyelinating and ischemic optic neuropathies using multifocal visual evoked potential recordings. Invest Ophthalmol Vis Sci. 2004;45 ARVO E-Abstract 5493-B817. [Google Scholar]
- 32.Odel JG, Rodarte C, Yang B, Zhang X, Behrens MM, Chen JY, et al. A quantitative measure of multifocal visual evoked potential latencies in ischemic optic neuropathy and optic neuritis. Invest Ophthalmol Vis Sci. 2005;46 ARVO E-Abstract 642-B616. [Google Scholar]
- 33.Wenick AS, Rodarte C, Yang EB, Greenstein VC, Odel JG, Behrens MM, et al. Latencies of the multifocal visual evoked potential and the diagnosis of optic neuritis and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2007;48 ARVO E-Abstract 917-B892. [Google Scholar]
- 34.Semela L, Yang EB, Hedges TR, Vuong L, Odel JG, Hood DC. Multifocal visual-evoked potential in unilateral compressive optic neuropathy. Br J Ophthalmol. 2007;91:445–8. doi: 10.1136/bjo.2006.097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders EA, Volkers AC, van der Poel JC, van Lith GH. Visual function and pattern visual evoked response in optic neuritis. Br J Ophthalmol. 1987;71:602–8. doi: 10.1136/bjo.71.8.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildberger H, Robert Y, Bar P. A comparative study of visual evoked potentials and of retinal nerve fiber layer photography in neuropathies of the optic nerve. Doc Ophthalmol. 1989;73:133–8. doi: 10.1007/BF00155031. [DOI] [PubMed] [Google Scholar]
- 37.Pakrou N, Casson R, Kaines A, Selva D. Multifocal objective perimetry compared with Humphrey full-threshold perimetry in patients with optic neuritis. Clin Experiment Ophthalmol. 2006;4:562–7. doi: 10.1111/j.1442-9071.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 38.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Assessing visual pathway function in multiple sclerosis patients with multifocal visual evoked potentials. Mult Scler. 2009;15:1431–41. doi: 10.1177/1352458509350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hood DC, Odel JG, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: A Multifocal VEP Study. Invest Ophthalmol Vis Sci. 2000;41:4032–8. [PubMed] [Google Scholar]
- 40.Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM. Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Invest Ophthalmol Vis Sci. 2007;48:692–8. doi: 10.1167/iovs.06-0475. [DOI] [PubMed] [Google Scholar]
- 41.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Comparison of multifocal visual evoked potential, standard automated perimetry and optical coherence tomography in assessing visual pathway in multiple sclerosis patients. Mult Scler. 2010;16:412–26. doi: 10.1177/1352458509359782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinalduzzi S, Brusa A, Jones SJ. Variation of visual evoked potential delay to stimulation of central, nasal, and temporal regions of the macula in optic neuritis. J Neurol Neurosurg Psychiatry. 2001;70:28–35. doi: 10.1136/jnnp.70.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser CL, Klistorner A, Graham SL, Garrick R, Billson F, Grigg JR. Multifocal visual evoked potential analysis of inflammatory or demyelinating optic neuritis. Ophthalmology. 2006;113:315–23. doi: 10.1016/j.ophtha.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Fraser C, Klistorner A, Graham S, Garrick R, Billson F, Grigg J. Multifocal visual evoked potential latency analysis: Predicting progression to multiple sclerosis. Arch Neurol. 2006;63:847–50. doi: 10.1001/archneur.63.6.847. [DOI] [PubMed] [Google Scholar]
- 45.Klistorner A, Graham S, Fraser C, Garrick R, Nguyen T, Paine M, et al. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:4549–56. doi: 10.1167/iovs.07-0381. [DOI] [PubMed] [Google Scholar]
- 46.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, et al. Fellow eye changes in optic neuritis correlate with risk of multiple sclerosis. Mult Scler. 2009;15:928–32. doi: 10.1177/1352458509105228. [DOI] [PubMed] [Google Scholar]
- 47.Danesh-Meyer HV, Carroll SC, Gaskin BJ, Gao A, Gamble GD. Correlation of the multifocal visual evoked potential and standard automated perimetry in compressive optic neuropathies. Invest Ophthalmol Vis Sci. 2006;47:1458–63. doi: 10.1167/iovs.05-1146. [DOI] [PubMed] [Google Scholar]
- 48.Chan HH, Ng FY, Chu PH. Clinical application of mfERG/VEP in assessing superior altitudinal hemifield loss. Clin Exp Optom. 2005;88:253–7. doi: 10.1111/j.1444-0938.2005.tb06704.x. [DOI] [PubMed] [Google Scholar]


