Abstract
Obesity currently affects about one third of the U.S. population, while another one third is overweight. The importance of obesity for certain conditions such as heart disease and type 2 diabetes is well appreciated. The effects of obesity on the respiratory system have received less attention and are the subject of this chapter. Obesity alters the static mechanic properties of the respiratory system leading to a reduction in the functional residual capacity (FRC) and the expiratory reserve volume (ERV). There is substantial variability in the effects of obesity on FRC and ERV, at least some of which is related to the location, rather than the total mass of adipose tissue. Obesity also results in airflow obstruction, which is only partially attributable to breathing at low lung volume, and can also promote airway hyperresponsiveness and asthma. Hypoxemia is common is obesity, and correlates well with FRC, as well as with measures of abdominal obesity. However, obese subjects are usually eucapnic, indicating that hypoventilation is not a common cause of their hypoxemia. Instead, hypoxemia results from ventilation perfusion mismatch caused by closure of dependent airways at FRC. Many obese subjects complain of dyspnea either at rest or during exertion, and the dyspnea score also correlates with reductions in FRC and ERV. Weight reduction should be encouraged in any symptomatic obese individual, since virtually all of the respiratory complications of obesity improve with even moderate weight loss.
The prevalence of obesity in the U.S. has been rising for the last 3 decades. According to the U.S. Center for Disease Control and Prevention (CDC), in the late 1970’s obesity prevalence (obesity defined as a body mass index (BMI) ≥ 30) was approximately 15% among adults aged 20–74 (www.cdc.gov/nccdphp/dnpa/obesity/trend/index.htm). By 1990, it had increased to about 23%, and by 2000, obesity prevalence averaged 31%. Current CDC estimates are that approximately 35% of the U.S. population is obese, while another third is overweight (BMI ≥ 25), with even greater prevalence in certain ethnic groups. Approximately 6% of the U.S. population is classified as extremely obese (BMI ≥ 40), versus 1.4% in the late 1970’s. Since it is likely that abdominal obesity is of greatest relevance for the respiratory complications of obesity (see below), it is noteworthy that abdominal obesity (defined as a waist circumference of greater than 88 cm for women or 102 cm for men) has also increased over the same time period. Data from the National Health and Nutrition Examination Survey (NHANES), indicates that in 2003–2004 approximately 52% of the U.S. population had abdominal obesity (96). Perhaps of even greater concern, according to the CDC, the prevalence of obesity has tripled in children and adolescents over the same time period. Currently, approximately 10% of infants and toddlers and 18% of adolescents are obese. The problem is not restricted to the U.S. Obesity prevalence has also been rising worldwide in both developed and developing nations (112, 128). In 2005, the World Health Organization estimated that worldwide, 1.6 billion adults were overweight, and at least 400 million were obese.
Obesity is an important risk factor for atherosclerosis, hypertension, type 2 diabetes, musculoskeletal disorders, and some types of cancer. The effects of obesity on the respiratory system have received less attention and are the subject of this chapter. Obesity induces respiratory impairments that can lead to alterations in blood gases and cause dyspnea. These effects are magnified in the supine posture. Obesity also increases the risk for a number of respiratory diseases, including asthma. Each of these issues is discussed below. We also address the marked heterogeneity in the impact of obesity on the respiratory system. Some of this heterogeneity is likely the result of the imperfection of weight or even BMI as a measure of obesity, since fat mass and fat free mass have opposing effects on pulmonary function (89, 109). However, the location, rather than the total mass, of body fat is likely also important, just as it is for the etiology of other obesity-related conditions (96). We consider both mechanical and inflammatory factors in the etiology of the respiratory impairments of obesity. The mechanical effects of added adipose tissue mass on the thorax and abdomen are likely to contribute via their effects on absolute lung volume and subsequent airway closure. However, it is now recognized that adipose tissue is not just a passive space filling organ. It is also has important endocrine functions and can even play a role in immune regulation. Derangements of these functions in obesity lead to low grade systemic oxidative stress and inflammation, factors that may both contribute to and interact with respiratory impairment.
OBESITY, SYSTEMIC INFLAMMATION, AND OXIDATIVE STRESS
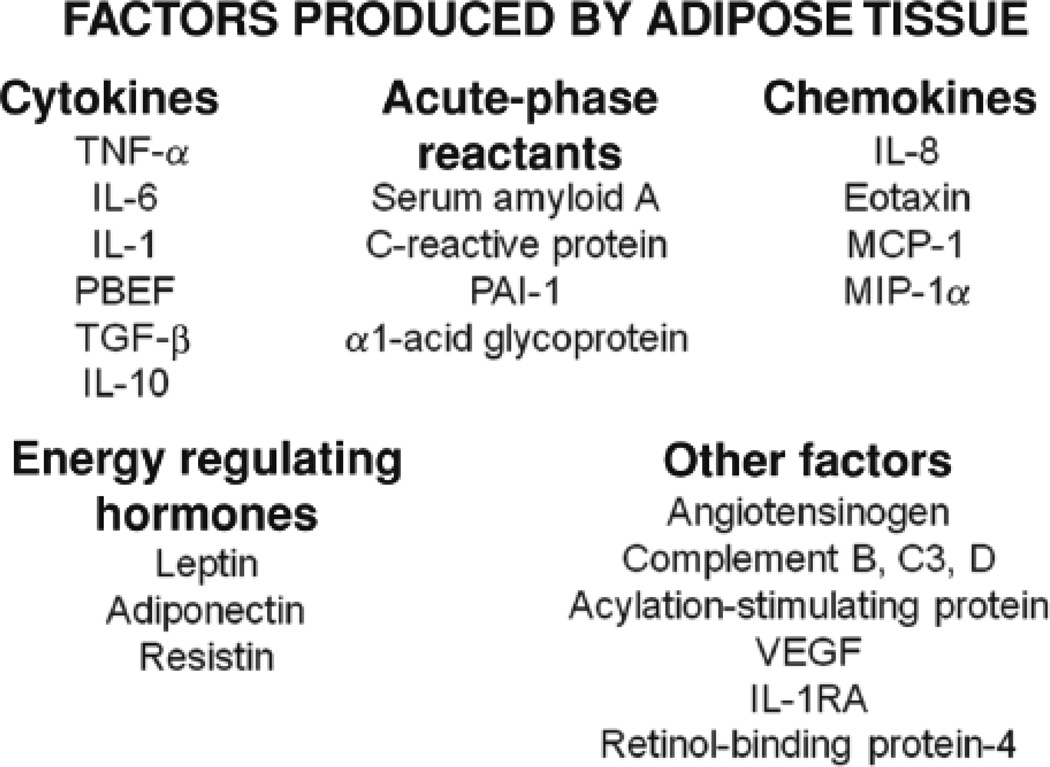
Adipose tissue is an important endocrine organ. It produces hormones, such as adiponectin and leptin, that play a role in satiety and metabolism but also have effects on the immune system. In obesity, there are changes in both the release of these hormones and in the sensitivity of various organs and tissues to their effects. There is also a marked increase in the expression of inflammatory genes within obese adipose tissue (194, 197). These genes include chemokines, such as IL-8 and MCP-1, cytokines, such as TNFα and IL-6, complement proteins, and other acute phase moieties typically associated with the liver. A partial list is provided in Figure 1. Together with adipose derived hormones, these moieties are collectively termed adipokines. The current paradigm is that these adipokines spill over into the blood. For example, serum levels of numerous pro-inflammatory molecules increase in obesity, in proportion to the BMI, and decline with weight loss (14, 26, 72, 75, 148, 173, 183, 185, 201). Circulating leukocytes are also increased (203). Hence, obesity has come to be described as a state of low grade systemic inflammation.
Figure 1.
Some of the proteins produced by adipose tissue (adipokines). IL, interleukin; TNF, tumor necrosis factor; PBEF, pre-B-cell colony-enhancing factor; TGF, transforming growth factor; PAI, plasminogen activator inhibitor; VEGF, vascular endothelial growth factor; IL-1RA, interleukin-1 receptor antagonist. Reproduced with permission of the American Physiological Society from Figure 4 of Shore, 2007 (155).
Adipose tissue macrophages (ATM) either alone or via interactions with fatty acids released from adipocytes appear to be the source of many of the inflammatory molecules produced by obese adipose tissue. Some ATM exist even in lean individuals (104, 207), but in obesity, the adipose tissue becomes infiltrated with macrophages. In some studies, up to 50% of the cells isolated from adipose tissue are macrophages (194). These ATM are recruited from blood monocyte derived precursors, likely in response to adipocyte necrosis (see below), and have an increased capacity to produce pro-inflammatory cytokines (104, 116, 168).
Obesity is also a condition of systemic oxidative stress. There are reductions in antioxidant capacity, elevated reactive oxygen species (ROS) production, and increased lipid oxidation products in blood of obese human subjects that correlate with BMI (7, 12, 85) and subside with calorie restriction (78). Systemic lipid peroxidation products and plasma ROS are also increased in obese mice (58, Lee, 2008 #1828), (92). The adipose tissue appears to be a major source of systemic oxidative stress in obesity (92), and the physiological significance of this oxidative stress is illustrated by studies showing that ROS production by treatment with an NADPH oxidase inhibitor attenuates obesity-related hyperlipidemia and hepatic steatosis improves insulin sensitivity (58). In the Framingham Heart Study, markers of systemic oxidative stress were correlated with both diabetes and a history of cardiovascular disease (85). The importance of oxidative stress for the respiratory complications of obesity remains to be established, but it is noteworthy that 8-isoprostane, a marker of oxidative stress, increases with BMI in the exhaled breath condensate of asthmatics (87).
There is evidence that the oxidative stress and systemic inflammation associated with obesity impact cells and organs distal to the adipose tissue, and may thus also impact the lung, the upper airways, and the respiratory muscles. For example, adipokines act on circulating leukocytes impacting their activation state (43), and can also alter endothelial cell function leading to increased endothelin expression, reduced nitric oxide expression, and corresponding changes in vascular function (3, Miyaki, 2008 #1703, 105). In humans, obesity-related elevations in many adipokines correlate with the presence of obesity-related diseases, including type 2 diabetes and atherosclerosis (51, 148). In animal models, aspects of the systemic inflammation of obesity are required for insulin resistance, hypertension, and atherosclerosis (73, 91, 179–180), suggesting that this inflammation is functionally important. The observation that in mice, myeloid specific knockout of IkappaB kinase beta, a central regulator of inflammatory responses, protects against obesity-related insulin resistance in mice also establishes the function importance of inflammation in obesity (6).
ELASTIC PROPERTIES OF THE RESPIRATORY SYSTEM
During breathing, effort is expended to overcome the elastic properties of the lungs and chest wall, as well as the resistance to gas flow. This section deals with the elastic properties of respiratory system. The effect of obesity on the airways is described below.
Chest wall mechanics
Most investigators report a reduction in chest wall compliance (CCW) in the obese (102, 115, 125–126, 152, 181), although this is not universally observed (16, 66, 170). Naimark and Cherniack (115) were the first to report measurements of CCW in obese subjects. Measurements were made using the method of Heaf and Prime (65). Subjects sat in a sealed plethysmograph and breathed tidally while continuous changes in pressure within the plethysmograph were effected with a pump. When a new steady state was reached, the changes in end-expiratory lung volume (EELV) and pressures were recorded. This procedure was repeated at several different plethysmograph pressures so that a pressure volume (PV) relationship could be constructed using pressure across the lung, chest wall, or total respiratory system, and relevant compliances estimated from the slope of these relationships. When subjects were in the upright posture, there was a marked (approximately 65%) decrease in CCW in the obese versus lean individuals, that was further exacerbated when the subjects were supine. Sharp et al (152) also used this method to measure the effects of obesity on respiratory system mechanics, although they studied their subjects only in the supine posture. They also observed a reduction in CCW in their obese subjects, although this reduction (approximately 33%) was smaller than that reported by Naimark and Cherniack. Lourenco et al (102) noted reduced CCW in obese subjects using the relaxation method to measure chest wall stiffness. In contrast, Suratt et al (170) using a pulse flow apparatus which they developed, observed no difference in CCW between upright obese and lean subjects.
Several technical issues require discussion. First, these investigators used esophageal balloon pressure (Pes) to estimate pleural pressure (Ppl). While there has been concern about such estimates particularly in the supine posture, Behazin et al (16) have now provided evidence that Pes does indeed provide a reasonable estimate of Ppl in the obese. Second, computation of CCW requires that the respiratory system be relaxed at the points where pressure and volume are measured. In the studies of Naimark and Cherniack (115) and Sharp et al (152), confirmation of relaxation was assessed from surface EMG measurements on various respiratory muscles. They did not assess or verify diaphragmatic relaxation, but Lourenco (102) did (using a diaphragmatic EMG electrode introduced via the esophagus), and even excluded subjects who could not relax from analysis. Suratt et al (170) only confirmed diaphragmatic relaxation with EMG in a few of their subjects, and did not assess relaxation of other respiratory muscles. Others have since demonstrated that the assumption of respiratory muscle relaxation using the method of Heaf and Prime may not be valid (181). Nevertheless, even after muscle paralysis, Van Lith et al (181), noted a reduction in CCW in obese versus lean subjects, although the effect was not statistically significant due to very small numbers of subjects in the obese group. Similarly, Pelosi et al (125–126) observed a reduction in CCW even in supine paralyzed obese subjects, although others did not (16, 66). Pelosi et al (125) noted little change in CCW in mildly obese subjects: only with marked obesity was there a decline in CCW, suggesting that differences in the magnitude of obesity could explain at least part of this apparent discrepancy.
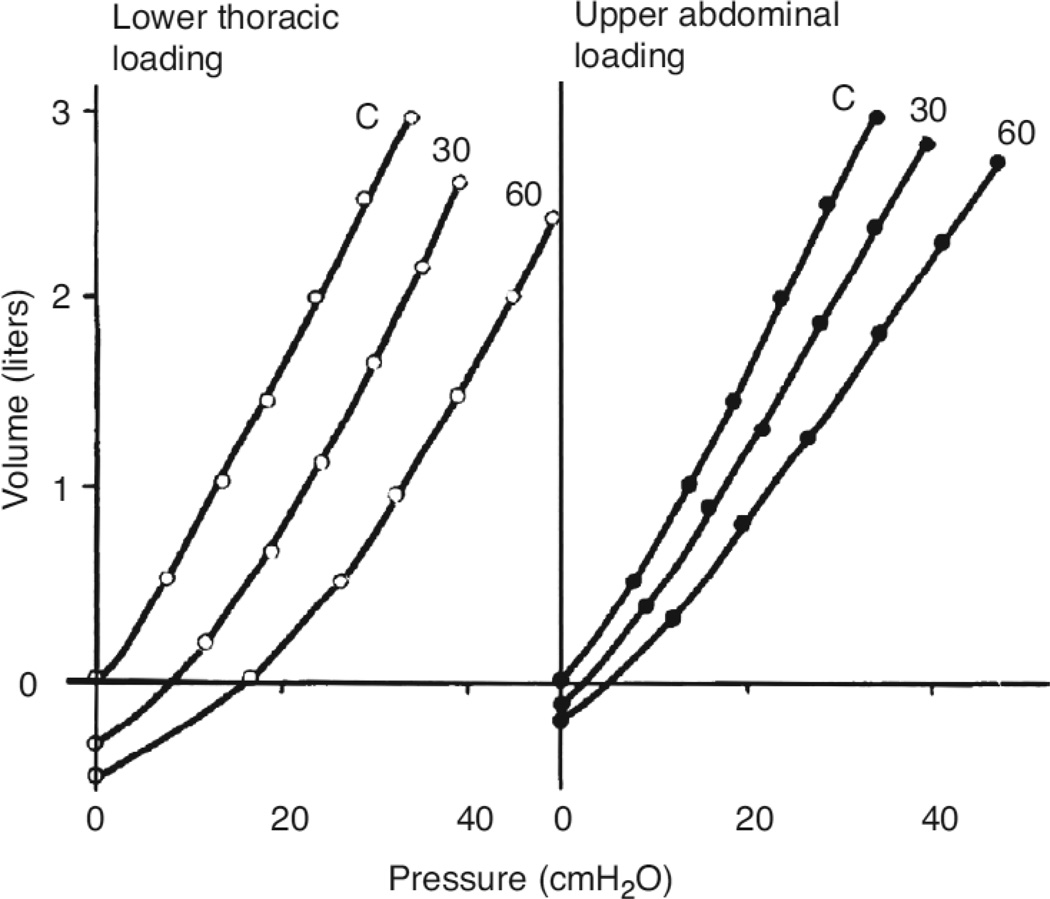
Sharp et al (151) simulated the effects obesity on respiratory system compliance by applying weights to the upper thorax, the lower thorax, or the abdomen of normal supine anesthetized and paralyzed men. They noted that applying weights to the lower thorax resulted in a parallel shift in the PV curve of the respiratory system towards higher pressures (Fig.2, left). Adding weight to the upper thorax produced a similar parallel displacement, but of smaller magnitude.. Adding weight to the abdomen not only shifted the PV curve, but also reduced the slope (Figure 2 right). When the investigators examined PV curves from obese subjects, most demonstrated a pattern consistent with lower thoracic loading. Waltemath et al (190) came to a similar conclusion in comparisons of obese subjects and mass loaded lean subjects. Should such changes in the PV relationship of the chest wall result in alterations in the measured CCW or not? It depends on where in this PV relationship the chest wall is operating. Although the lung volume at end expiration is clearly reduced in the obese, whether they are upright or supine (see below), the volume of the chest wall may not be, since it includes not just the volume of the lung, but also the volume of the additional substances now filling the chest wall, including the adipose tissue.
Figure 2.
Effects of lower thoracic (left) and abdominal (right) loading on the total respiratory static volume pressure curve in an anesthetized paralyzed normal subject. Reproduced with permission of the American Physiological Society from Figure 5 of Sharp et al, 1964 (151).
Lung mechanics
It is notable that in the earliest studies of respiratory mechanics in obesity, both Naimark and Cherniack (115) and Sharp et al (152) reported a decrease in the compliance of the lung (CL), but did not focus upon it, discounting it as either small or not statistically significant, even though in the data of Sharp et al (152), the reduction in CL was of approximately the same magnitude as that for CCW. Indeed, other investigators have subsequently reported substantial and significant reductions in CL in the obese that are at least as great or greater than the reductions in CCW (16, 125–126, 181), averaging approximately 50% of values obtained in lean subjects. Rochester et al (135) suggested that the increased stiffness of the lung might be the result of increased blood volume or increased fluid flux into the lung resulting from increased pulmonary artery pressure. However, Behazin et al (16) recently provided an alternative explanation. They reported that in many obese subjects, the pleural pressure surrounding the lungs is greater than atmospheric at end expiration. Steier et al (166) also reported that Ppl can be positive at end expiration in the obese, even in the upright posture. Positive Ppl will cause closure of most small airways, with the possible exception of those in the most superior parts of the lung, so that pressure losses across the lung in phase with volume reflect not just the pressure required to overcome the stiffness of the lung, but also the pressure required to open closed airways. So is the stiffness of the lung tissue actually different? Douglas et al (48) are among the few studies to report full PV curves of the lungs of obese subjects, wherein the measurements follow an inflation to total lung capacity (TLC). After inflation to TLC, there is presumably full recruitment of all lung regions. The results of Douglas et al (48) indicate that the static PV curves of the lungs of obese subjects fall within the normal range, indicating that the stiffness of the lung tissue of obese individuals, once recruited, is not substantially different from that of lean subjects.
Intraabdominal hypertension
A recent consensus statement defines intraabdominal hypertension (IAH) as an increase in an abdominal pressure ≥ 12 mm Hg above atmospheric (about 16 cm H2O). Abdominal pressure measured either from a gastric balloon (16, 144, 166) or from a catheter in the bladder (169) is increased in the obese, both in the upright and supine postures, although the effect is particularly apparent when subjects are supine. Behazin et al (16) reported that approximately 10% of the obese subjects they examined had evidence of IAH. In a study of subjects undergoing surgery for morbid obesity, Sugarman et al (169) reported that the average bladder pressure of these subjects was 18 cm H2O: more than half would have been classified as having IAH. Abdominal pressure correlated with the sagittal abdominal diameter, and was greater in men than women, despite identical BMI, consistent with the typically greater deposition of abdominal adipose tissue in men. Notably, elevations in abdominal pressure were associated with an increased propensity to certain obesity co-morbidities including gastro- esophageal reflux, lower limb venous stasis, hypoventilation, and stress overflow urinary incontinence suggesting that these are dependent on the change in pressure, whereas other obesity co-morbidities including joint problems and gallstones were not associated with abdominal pressure. The observation that reflux, venous stasis, hypoventilation, and incontinence, are also associated with other conditions in which abdominal pressure is increased, including pregnancy and the ascites of cirrhosis, also supports the hypothesis that these problems are related mechanistically to the change in pressure.
Increased intraabdominal pressure (IAP) can be transmitted to the pleural space resulting in changes in cardiopulmonary physiology. The degree of transmission will depend upon the tension developed in the diaphragm. Stretch of the diaphragm resulting from an acute increase in IAP will increase the passive tension in the diaphragm yielding little change in Ppl. However, since obesity develops slowly over time, the diaphragm likely adapts to this stretch, yielding lesser passive tension for the same IAP, and Ppl increases, an event that likely contributes to closure of small compressible airways (see below). Compression of intraabdominal blood vessels by IAH and associated reductions in blood flow to abdominal adipose tissue may also contribute to systemic inflammation (see below).
EFFECT OF OBESITY ON STATIC LUNG VOLUMES
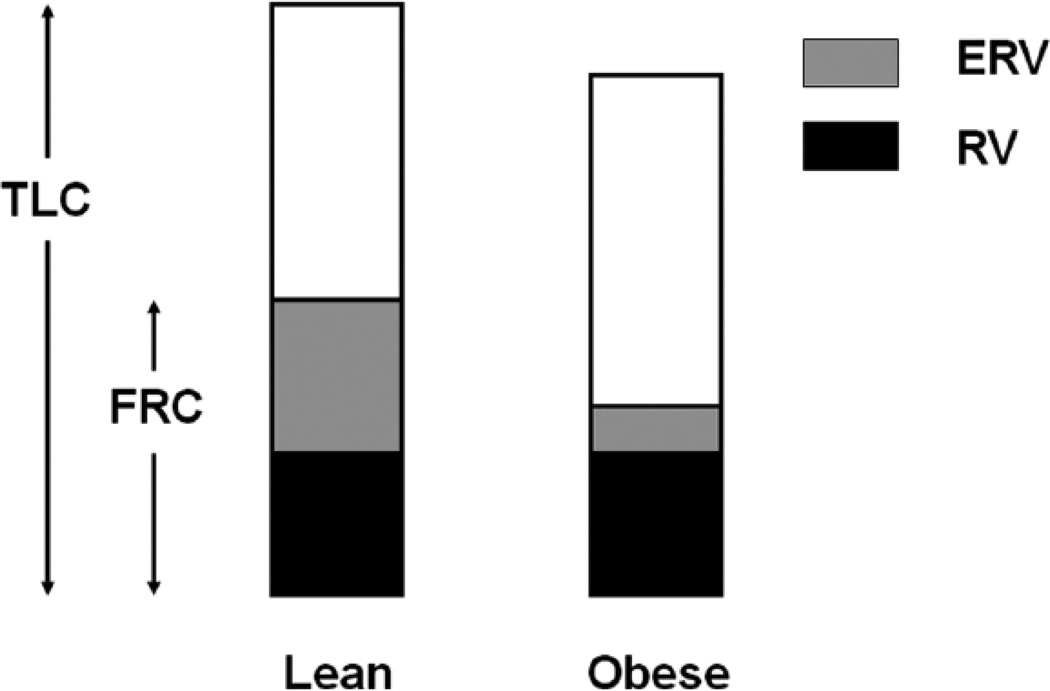
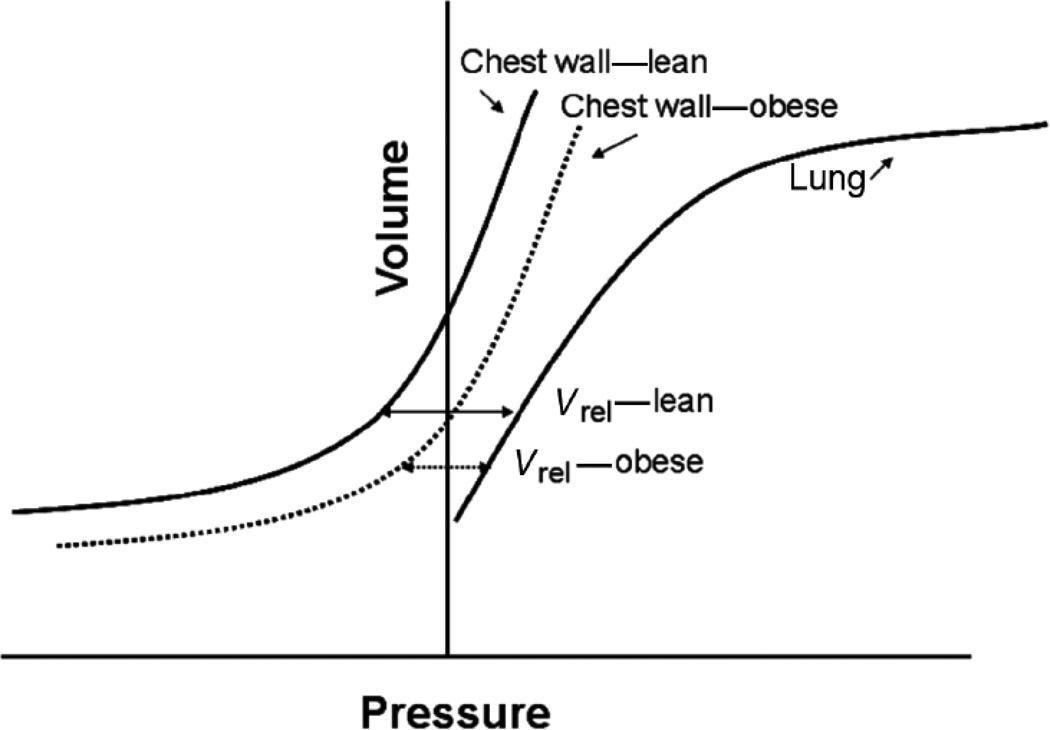
Obesity alters static lung volumes, especially the functional residual capacity (FRC) and the expiratory reserve volume (ERV) (81, 95, 137–138, 200). These changes are represented schematically in Figure 3. Jones et al (81) measured static lung volumes in 373 otherwise normal adults with a range of BMI’s. They reported declines in FRC averaging 22% and 33%, respectively, in mild (BMI 30–35) and morbid (BMI > 40) obesity. Even overweight resulted in a decline in FRC by approximately 10% of predicted values (77, 81). FRC usually occurs at the relaxation volume of the respiratory system, the volume at which the outward recoil of the relaxed chest wall balances the inward recoil of the lung (Vrel). As described above, in obesity there is a shift in the pressure volume relationship of the respiratory system, such that this point occurs at a lower lung volume, and FRC declines (see Figure 4). Caro et al (25) also noted a marked reduction in FRC using chest strapping to model the effects of obesity on respiratory system compliance. However, in obesity, FRC may not be statically determined, especially in the supine posture. Consequently, from this point forward, where relevant, we will sometimes refer to the volume at which inspiration is typically initiated as the EELV. Ofir et al (120) and Romagnoli et al (136) reported that about half of obese subjects exhibit some degree of expiratory flow limitation at rest in the upright posture (i.e. tidal flows overlapped the maximal expiratory flow volume loop) (see Figure 5). Similarly, Ferretti et al reported that approximately 22% of seated obese individuals had flow limitation at rest (53). Under such circumstances, subjects may not be able to expire completely before the next inspiration is initiated, so that absolute lung volume gradually increases until a lung volume is achieved at which expiratory flow rates are sufficient to permit full expiration of the tidal breath. During such dynamic hyperinflation, EELV is above Vrel. Thus, in obesity, dynamic hyperinflation likely prevents even greater reductions in EELV than would otherwise occur. This dynamic hyperinflation is magnified in the supine posture (53). Indeed, whereas normal weight subjects have substantive changes in FRC in going from sitting to supine, FRC decreases very little in the obese (11, 17, 53, 191, 200), likely because of increased flow limitation. Inspiratory muscle braking during expiration may also keep EELV above Vrel in the obese. Sampson and Grassino (144) described increased EMG activity in inspiratory muscles in the early part of expiration in most obese subjects breathing quietly in the upright posture.
Figure 3.
Schematic representation of lung volumes in lean and obese subjects. TLC: total lung capacity; FRC: functional residual capacity; ERV: expiratory reserve volume; RV: residual volume. Reproduced with permission of Elsevier publishers from Figure 1 of Shore and Johnston, 2006 (157).
Figure 4.
Pressure volume relationship of the lung and of the lean and obese chest wall showing how static lung volumes change when obesity alters the pressure volume curve of the chest wall. Vrel: relaxation volume of the respiratory system, volume at which the inward recoil of the lung balances the outward recoil of the chest wall. Obesity-related changes in the relaxation PV curve of the chest result in a reduction of Vrel.
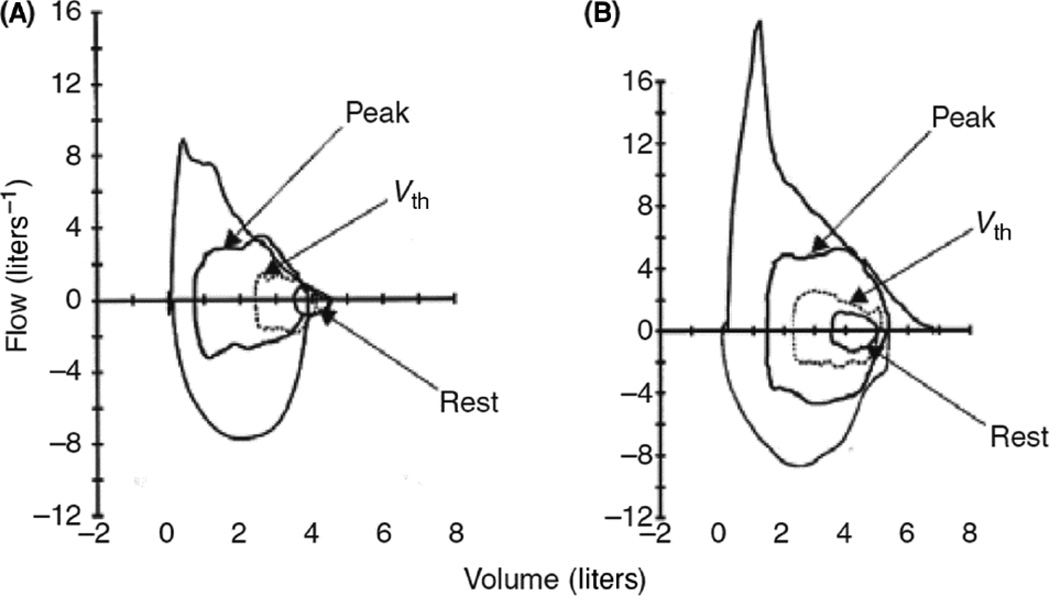
Figure 5.
Flow volume loops during tidal breathing at rest, at anaerobic threshold (Vth), and at peak exercise, as well as the maximum expiratory flow volume loop of an obese subject who was flow limited during tidal breathing both at rest and during exercise (a), and one who was not (b). Reproduced with permission of the Scandinavian Physiological Society from Figure 3 of Romagnoli et al, 2008 (136).
Jones et al (81) reported marked heterogeneity in the impact of obesity on FRC. Such heterogeneity may depend on the extent to which EELV becomes dynamically rather than passively determined, as described above. However, at least some of this heterogeneity depends on the locus of fat distribution. Li et al (95) observed no correlation between the degree of reduction of EELV and the BMI in children, whereas there was a correlation with trunk fat. Babb et al (10) also examined changes in the EELV relative to the distribution of body fat in a small study of adult men and women. They noted that the decrease in EELV was related to visceral fat and anterior subcutaneous fat, and concluded that it was the cumulative effect of increased fat on the chest wall that mattered. Sutherland et al (172) also noted an inverse correlation between EELV or ERV and measures of abdominal obesity in both men and women, whereas there was no correlation with BMI. Similar results were obtained by others (38, 119). Incorporating such data into models of chest wall mechanics in the obese leads to the hypothesis that the reduction in EELV depends on the extent to which adipose tissue mass impinges on the volume of the chest cavity. For example, fat in the buttocks and hips would be expected to have little impact on Vrel. However, the timing of the development of obesity may also be important. Observations on the barrel shaped chests of many patients with emphysema indicate that the thoracic cage has the capacity to adapt its shape over time to accommodate its contents. Similarly, with very gradual increases in abdominal fat, the thorax may slowly remodel so that Vrel changes very little. In contrast, in cases where weight gain is too rapid to permit such remodeling, abdominal fat may encroach into that part of the chest wall cavity normally taken up by the lung, leading to reductions in FRC.
Since residual volume (RV) is usually normal or even elevated in obesity (77, 138, 176), ERV also decreases (see Figure 3). In overweight, mild, and morbid obesity, Jones et al (81) reported declines in ERV averaging 28%, 68%, and 75% respectively of predicted values, such that morbidly obese subjects breathed almost at RV. Under such conditions, the closing volume may exceed the FRC, resulting in gas trapping (16–17, 39, 67). As discussed below, reductions in ERV appear to drive many of the other respiratory system complications that occur with obesity. Importantly, ERV increases with weight loss, as does FRC (15, Refsum, 1990 #993, 52, 131, 177, 184).
In obesity, TLC is usually within the normal range (10, 15, 77, 101, 131, 136, 138, 176). While there are reports of a decrease in total lung capacity (TLC) with obesity, the effect is typically small, and restricted to subjects with morbid obesity (131). Even in those subjects, TLC is usually within 10% of the predicted value (81). TLC is determined by maximal inspiratory muscle strength and the inward recoil of the respiratory system at high lung volume. The mechanistic basis for the decline in TLC is likely the altered mechanics of the chest wall with the increased abdominal mass restricting descent of the diaphragm. There is also increased pulmonary blood volume in obesity (135), which could also reduce TLC by encroaching upon the volume of the chest cavity. Maximum inspiratory pressure (PImax) is often normal in obesity (106, 120, 166), but there are reports of reductions in PImax (36, 193), particularly in morbid obesity (37). As discussed above, systemic oxidative stress is observed in obesity, and oxidative stress is known to impair skeletal muscle function (68). Diaphragmatic contraction is also reduced in the presence of TNFα (64) and elevations in TNFα are also a common component of the systemic inflammation of obesity (83, 113, 198). Metabolic complications within the muscle fibers, for example, insulin resistance, resulting in reduced glucose uptake, and decreased glycogen synthase activity may also contribute (41, 54). If there are changes in diaphragmatic length as a result of the abdominal load, this could place the muscle at a mechanical disadvantage and also lead to reduced PImax (150).
OBESITY AND THE AIRWAYS
There are consistent reductions in FEV1 in the obese. However, the primary determinant of the low FEV1 appears to be not absolute body weight, but waist circumference (93). In a study of approximately 130,000 Parisians, Leone et al (93) noted an approximate 2-fold increased risk of having an FEV1 below the lower limit of normal in subjects who had large waist circumferences, and this was true even in subjects with normal BMI. Similarly, Chen et al (31) reported that in a random sample of 1836 Scottish men and women, FEV1 was inversely associated with waist circumference even after controlling for a number of factors, including weight. Ochs-Balcom et al (119) also noted that FEV1 correlated (inversely) better with measures of abdominal obesity, including abdominal height and waist circumference than with weight or BMI. Lazarus et al obtained similar results both in adults (90) and in children (89). The reason why BMI and weight fair more poorly as predictors of lung function is that fat mass and fat free mass have opposing effects on pulmonary function (89, 109). For example, in children, FVC and FEV1 increase with body weight (89). The effect is likely the result of lean body weight, since after adjustment for height and weight, these measures of pulmonary function actually decrease with increasing % total body fat predicted from skinfold thickness. Lean body weight likely correlates with overall somatic growth including growth of the thoracic cavity.
While FEV1 is typically reduced, the forced vital capacity (FVC) is comparably reduced, and the FEV1/FVC ratio is usually normal (24, 31, 35, 81, 93, 119, 122, 165, 172), although Leone et al (93) did note a small (about 13%) but statistically significant increased risk of having a lower than normal FEV1/FVC ratio in subjects with elevated waist circumference. Both FEV1 and FVC improve with weight loss, although the changes are usually small (15, 40, 42, 52, 131–132).
Others have emphasized that the typically normal FEV1/FVC ratio associated with this pattern of pulmonary function changes does not necessarily exclude airway obstruction (74, 140, 164). Indeed, Hyatt et al (74) noted that 56% of subjects with a similar “nonspecific pattern” of pulmonary function tests had airway hyperreactivity and suggested that this “volume derecruitment” pattern comes about as a result of scattered diseased airways with low ventilation in an otherwise normal lung. These airways close soon after a forced expiration begins, but the remaining lung empties through normal airways, producing a normal FEV1/FVC ratio. As described above, based on measurements of ventilation distribution using xenon, Holley et al (70) concluded that airways in the dependent parts of the lungs of upright obese individuals are likely to be closed, at least at FRC. Other evidence of peripheral airway narrowing or closure in the obese is discussed below.
Douglas et al (48) also noted marked frequency dependence of dynamic compliance as well as evidence of gas trapping (measured by nitrogen washout) in group of obese subjects who had low ERV, and concluded that there was closure of small peripheral airways in dependent lung regions. Rubinstein et al (138) noted that maximal expiratory flow rates (V̇max) were reduced in obese versus lean individuals. As discussed by Salome et al (142), such reductions could be expected simply on the basis of reductions in VC, so it is notable that in the male subjects, V̇max at 25% of VC was reduced even after normalization for VC, consistent with peripheral airways obstruction. There was also an increase in the RV and the RV/TLC in both men and women ratio consistent with small airway closure and gas trapping. An increase in RV or the RV/TLC ratio in obesity has also been reported by others (140, 151). The observations of others (39, 52, 62) that closing capacity exceeds the EELV in many obese individuals, even in the upright posture, also indicates small airway closure in the obese during tidal breathing.
Airway resistance (Raw) is elevated (86, 191, 200, 206) in the obese. Because the retractive forces of the lung parenchyma on the airways are reduced at low volume, airway caliber decreases as absolute lung volume declines (23). Consequently, obesity-related reductions in airway caliber may simply be the result of reductions in EELV. To address this issue, Zerah et al (206) measured airway conductance (Gaw, the inverse of Raw) in a group of 46 overweight and obese subjects. No normal weight subjects were included. They noted a linear relationship between airway conductance (Gaw) and EELV among this group, and when specific conductance (sGaw) (which takes into account the volume dependence of Gaw) was assessed, no relationship between sGaw and BMI was observed, although there was marked variability in sGaw for any given BMI. No measurements of the distribution of body mass were made, so it is not possible to determine whether a relationship would have been observed between sGaw and measures of abdominal obesity, as has been observed with FEV1 and FVC (93). In contrast to the results of Zerah et al (206), King et al (86) did observe a significant decline in Gaw with BMI, even after correction for absolute lung volume, and concluded that other non volume-related effects on airway caliber must also contribute to increases in resistance among the obese. Indeed, by their calculations, only 10% of the obesity-related increase in Raw could be explained on the basis of the reduction in FRC. The study by King et al was larger (256 subjects), and the subjects restricted in age (28–30 years), thus reducing the variance, so the study may simply have been better powered to detect differences. It may also be important that King et al selected their subjects randomly, whereas Zerah et al restricted their subjects to those without respiratory symptoms. Obese subjects with respiratory symptoms may be those in whom declines in airway caliber are most marked.
Yap et al (200) used changes in lung volume that occur between the sitting and supine position to determine whether obesity-related changes in lung volume account for observed increases in Raw. They noted that in lean subjects, FRC decreased by about 0.65 L upon adopting the supine posture, consistent with gravitational effects of the abdomen and their impact on Vrel. The decrease in FRC was associated with a reduction in the total respiratory system conductance (Grs, the inverse of resistance) measured by forced oscillation at 6 Hz, from 0.48 to 0.34 L/s/cm H2O, that was consistent in magnitude with the fall in FRC (200). In the obese subjects, Rrs averaged 0.20 L/s/cm H2O even in the sitting position. Since the absolute volume of the seated obese and the supine lean subjects were similar, this Grs was substantially lower than could be expected on the basis of changes in lung volume alone. Watson and Pride (191) obtained similar results (see Figure 6). These results, similar to those of King et al (86), suggest that factors other than changes in lung contribute to airflow obstruction in the obese.
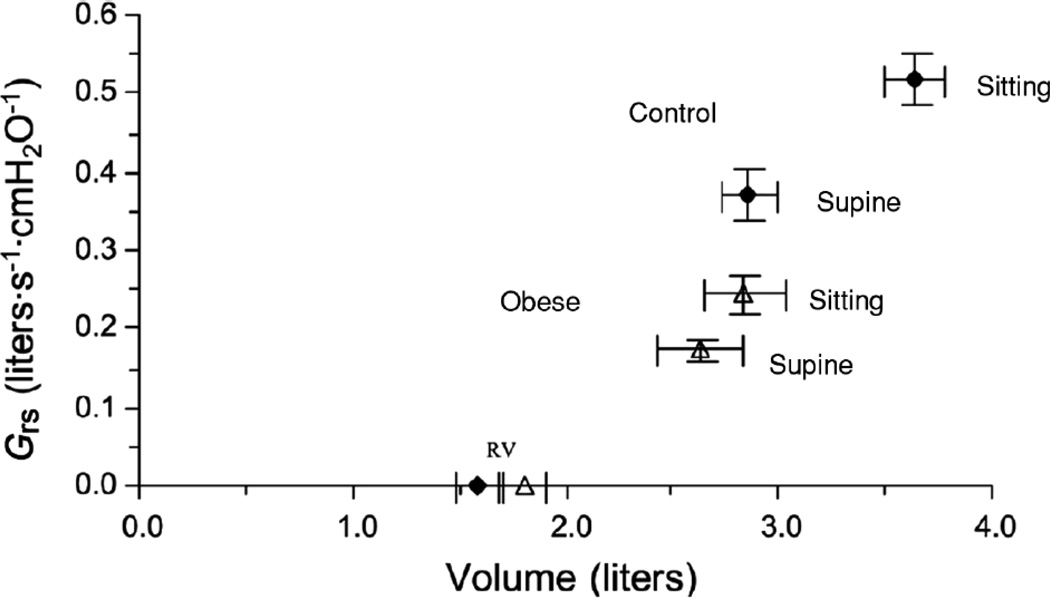
Figure 6.
Postural changes in mean total respiratory conductance (Grs) plotted against the midtidal lung volume (MTLV) in 10 obese subjects and 13 control subjects, both sitting and supine. Symbols on horizontal axis indicate mean values of RV. Control subjects are represented by closed symbols and obese subjects are represented by open symbols subjects. MTLV in control subjects was from records of tidal volume and inspiratory capacity, assuming that TLC measured by body plethysmography in the seated position fell by 200 ml when supine. In the obese subjects, TLC measured by multibreath helium dilution in both postures was used as the reference volume to derive MTLV. Reproduced with permission of the American Physiological Society from Figure 2 of Watson and Pride, 2005 (191).
What are these non volume dependent factors? It has been postulated that airway narrowing in the obese may be the result of increased intrapulmonary blood volume (135). Higher pulmonary artery pressures would be expected to increase fluid flux into the pulmonary interstitium, leading to cuffing of fluid around airways, an event that would uncouple the airways from the parenchyma, exacerbating airway narrowing. As discussed above, small airway closure is observed in many obese subjects during tidal breathing (62). Others have suggested that repeated opening and closing of peripheral airways could cause rupture of alveolar attachments to bronchioles (108), which would also uncouple the airways from parenchymal tethering. Such uncoupling, whether from loss of alveolar attachments or from airway edema may explain observed reductions in the bronchodilating effect of deep breath in the obese (21, 69). Other effects of breathing chronically at low lung volume may also occur, such as remodeling of airway smooth muscle (ASM) cells, allowing them to generate increased force at shorter lengths. The lower than normal tidal volume (VT) observed in many obese (see below) may also contribute to airway narrowing. When activated, for example by normal parasympathetic tone, ASM shortens as a result of actin-myosin crossbridge cycling. Tidal breathing stretches ASM causing crossbridges to detach and resulting in bronchodilation (60). Lowering VT in obesity would be expected to reduce the efficacy of this natural bronchodilating mechanism. Crossbridge attachment also stiffens ASM, making it harder to stretch. Hence, obesity could lead to a self sustaining loop: small ASM strain (because of small VT) leads to greater ASM stiffness, and greater stiffness leads to even less ASM strain with each tidal breath (see Figure 7). The net result would be more substantial ASM contraction and consequent airway narrowing. It is also possible that aspects of the systemic inflammation that characterizes obesity (see above) may contribute to airway narrowing in obesity. For example, endothelin is elevated in serum of obese individuals (105, 195), likely as a result of systemic oxidative stress acting on the vascular endothelium (99). Endothelin is a potent bronchoconstrictor (29, 178).
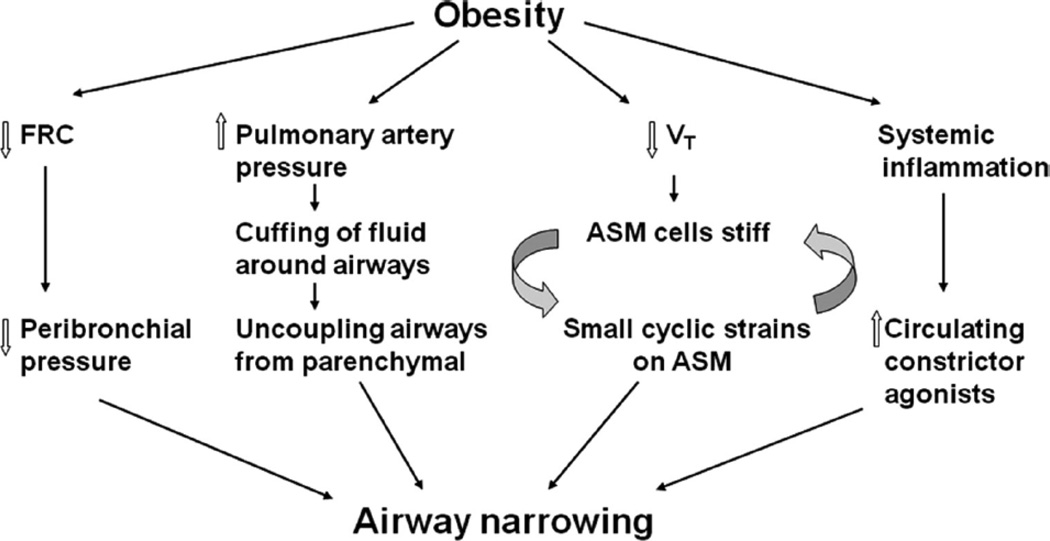
Figure 7.
Factors promoting airway narrowing in obesity. In the obese, functional residual capacity (FRC) is reduced leading to decreased peribronchial pressure. Tidal volume (VT) is also reduced. The decrease in VT reduces the amount of airway smooth muscle (ASM) strain that occurs with each breath. Less strain results in fewer detachments of myosin from actin, and a stiffer muscle. The stiffer the muscle, the more difficult it is to strain it. There is increased pulmonary blood volume in obesity Consequent increases in pulmonary artery pressure will increase fluid flux into the pulmonary interstitium resulting in cuffing of fluid around airways. This uncouples the airways from the retractive forces of the lung parenchyma further increasing ASM shortening. There may also be increases in circulating factors, such as endothelin, that can directly constrict ASM. The result is more ASM shortening and consequent reductions in airway caliber.
Age-related decline in lung function
Obesity also impacts the normal age-related loss of lung function. Pistelli et al (127) measured FEV1 and FVC in 1212 adult subjects on two occasions, approximately 8 years apart. Compared to subjects who were never obese during this 8 year period, subjects who became obese had a more rapid decline in lung function, whereas subjects who were initially obese but lost weight during this period had a lower rate of loss of lung function. Longitudinal studies by Chen et al (32) and Chinn et al (33) obtained similar results. In each of these latter studies, the investigators examined FEV1 and FVC changes in association with changes in body weight in approximately 1000 adults over 6–7 years. After controlling for other factors including age, a 1 kg increase in weight was associated with a reduction in FEV1 of 17.6 (33) or 23 ml (32) in men. The effect was smaller in women (14 ml/kg) (32). There are two possible explanations for these results. First, since obesity itself is known to reduce FEV1 and FVC (see above), subjects who gain weight would be expected to have greater declines in lung function over time than subjects who do not: the effects of weight gain on lung function would be added to the normal age-related decline in lung function. Alternatively, obesity may actually accelerate the normal age-related decline in lung function. The data of Pistelli et al (127) provide some insight into this issue, since in their study, they also included a group who were always obese. This group also had a significantly greater decline in FVC and VC but not FEV1 than the group who were never obese. The data of Chen et al (32) also indicate that the increase in the rate of decline in lung function in subjects who gained weight compared to those who did not is more than twice that which would be expected based solely on the increase in weight. Taken together, the data suggest that the presence of obesity does indeed accelerate the rate of decline in VC that typically occurs with aging, In other studies, declines in lung function over time in young adults aged 20–29 have been related to aspects of systemic inflammation (e.g. serum C-reactive protein (CRP)), even after controlling for BMI (129). These results suggest that the more rapid decline in lung function observed in the obese may be the result of their low grade systemic inflammation.
Airway responsiveness
Several investigators have examined the impact of obesity on airway responsiveness in adults or in children (see (156) for review). The results of these studies are mixed. Some have shown that obesity is a risk factor for airway hyperresponsiveness (AHR), while others report no effect of BMI on responsiveness (28, 34, 147, 163). As discussed (156), there are methodological issues that may contribute to the lack of consistency in the effect of obesity on airway responsiveness, including the method of accrual of the subjects, the agonist used to induce bronchoconstriction, and whether or not gender differences were considered. Also, studies addressing the impact of obesity on airway responsiveness have used BMI but not measures of the distribution of adiposity as the measure of obesity. Abdominal obesity appears to be a better determinant of other changes in lung function than BMI. The outcome indicator used to assess airway narrowing may also be important. For example, Salome et al (143) were unable to observe any difference between obese and non-obese individuals in methacholine-induced changes in FEV1 or Rrs, whereas obese subjects did have greater increases in respiratory system reactance. In a follow up study, the authors demonstrated that this was likely the result of greater methacholine-induced airway closure and consequent hyperinflation in the obese (30). Similarly, Sutherland et al (171) reported greater methacholine induced increases in FRC and reductions in inspiratory capacity (IC) in obese versus lean asthmatics. One of the consequences of localized airway closure in dependent regions of the lung is dilation of the airways in the rest of the lung, since these areas now receive a greater portion of VT (70), leading to greater stretch of airway smooth muscle and subsequent bronchodilation (196) (see above). The FEV1 is unlikely to capture the complexity of such heterogeneous airway changes.
Aaron et al (1) examined the effect of weight loss on AHR in obese individuals. Although there was a trend towards an improvement in AHR after an average 17.4% reduction in body weight, the effect was not significant. The study was small (50 patients), and may not have been sufficiently powered to observe an effect on AHR, especially given the heterogeneity of the response. Indeed, within their population, the authors noted several individuals with fairly marked reductions in airway responsiveness after weight loss.
In contrast to the inconsistent results in human subjects, AHR to intravenous methacholine is observed in both genetically obese mice and in mice with diet induced obesity (79–80, 103, 134, 158) (see Figure 8). The mechanistic basis for the AHR observed in obese mice has not yet been established. As previously discussed, it is possible that mechanical factors related to reductions in absolute lung volumes may be involved (80, 155). There is no overt cellular inflammation in the lungs of unchallenged obese mice (80, 103), but it is possible that there are differences in the inflammatory state of resident immune and inflammatory cells that may promote AHR. It is also possible that adipokines contribute to obesity-related AHR (156). For example, exogenous administration of adiponectin attenuates (160), while leptin augments (159) allergen induced AHR in mice.
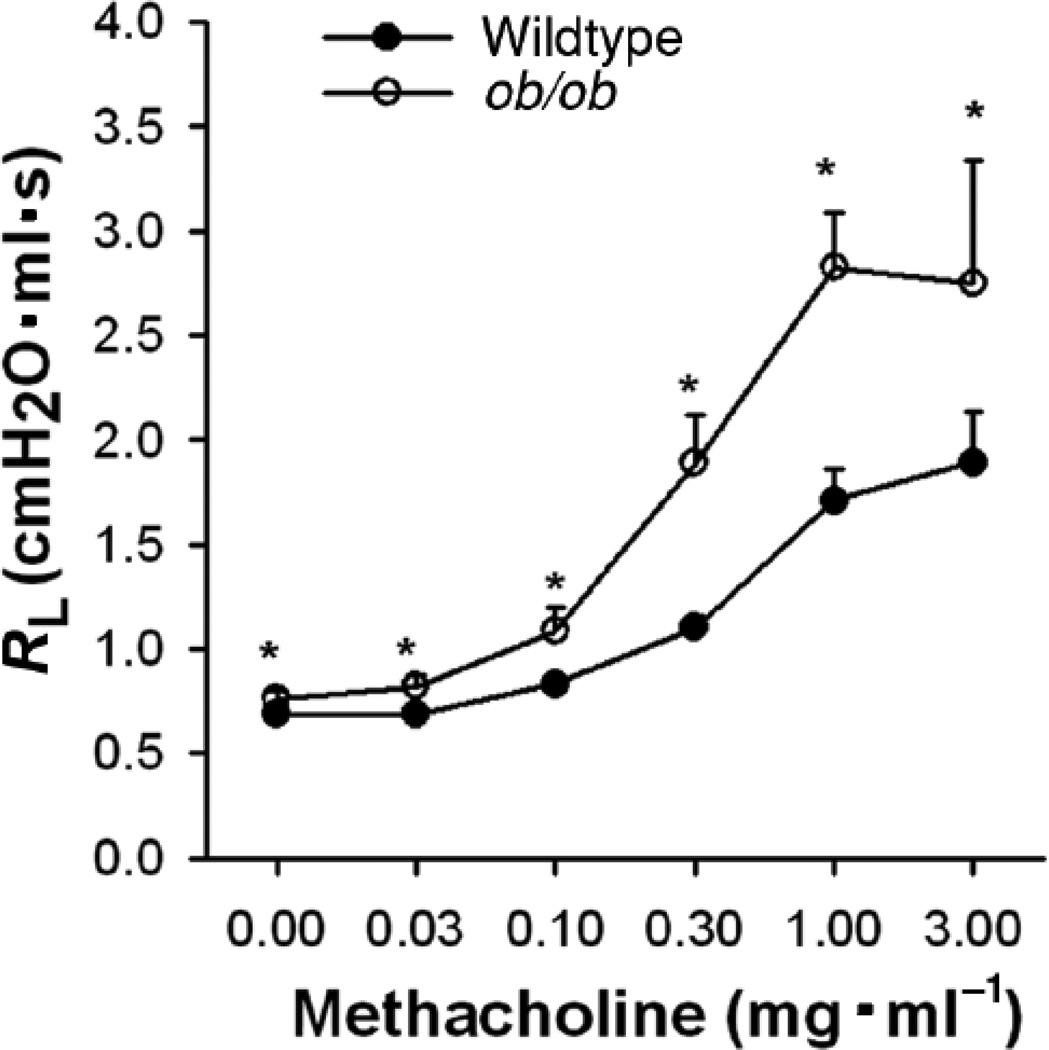
Figure 8.
Changes in pulmonary resistance (RL) induced by i.v. methacholine in obese ob/ob mice and their lean age and gender matched wildtype controls. Data are mean ± SE. *p<0.05 versus wildtype. Adapted from Figure 1 of Shore et al, 2003 (158) and reproduced with permission of the American Physiological Society.
Obesity and asthma
Within the last decade, it has become apparent that obesity is a risk factor for asthma. More than 50 cross-sectional studies performed in adults and children throughout the world demonstrate an increased prevalence of asthma in the obese and overweight (reviewed in (18, 55, 154, 157)). Importantly, 16 of 17 prospective studies involving more than 200,000 adults and children indicate that obesity antedates asthma (18, 55, 98, 154, 157). It unlikely that these results are simply the result of differences in symptom perception in the obese or misdiagnosis of exertional dyspnea as asthma. Obese and lean subjects do not differ in their ability to perceive bronchoconstriction (44, 94). Moreover, extensive phenotyping of subjects with a doctor’s diagnosis of asthma showed no difference in the rate of misdiagnosis of asthma between obese and lean subjects (2). Obesity also worsens asthma control, and some, but not all studies indicate an increased severity of asthma in the obese (see (153) for details). The observation that either surgical or diet-induced weight loss improves asthma outcomes, including prevalence, severity, use of asthma medications, hospitalizations, and flow rates (1, 45–47, 63, 107, 114, 117, 167), provides additional support for a relationship between obesity and asthma.
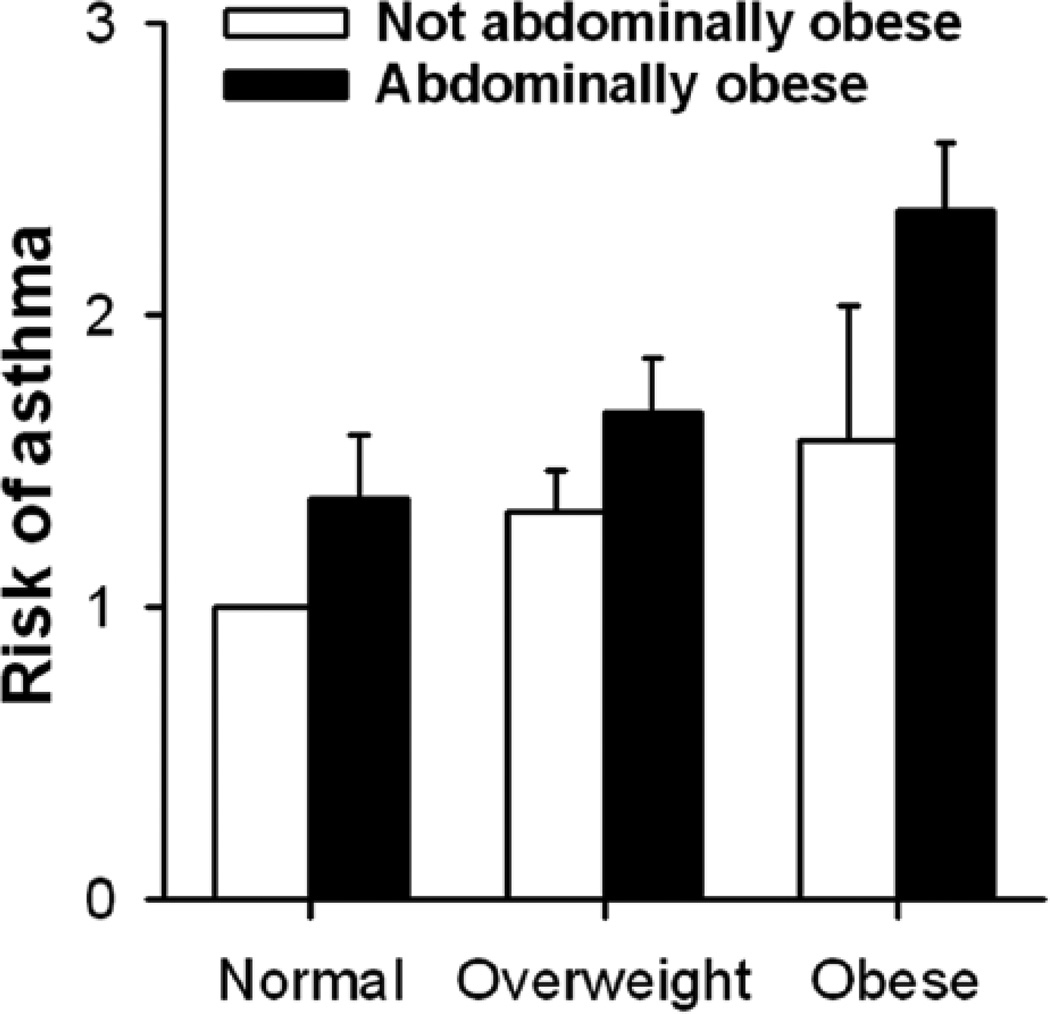
As discussed elsewhere in this chapter, abdominal obesity appears to be particularly important for obesity-related changes in lung volume, in the partial pressure for oxygen in arterial blood (PaO2), and in dyspnea. The same is true for obesity-related asthma. In a large epidemiological study involving approximately 88,000 women in the California Teachers Study cohort, Von Behren et al (186) reported increased asthma prevalence in obese versus lean women. Even among the obese women, the odds ratio for having asthma was greater in those who were abdominally obese versus those with normal waist circumference. The risk of asthma was also increased in those women who had a large waist circumference but a normal BMI (see Figure 9).
Figure 9.
Odds ratios (adjusted for race/ethnicity, age, and smoking) for adult-onset current asthma as a function of body mass index, stratified by abdominal obesity, among California Teachers Study cohort members. Errors bars indicate the upper limit of the 95% confidence interval. Adapted from Table 3 of von Behren et al, 2009 (186).
In adults, there is increasing evidence that obesity-related asthma is typically non-allergic in nature. For example, in adults, obesity increases the risk of asthma, but not other atopic diseases (22, 76, 149, 187–188), though the data are less consistent in children (27, 146). Similarly, increases in asthma morbidity in overweight and obese subjects are found almost exclusively in the non-atopic asthma patients (5, 88). Finally, sputum eosinophils, a marker of allergic asthma, have been reported to decline with increasing BMI in asthmatics (182).
OBESITY AND GAS EXCHANGE
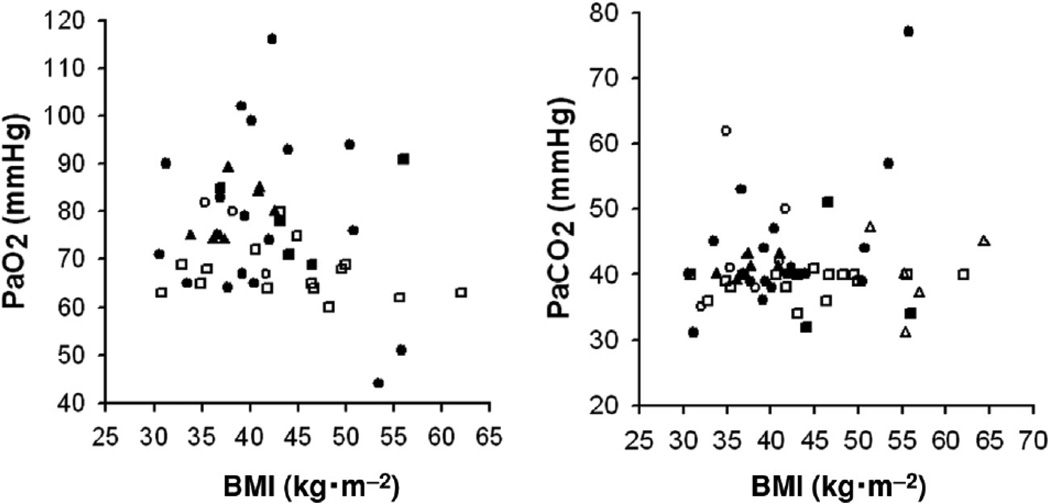
Hypoxemia is common in the obese. Kaufman et al (84) were among the first to systematically examine gas exchange in upright obese subjects. They measured arterial blood gases in 18 obese subjects with a mean BMI of 41 (range 30.5 – 50.8). Mean PaO2 was 78 mm Hg and mean PaCO2 was 44 mmHg. However, there was substantial heterogeneity in their population. One third had normal blood gases. Two thirds had hypoxemia, which they defined as a PaO2 less than 90 mmHg. In many individuals, PaO2 was low enough to elicit polycythemia. Of those with hypoxemia, two thirds had normal PaCO2, while one third had hypercapnia, which they defined as a PaCO2 > 45 mmHg. The term “obesity hypoventilation syndrome” (OHS) is used to describe this hypoxemic and hypercapnic group which will be discussed separately below. A recent analysis combining data from 41 studies confirms that obesity causes hypoxemia (204): in 626 awake, morbidly obese individuals (average BMI of 48) studied in the upright posture, PaO2 averaged 81 mmHg. In contrast, average PaCO2 was 41 mm Hg, within the normal range. Analysis of data from 6 studies of obese subjects studied in the upright posture and for which individual values of height, weight, and PaO2 were reported (57, 70, 84, 101, 141, 144), indicates that even mild obesity can be associated with hypoxemia (Figure 10). Obese subjects also undergo more desaturation at altitude than lean subjects (133).
Figure 10.
PaO2 (left) and PaCO2 (right) as a function of BMI. Data are compiled from 6 studies (57, 70, 84, 101, 141, 144) in which either PaO2 or PaCO2 or both were available on individual obese subjects in the seated upright posture. Each study is indicated by a different symbol: closed circles, Kaufman et al; open circles, Holley et al; closed squares, Said; open squares, Sampson and Grassino; closed triangles, Lotti et al; open triangles, Fritts et al.
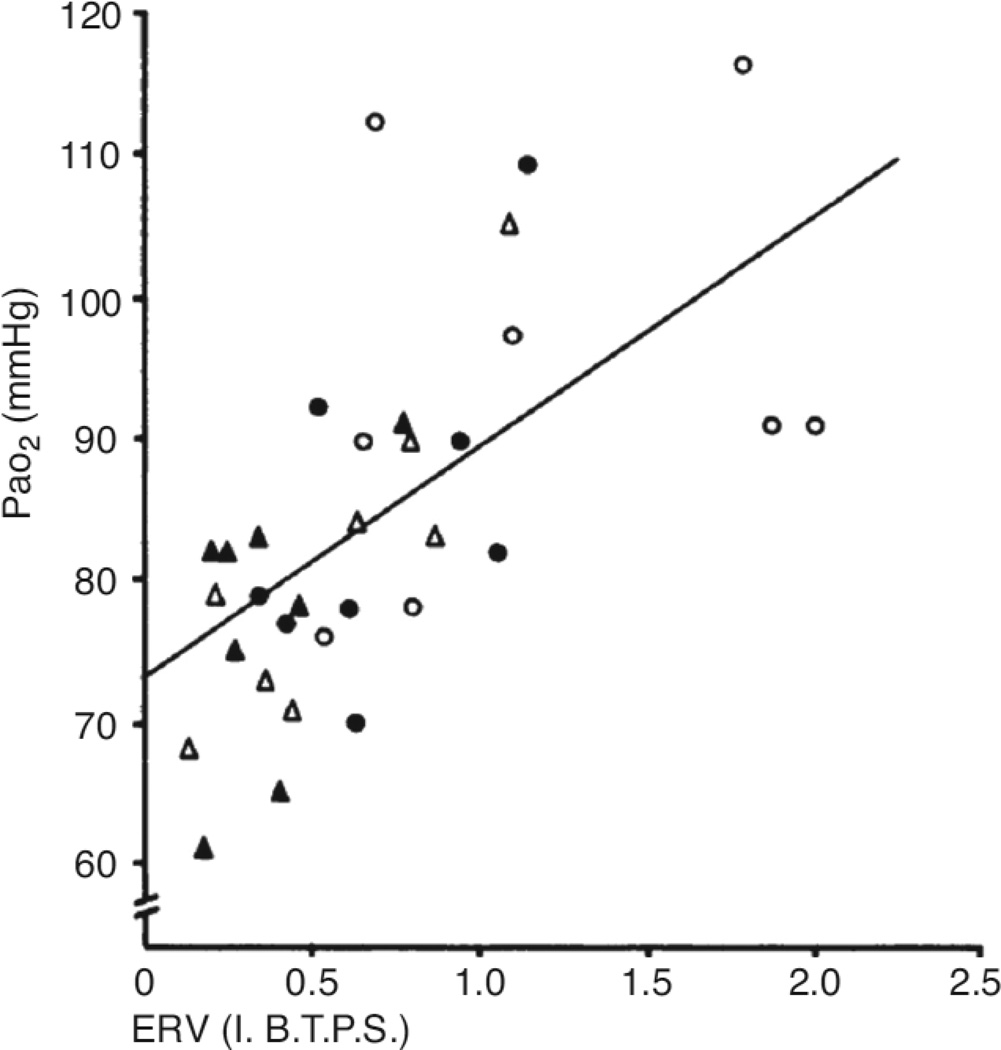
As indicated above, there is heterogeneity in the impact of obesity on PaO2. However, the magnitude of hypoxemia does not appear to depend on the magnitude of body weight. As shown in Figure 10, there is no strong relationship between PaO2 and BMI: individuals with BMI’s in the low 30’s can be substantially hypoxemic, while others with BMI’s in the 40’s or 50’s are not (Figure 10). Indeed, Jenkins and Moxham (77) noted mild hypoxemia even in overweight individuals. Instead, the distribution of body fat and its impact on absolute lung volume appears to be what drives changes in PaO2. For example, Zavorsky et al (204–205) noted that in obesity, there was a strong correlation between PaO2 and the waist:hip ratio: 30% of the variance in PaO2 could be explained on the basis of this ratio. They also noted that on average, obese women had better oxygenation than obese men, in large part because of differences in the distribution of body fat (205). For the same BMI, women had lower waist/hip ratio’s. In women, fat mass is more often concentrated around the hips, whereas in men, it is usually concentrated around the abdomen. As discussed above, others have shown that this central abdominal pattern is associated with reductions in ERV (10, 38, 95, 120, 172). Importantly, in obesity, PaO2 correlates well with FRC and with ERV (125) (see figure 11). Following weight loss, changes in PaO2 also correlate well with changes in ERV (15, 52, 177, 184). Pelosi et al (124) also noted that moving obese subjects from the supine to the prone position, which was associated with a marked (almost 1 L) increase in FRC, also resulted in an increase in PaO2 (124). At low lung volumes the closing volume may exceed the FRC, resulting in airway closure and consequent reductions in PaO2 (see below).
Figure 11.
Relation between arterial PaO2 and expiratory reserve volume (ERV) in eight obese subjects. Regression line of closest fit is shown (y = 73.9 + 15.8 x; n = 32; S.D. from regression ± 10–8 mm Hg). For key to symbols see fig. 1. Closed circles: sitting before fasting. Open circles: sitting after fasting. Closed triangles: supine before fasting. Open triangles: supine after fasting. Reproduced from Figure 2 of Farebrother et al, 1972 (52) with permission of the British Medical Association.
As discussed above, in the majority of cases, obesity-related hypoxemia occurs in the absence of hypercapnia, indicating that alveolar hypoventilation is not a major cause of hypoxemia in most obese individuals. Instead, alterations in the ventilation to perfusion (V/Q) ratio appear to account for the hypoxemia associated with simple obesity. Holley et al (70) measured the distribution of ventilation and perfusion in 8 seated resting obese subjects using radioactive xenon. In 4 subjects, tidal ventilation increased with vertical distance down the lung, similar to the situation in lean individuals. However, in the other 4 subjects, the opposite occurred: the upper regions of the lung received relatively more of the tidal breath than the lower regions. The variability in the distribution of ventilation did not appear to be related to the degree of obesity, but was related to obesity-related changes in the ERV. Those 4 obese subjects with the reversed pattern of ventilation distribution had substantially lower ERV than the 4 with relatively normal ventilation distribution. (21% vs 49% of predicted ERV). Indeed, the authors noted that a similar reversal of ventilation distribution occurs when lean subjects take a tidal breath from low lung volume. By contrast, when the obese subjects took a full rather than a tidal inflation, the abnormal distribution of ventilation was reversed, presumably because this inflation opened otherwise closed airways at the bases of the lungs. The authors also observed a linear increase in perfusion with vertical distance down the lungs, consistent with observations in lean subjects. However, the slope of this relationship was only about half that observed in lean subjects, indicating that the distribution of perfusion was more uniform than in lean subjects. As described by Rochester et al (135), total blood volume increases in obesity, as does cardiac output, and the pulmonary circulation must accommodate the increase in venous return. Consequently, there is likely recruitment of previous underperfused vessels. However, it is also possible that the systemic inflammation that accompanies obesity (see above) interferes with the hypoxic pulmonary vasoconstriction that should have occurred in the lower, less well ventilated parts of the lungs.
Barrera et al (13) used nitrogen and helium washout curves to measure regional ventilation and perfusion in obese and lean subjects and also concluded that the hypoxemia observed in their obese subjects resulted from overperfusion of either underventilated or completely unventilated regions of the lung. The alveolar-arterial O2 tension difference ((A-a)DO2) measured in their obese subjects averaged 32.5 versus 10.5 mmHg in their lean subjects. Others have also reported a marked increase in (A-a)DO2 in obesity, and noted that the (A-a)DO2 correlates well with waist/hip ratio (204–205). This elevated (A-a)DO2 declines with exercise in obese subjects (204), likely because of recruitment of previously atelectatic lung units with increases in VT.
Yamane et al (199) measured blood gases in superior and inferior pulmonary veins of normal supine human subjects with varying BMI’s. PO2 averaged only 71 and 73 mm Hg in the left and right inferior veins, versus 92 mmHg in both the left and right superior veins. The mean PO2 in the inferior veins was related to the subject’s BMI, whereas this was not true of the PO2 in the superior veins. They concluded that the regional hypoxemia resulted from V/Q mismatch caused by closure of dependent (inferior) airways. Importantly, none of their subjects had a BMI exceeding 30, indicating that even overweight can induce hypoxemia in the supine posture.
Although overall minute ventilation (V̇E) appears to be well matched to resting metabolism in obese subjects, and most are normocapnic (see above), alterations in the pattern of tidal breathing are often observed. In particular, VT is often reduced and breathing frequency increased (15, 35, 84, 120, 135, 144), although this is not universally observed (101, 166). Obese rodents also use this pattern of breathing (157, 174). During resting tidal breathing, such a breathing pattern minimizes the work of breathing in situations such as obesity when respiratory system compliance is reduced. Obese subjects may adopt this rapid shallow breathing pattern for this reason (59, 152). Indeed this breathing pattern is typically adopted when experimental subjects breathe against an elastic load (20).
Sampson and Grassino (144) used magnetometers to measure rib cage and abdomen displacements during tidal breathing in upright obese subjects. Compared to lean subjects, displacements of the rib cage accounted for more of the tidal breath in the obese. Indeed, abdominal displacements contributed only 11% of the total breath, whereas in lean subjects the abdominal component typically averages 35% of VT. The results are consistent with the abdomen being much less compliant than the rib cage in obesity, and are also consistent with the reduced ventilation to dependent regions of the lung observed by Holley et al (70).
Implications of hypoxemia for the systemic inflammation of obesity
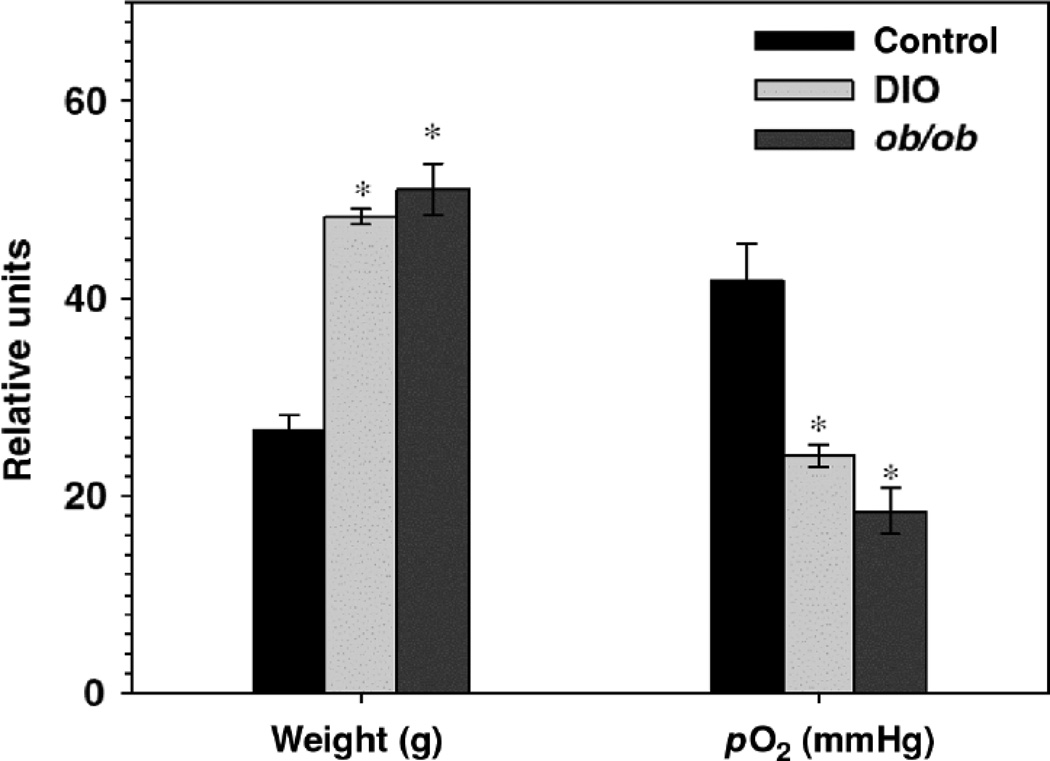
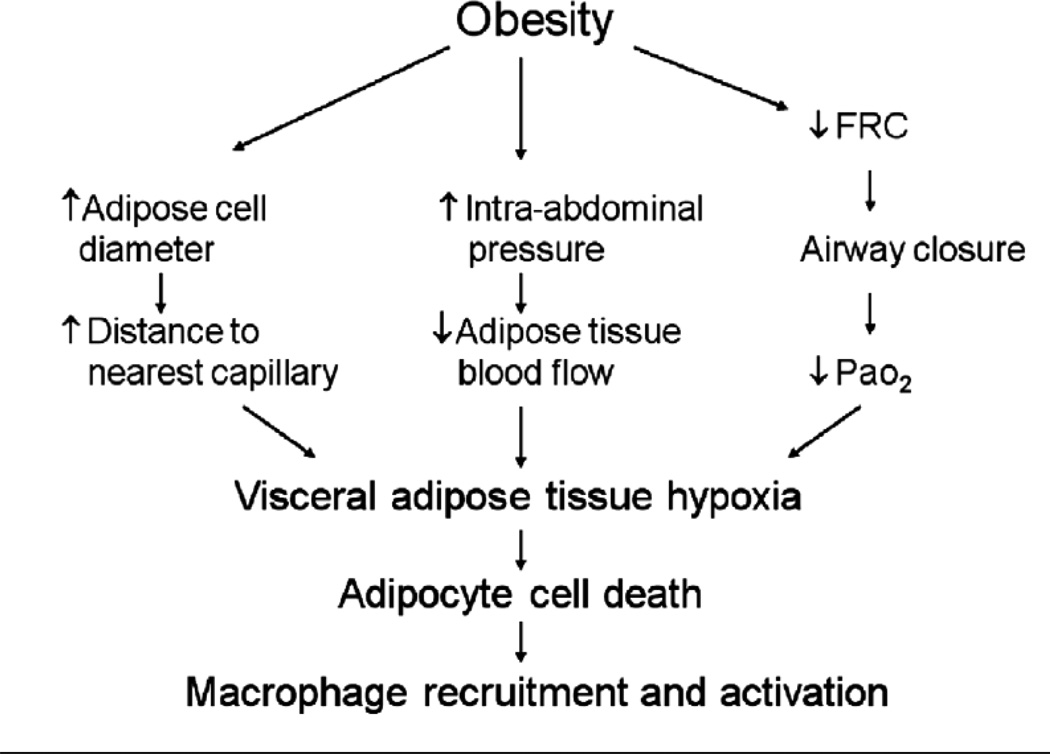
As described above, in obesity, macrophages infiltrate the adipose tissue and are thought to be the source of many of the inflammatory moieties that are elevated in the serum. Histological data indicate that these ATM’s surround necrotic adipocytes (168). The current paradigm is that as expansion of adipocytes occurs, the distance between adipocytes and capillaries increases. The adipose tissue thus becomes hypoxic (see Figure 12), which may lead to adipocyte cell death and subsequent recruitment of macrophages (71, 130, 202). Adipose tissue hypoxia also appears to promote systemic oxidative stress, since in mice, adipose expression of glutathione peroxidase 3 (GPx3), the major anti-oxidant activity in plasma, is suppressed by hypoxia. The functional significance of GPx3 is illustrated by data showing that in mice, overexpression of GPx3 in adipocytes improves high glucose-induced insulin resistance (92).
Figure 12.
In vivo adipose tissue oxygen status. Body weight and adipose tissue pO2 determined by a fluorometric tissue oxygen sensor for lean (control, C57BL/6J) and obese ob/ob mice as well as mice with diet induced obesity (DIO). (n=4 per group; all data expressed as mean+/−s.e.m.). Reproduced from Figure 6d of Rausch et al, 2008 (130) with permission of the Nature Publishing Group.
It is increasingly recognized that systemic inflammation contributes to the development of obesity-related conditions such as type 2 diabetes and cardiovascular disease, and that abdominal obesity has a greater impact on disease risk than other types of body fat distribution. It has been argued that this increased risk is the result of greater inflammatory potential of visceral than subcutaneous fat (4, 189). Such arguments ignore the respiratory complications of obesity. As discussed above, the hypoxemia of obesity appears more closely related to changes in the ERV than to measures of adiposity per se, and patterns of abdominal obesity promote changes in the ERV. Hence, it is conceivable that abdominal obesity increases disease risk by reducing ERV, leading to airway closure in dependent regions of the lung, and consequent V/Q mismatch. The resulting hypoxemia could be expected to amplify tissue hypoxia, resulting in increased adipocyte cell death and its attendant inflammation. As discussed above, IAH is also frequently observed in the obese, at least in the supine posture. With IAH, abdominal blood vessels may be compressed leading to reduced blood flow to intra-abdominal adipose tissue, which may further amplify tissue hypoxia and adipocyte cell death (Figure 13). The observation that perfusion of abdominal adipose tissue, but not skeletal muscle, is reduced in obese versus lean mice, supports this hypothesis (71).
Figure 13.
Schematic diagram showing potential mechanisms whereby obesity may lead to visceral adipose tissue hypoxia, and subsequent adipocyte necrosis and macrophage recruitment. PaO2: arterial partial pressure for oxygen.
Obesity hypoventilation syndrome
As described above, in most obese subjects, PaCO2 is in the normal range. However, in about 10% of obese subjects, hypercapnia does occur. Obesity hypoventilation syndrome (OHS) is defined as a BMI ≥ 30 in conjunction with awake arterial hypercapnia (PaCO2 ≥ 45 mm Hg) in the absence of other known causes of hypoventilation. The term “Pickwickian Syndrome” was coined to describe these patients (19) who often also have obstructive sleep apnea syndrome (OSAS) and exhibit excessive daytime, sleepiness, fatigue, and morning headaches. In addition, severe hypoxemia leads to pulmonary hypertension and right sided heart failure and this syndrome is associated with greater mortality and morbidity than simple obesity (110–111, 121).
The mechanistic basis for hypoventilation in these subjects is multifactorial. Respiratory system compliance is reduced to a greater extent than in subjects with simple obesity (152), and both lower (see above) and upper airway resistance are also elevated (97). These factors lead to an approximate 3-fold increase in the work of breathing. It has been speculated that subjects with OHS choose to hypoventilate in order to avoid expending large amounts of energy breathing, similar to some patients with chronic obstructive pulmonary disease. Changes in central respiratory sensitivity to CO2 also appear to play an important role. Various investigators have used either changes in the diaphragmatic EMG, or changes in the P0.1 to assess central respiratory sensitivity to CO2. P0.1 is the pressure measured at the airway opening in the first 0.1 seconds after an airway occlusion that occurs at the onset of inspiration, and is a measure of respiratory muscle activation. By making the measurement in the 1st 0.1 seconds after occlusion, reflex changes in muscle activation that occur in response to the occlusion are avoided. In obese subjects without OHS, the diaphragmatic EMG and P0.1 responses to CO2 are actually greater than in lean subjects (100, 144–145, 166). This increased drive may be what allows these obese subjects to maintain eucapnia despite an increased load on the respiratory system that would otherwise reduce their ventilatory output. In contrast, in subjects with OHS, the EMG and P0.1 response to CO2 is similar to what is observed in lean subjects (100, 145). In the face of their increased respiratory load, this results in a reduced ventilatory response, and hypercapnia ensues.
Several mechanisms have been proposed to account for the altered hypercapnic drive in patients with OHS. Mice genetically deficient in leptin, a satiety hormone, are substantially obese. These mice have reduced ventilatory responses to CO2, which can be corrected with exogenous administration of leptin, suggesting that leptin augments central respiratory drive (174–175). Although leptin deficiency is a very rare cause of obesity in humans, there can be central leptin resistance, which would also reduce ventilation. It has also been proposed that OSAS contributes to hypoventilation in these obese subjects, since other conditions that result in chronic hypoxemia and sleep disruption also cause a blunted ventilatory response to CO2 response (49, 192). It is unlikely that genetic differences are at the core of the blunted ventilatory response to CO2 in patients with OHS, since first degree relatives of these patients have normal responses to CO2 when compared to control, unaffected individuals.
OBESITY AND DYSPNEA
Many obese subjects complain of dyspnea during exercise or even at rest (9, 50, 120, 139, 176). The mechanistic basis for this dyspnea is important to understand, as it is an extremely unpleasant sensation. In addition, it limits exercise, which promotes the development of even greater obesity. Below we consider dyspnea both at rest, and during exercise.
Dyspnea at rest
Many obese subjects experience dyspnea even while at rest. In otherwise healthy individuals, Sahebjami et al (139) reported that 8 of 23 obese subjects were dyspneic, while Teixeira et al (176) reported dyspnea in 37 of 49 obese subjects. Whether or not dyspnea is reported appears at least partly related to obesity-related changes in static lung volumes. El-Gamal et al (50) examined dyspnea in 25 otherwise healthy obese subjects with a range of BMI’s. They observed a correlation between resting dyspnea score and the reduction in EELV and ERV, but not with BMI or weight. Similarly, Teixeira et al (176) observed that the only pulmonary function abnormality that differentiated obese subjects who complained of dyspnea at rest from those who did not, was a greater reduction in ERV in the dyspneic individuals. As described above, the extent of reduction in ERV also determines the magnitude of hypoxemia in the obese, and Teixeira et al (176) noted a significant correlation between the dyspnea score and the PaO2. Sahebjami et al (139) also noted lower PaO2 in obese subjects who experienced dyspnea at rest versus those who did not, although the effect did not reach statistical significance.
Since current concepts emphasize the importance of respiratory muscle effort in the pathogenesis of dyspnea (118), it is perhaps not too surprising that many obese subjects experience dyspnea: normocapnic obese subjects have increased respiratory drive even at rest (100, 144–145, 166). The sensation of dyspnea occurs especially when there is a dissociation between effort and ventilatory output assessed from mechanosensory afferents (118). Such dissociation could arise from the additional load imposed on the respiratory muscles in obesity by the elastic and resistive changes in the respiratory system described above. As described above, increased respiratory drive is observed in normocapnic obese subjects (100, 144–145, 166). El-Gamal (50) could not demonstrate a relationship between dyspnea and resting O2 consumption, but did find that magnitude of dyspnea correlated with respiratory drive assessed during CO2 rebreathing. Respiratory muscle dysfunction could also lead to a dissociation between respiratory effort and ventilatory output. As described above, most studies that have assessed respiratory muscle strength in obesity do not observe differences (101, 120 Magnani, 2007 #2186, 166), but that is not always the case, particularly in morbidly obese subjects (36–37, 193). Indeed, Sahebjami et al (139) observed that measures of respiratory muscle strength, particularly maximum expiratory pressure (PEmax) and maximum voluntary ventilation (MVV), were inversely associated with dyspnea. Teixeira et al (176) also observed a reduction in PEmax in the obese subjects who were dyspneic at rest, while Collet et al (37) found a relationship between dyspnea and inspiratory muscle strength.
Dyspnea during exercise
Breathlessness upon exertion is also very common in the obese. After climbing two flights of stairs, 80% of obese individuals report shortness of breath, versus only 16% of age-matched lean individuals (162). Similarly, Sin et al (161) reported that among subjects in the Third National Health and Nutrition Examination Survey (NHANES III), about 36% of subjects in the highest quintile of BMI experienced dyspnea upon walking up a hill, versus 19% of subjects in the lowest quintile of BMI. Even normal daily activities can be limited by breathlessness in obese subjects (120).
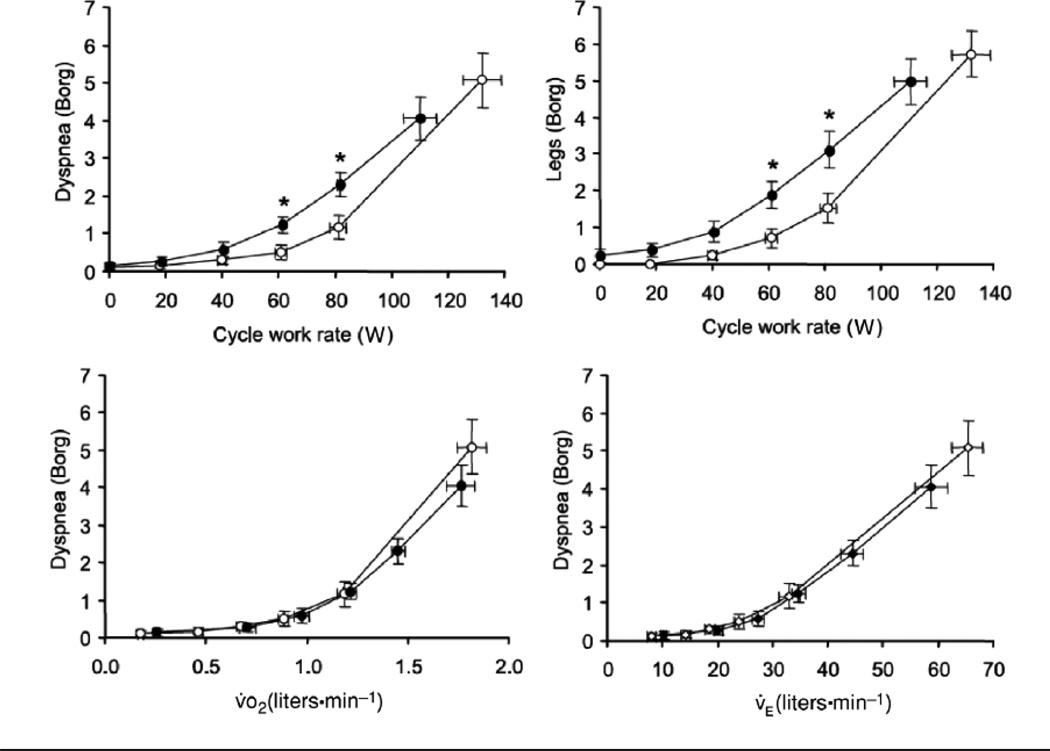
Several investigators have examined the mechanistic basis for exertional dyspnea in the obese. Babb et al (9) examined two groups of obese subjects, one with exertional dyspnea and one without, that were matched on the basis of age, height, BMI, and distribution of adiposity. The peak exercise work rate, oxygen consumption (V̇O2), and heart rate were not different in those that experienced dyspnea versus those that did not. The results suggest that exertional dyspnea in the obese is not the result of cardiovascular deconditioning. Using CO2 rebreathing to increase respiratory effort, Lotti et al (101) demonstrated the magnitude of respiratory effort, as indexed by esophageal pressure swings increases with EELV in obese subjects and is related to dyspnea score (101). Ofir et al (120) examined the hypothesis that dyspnea arises from the increased loads on the respiratory muscles imposed by obesity-related changes in respiratory system elastance and resistance. Such loads could be expected to reduce the ventilatory output for a given degree of respiratory system effort, leading to dyspnea. They used cycling, rather than treadmill exercise, to reduce the work of weight bearing exercise. To achieve the same external cycle work rate as lean subjects, obese subjects required a greater V̇O2 and a greater V̇E. However, the relationships between dyspnea (measured on a Borg scale) and either V̇O2 or V̇E were superimposed in obese and normal subjects (see Figure 14). At a given V̇O2 or V̇E, the obese subjects were not more breathless. Romagnoli et al obtained similar results (136). This was a surprising observation, since the central respiratory drive needed to achieve that VE was expected to be greater in the obese subjects. Both groups considered the role that exercise induced changes in EELV might play in the sensation of dyspnea during exercise in these obese subjects. During peak exercise, EELV increases in most obese subjects, whereas in lean subjects, EELV often decreases as expiratory muscles are recruited during expiration (8, 101, 120, 136). However, exercise-related increases in EELV are not universal in the obese: Roamagnoli et al (136) reported that 6 of 20 obese individuals in their study decreased EELV during peak exercise, just as the lean subjects did. The only factor that discriminated those obese subjects who hyperinflated from those that did not, was the initial ERV, which was lower in those who hyperinflated. In those who hyperinflated, tidal flow reached the maximum expiratory flow early in expiration both at rest and during peak exercise, whereas both in the lean subjects and in the obese subjects who did not hyperinflate, there was no flow limitation during tidal breathing (120, 136). It is notable that even at rest, expiratory flow limitation and dynamic hyperinflation may occur in some obese subjects in the upright posture, and in most obese subjects in the supine posture, and thus contribute to dyspnea even in the absence of exertion (53, 123). Indeed, Ferretti et al (53) noted that orthopnea (an increase in dyspnea upon assuming the supine posture) was more common in obese subjects with expiratory flow limitation than in those without it.
Figure 14.
Relationship between intensity of breathing and leg discomfort (assessed by the modified Borg scale) and work rate during symptom-limited incremental cycle exercise in obese (OB) women (closed circles) and in normal weight (NW) women (open circles). Relationships between intensity of breathing discomfort and both VE and VO2 during exercise were similar in OB and NW weight. Values are means +/− SE. *P < 0.05 OB vs. NW at a standardized cycle work rate. Reproduced from Figure 4 of Ofir et al, 2007 (120) with permission of the American Physiological Society.
Effect of weight loss on dyspnea
Weight loss improves the sensation of dyspnea (50). El-Gamal et al studied 10 subjects before and after gastric surgery for weight reduction. Twelve months after gastroplasty, subjects had reduced their average BMI from about 47 down to 32 kg/m2. Dyspnea score was computed from the dyspnea domain questions in the Chronic Respiratory Disease Questionaire (61). Dyspnea score improved markedly upon weight loss, while measures of respiratory drive declined, consistent with hypothesis that dyspnea in the obese may be related to increased respiratory drive. Others have also noted marked improvement in dyspnea upon surgically induced weight loss (56, 82, 132). For example, Karason et al (82) studied a group of 1210 obese subjects before and after surgically induced weight loss. They noted marked improvement in exertional dyspnea that correlated with the magnitude of weight loss. Compared to subjects in the lowest quartile of weight loss, the risk of exertional dyspnea was reduced by 98% in subjects in the highest quartile of weight loss, who lost an average of 41 kg. Even those in the third quartile of weight loss, who lost an average of 22 kg, the risk of exertional dyspnea was reduced by 94%. Those subjects who experienced the greatest weight loss were also much more likely to be physically inactive.
Conclusion
Obesity results in several important respiratory complications including increased work of breathing, reductions in lung volume, airflow obstruction, hypoxemia, and dyspnea. Most of these problems are amplified in the supine posture. Obesity also increases the risk of developing asthma. Gravitational effects on the abdominal mass result in a rightward shift in the PV curve of the respiratory system and a decrease in FRC. Since RV is usually normal, ERV declines markedly, and obese subjects often breathe close to RV, although in many, the reduction in lung volume results in flow limitation, and EELV becomes dynamically rather than statically determined. There is substantial heterogeneity in the impact of obesity on absolute lung volumes. This heterogeneity is at least partially dependent on the locus of adiposity, with adipose tissue in the buttocks and hips having less impact that adipose tissue in the trunk. Obesity also causes airflow obstruction, which is only partly explained by the reductions in lung volume. Obesity-related alterations in the pattern of breathing, especially rapid shallow breathing, or aspects of the systemic inflammation of obesity may contribute to this obstruction. Hypoxemia is much more common in obesity than is generally appreciated, even when subjects are in the upright posture, and occurs even in obese subjects without OHS. Indeed, in most obese subjects, ventilation is well matched to their metabolic needs, and subjects are eucapnic. Instead, hypoxemia results from overperfusion of poorly ventilated or even unventilated regions of the lung: closing volume exceeds FRC in many obese subjects and a tidal breath is often initiated from a volume at which many airways in the dependent parts of the lung are closed. Dyspnea is very common in obese individuals, even at rest and increases with exertion. Understanding the mechanistic basis for this unpleasant sensation is important, since dyspnea may limit the willingness of obese subjects to engage in exercise, which may further increase their propensity towards weight gain.
It is important to emphasize that most of the respiratory complications of obesity, including hypoxemia, dyspnea, asthma, and OHS are improved, sometimes dramatically, even with moderate weight loss. Weight loss should be an important goal for anyone dealing with the respiratory complications of obesity. However, it is increasingly apparent that abdominal obesity can exist even in subjects with a normal BMI. Since it is abdominal obesity that drives many of the respiratory complications of obesity, attention to waist circumference should be considered for anyone experiencing this type of respiratory symptoms. Since changes in EELV and ERV appear to contribute to both the hypoxemia and the dyspnea of obesity, strategies aimed at mechanically supporting elevations in FRC, some of which are already in use for the treatment of obstructive sleep apnea, may have also have substantial benefits for the general obese population, even those without apnea. The hypoxemia of obesity, which is sometimes marked, can be expected to impact the functioning of all organ systems, but may be especially important for the intraabdominal adipose tissue which may be additionally compromised by reduced perfusion. Since adipose tissue hypoxia is increasingly recognized as contributing to the systemic inflammation of obesity and its sequelae, therapeutic strategies aimed at reducing hypoxemia might also have benefits for the metabolic complications of obesity.
Acknowledgements
The author would like to acknowledge Dr. Steven Loring for helpful discussion. The author’s research is supported by National Heart, Lung, and Blood Institute grant HL-084044 and National Institute of Environmental Health Sciences grant ES-013307.
References
- 1.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 2.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, Lemiere C, Sharma S, Field SK, Alvarez GG, Dales RE, Doucette S, Fergusson D. Overdiagnosis of asthma in obese and nonobese adults. Cmaj. 2008;179:1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adya R, Tan BK, Punn A, Chen J, Randeva HS. VISFATIN INDUCES HUMAN ENDOTHELIAL VEGF AND MMP-2/9 PRODUCTION VIA MAPK AND PI3K/Akt SIGNALLING PATHWAYS: NOVEL INSIGHTS INTO VISFATIN-INDUCED ANGIOGENESIS. Cardiovasc Res. 2007 doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 4.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The Human Visceral Fat Depot Has a Unique Inflammatory Profile. Obesity (Silver Spring) doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 5.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 7.Atabek ME, Vatansev H, Erkul I. Oxidative stress in childhood obesity. J Pediatr Endocrinol Metab. 2004;17:1063–1068. doi: 10.1515/jpem.2004.17.8.1063. [DOI] [PubMed] [Google Scholar]
- 8.Babb TG, Buskirk ER, Hodgson JL. Exercise end-expiratory lung volumes in lean and moderately obese women. Int J Obes. 1989;13:11–19. [PubMed] [Google Scholar]
- 9.Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008;178:116–123. doi: 10.1164/rccm.200706-875OC. [DOI] [PubMed] [Google Scholar]
- 10.Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008;134:704–711. doi: 10.1378/chest.07-1728. [DOI] [PubMed] [Google Scholar]
- 11.Bae J, Ting EY, Giuffrida JG. The effect of changes in the body position obsese patients on pulmonary volume and ventilatory function. Bull N Y Acad Med. 1976;52:830–837. [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker SJ, RG IJ, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000;148:17–21. doi: 10.1016/s0021-9150(99)00329-9. [DOI] [PubMed] [Google Scholar]
- 13.Barrera F, Reidenberg MM, Winters WL, Hungspreugs S. Ventilation-perfusion relationships in the obese patient. J Appl Physiol. 1969;26:420–426. doi: 10.1152/jappl.1969.26.4.420. [DOI] [PubMed] [Google Scholar]
- 14.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 15.Bedell GN, Wilson WR, Seebohm PM. Pulmonary function in obese persons. J Clin Invest. 1958;37:1049–1060. doi: 10.1172/JCI103686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 108:212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedik PS, Baun MM, Keus L, Jimenez C, Morice R, Bidani A, Meininger JC. Effects of body position on resting lung volume in overweight and mildly to moderately obese subjects. Respir Care. 2009;54:334–339. [PubMed] [Google Scholar]
- 18.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bickelmann AG, Burwell CS, Robin ED, Whaley RD. Extreme obesity associated with alveolar hypoventilation; a Pickwickian syndrome. Am J Med. 1956;21:811–818. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- 20.Bland S, Lazerou L, Dyck G, Cherniack RM. The influence of the "chest wall" on respiratory rate and depth. Respir Physiol. 1967;3:47–54. doi: 10.1016/0034-5687(67)90023-0. [DOI] [PubMed] [Google Scholar]
- 21.Boulet LP, Turcotte H, Boulet G, Simard B, Robichaud P. Deep inspiration avoidance and airway response to methacholine: Influence of body mass index. Can Respir J. 2005;12:371–376. doi: 10.1155/2005/517548. [DOI] [PubMed] [Google Scholar]
- 22.Braback L, Hjern A, Rasmussen F. Body mass index, asthma and allergic rhinoconjunctivitis in Swedish conscripts-a national cohort study over three decades. Respir Med. 2005;99:1010–1014. doi: 10.1016/j.rmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest. 1958;37:1279–1285. doi: 10.1172/JCI103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, Khaw KT. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159:1140–1149. doi: 10.1093/aje/kwh155. [DOI] [PubMed] [Google Scholar]
- 25.Caro CG, Butler J, Dubois AB. Some effects of restriction of chest cage expansion on pulmonary function in man: an experimental study. J Clin Invest. 1960;39:573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartier A, Bergeron J, Poirier P, Almeras N, Tremblay A, Lemieux I, Despres JP. Increased plasma interleukin-1 receptor antagonist levels in men with visceral obesity. Ann Med. 2009:1–8. doi: 10.1080/07853890903022801. [DOI] [PubMed] [Google Scholar]
- 27.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 28.Celedon JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, Xu X. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164:1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers GW, Little SA, Patel KR, Thomson NC. Endothelin-1-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1997;156:382–388. doi: 10.1164/ajrccm.156.2.9702066. [DOI] [PubMed] [Google Scholar]
- 30.Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32:1563–1569. doi: 10.1183/09031936.00114007. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Tunstall-Pedoe H, Bolton-Smith C, Hannah MK, Morrison C. Association of dietary antioxidants and waist circumference with pulmonary function and airway obstruction. Am J Epidemiol. 2001;153:157–163. doi: 10.1093/aje/153.2.157. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48:375–380. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinn DJ, Cotes JE, Reed JW. Longitudinal effects of change in body mass on measurements of ventilatory capacity. Thorax. 1996;51:699–704. doi: 10.1136/thx.51.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chlif M, Keochkerian D, Choquet D, Vaidie A, Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol. 2009;168:198–202. doi: 10.1016/j.resp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Chlif M, Keochkerian D, Mourlhon C, Choquet D, Ahmaidi S. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int J Obes (Lond) 2005;29:1478–1483. doi: 10.1038/sj.ijo.0803030. [DOI] [PubMed] [Google Scholar]
- 37.Collet F, Mallart A, Bervar JF, Bautin N, Matran R, Pattou F, Romon M, Perez T. Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes (Lond) 2007;31:700–706. doi: 10.1038/sj.ijo.0803460. [DOI] [PubMed] [Google Scholar]
- 38.Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107:1298–1302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 39.Craig DB, Wahba WM, Don HF, Couture JG, Becklake MR. "Closing volume" and its relationship to gas exchange in seated and supine positions. J Appl Physiol. 1971;31:717–721. doi: 10.1152/jappl.1971.31.5.717. [DOI] [PubMed] [Google Scholar]
- 40.Crapo RO, Kelly TM, Elliott CG, Jones SB. Spirometry as a preoperative screening test in morbidly obese patients. Surgery. 1986;99:763–768. [PubMed] [Google Scholar]
- 41.Damsbo P, Vaag A, Hother-Nielsen O, Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- 42.De Lorenzo A, Maiolo C, Mohamed EI, Andreoli A, Petrone-De Luca P, Rossi P. Body composition analysis and changes in airways function in obese adults after hypocaloric diet. Chest. 2001;119:1409–1415. doi: 10.1378/chest.119.5.1409. [DOI] [PubMed] [Google Scholar]
- 43.de Mello VD, Kolehmainen M, Schwab U, Mager U, Laaksonen DE, Pulkkinen L, Niskanen L, Gylling H, Atalay M, Rauramaa R, Uusitupa M. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism. 2008;57:192–199. doi: 10.1016/j.metabol.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Deesomchok A, Fisher T, Webb KA, Ora J, Lam YM, Lougheed MD, O'Donnell DE. Effects of obesity on perceptual and mechanical responses to bronchoconstriction in asthma. Am J Respir Crit Care Med. 181:125–133. doi: 10.1164/rccm.200906-0934OC. [DOI] [PubMed] [Google Scholar]
- 45.Dhabuwala A, Cannan RJ, Stubbs RS. Improvement in co-morbidities following weight loss from gastric bypass surgery. Obes Surg. 2000;10:428–435. doi: 10.1381/096089200321594291. [DOI] [PubMed] [Google Scholar]
- 46.Dixon JB, Chapman L, O'Brien P. Marked improvement in asthma after Lap-Band surgery for morbid obesity. Obes Surg. 1999;9:385–389. doi: 10.1381/096089299765552981. [DOI] [PubMed] [Google Scholar]
- 47.Dixon JB, O'Brien PE. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. Am J Surg. 2002;184:51S–54S. doi: 10.1016/s0002-9610(02)01181-9. [DOI] [PubMed] [Google Scholar]
- 48.Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol. 1972;33:559–563. doi: 10.1152/jappl.1972.33.5.559. [DOI] [PubMed] [Google Scholar]
- 49.Edelman NH, Lahiri S, Braudo L, Cherniack NS, Fishman AP. The blunted ventilatory response to hypoxia in cyanotic congenital heart disease. N Engl J Med. 1970;282:405–411. doi: 10.1056/NEJM197002192820801. [DOI] [PubMed] [Google Scholar]
- 50.El-Gamal H, Khayat A, Shikora S, Unterborn JN. Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest. 2005;128:3870–3874. doi: 10.1378/chest.128.6.3870. [DOI] [PubMed] [Google Scholar]
- 51.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 52.Farebrother MJ, McHardy GJ, Munro JF. Relation between pulmonary gas exchange and closing volume before and after substantial weight loss in obese subjects. Br Med J. 1974;3:391–393. doi: 10.1136/bmj.3.5927.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119:1401–1408. doi: 10.1378/chest.119.5.1401. [DOI] [PubMed] [Google Scholar]
- 54.Fimognari FL, Pasqualetti P, Moro L, Franco A, Piccirillo G, Pastorelli R, Rossini PM, Incalzi RA. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:760–765. doi: 10.1093/gerona/62.7.760. [DOI] [PubMed] [Google Scholar]
- 55.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910. [DOI] [PubMed] [Google Scholar]
- 56.Frigg A, Peterli R, Peters T, Ackermann C, Tondelli P. Reduction in co-morbidities 4 years after laparoscopic adjustable gastric banding. Obes Surg. 2004;14:216–223. doi: 10.1381/096089204322857591. [DOI] [PubMed] [Google Scholar]
- 57.Fritts HW, Jr., Filler J, Fishman AP, Cournand A. The efficiency of ventilation during voluntary hyperpnea: studies in normal subjects and in dyspneic patients with either chronic pulmonary emphysema or obesity. J Clin Invest. 1959;38:1339–1348. doi: 10.1172/JCI103909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert R, Sipple JH, Auchincloss JH., Jr. Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961;16:21–26. doi: 10.1152/jappl.1961.16.1.21. [DOI] [PubMed] [Google Scholar]
- 60.Gump A, Haughney L, Fredberg J. Relaxation of activated airway smooth muscle: relative potency of isoproterenol vs. tidal stretch. J Appl Physiol. 2001;90:2306–2310. doi: 10.1152/jappl.2001.90.6.2306. [DOI] [PubMed] [Google Scholar]
- 61.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hakala K, Mustajoki P, Aittomaki J, Sovijarvi AR. Effect of weight loss and body position on pulmonary function and gas exchange abnormalities in morbid obesity. Int J Obes Relat Metab Disord. 1995;19:343–346. [PubMed] [Google Scholar]
- 63.Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 64.Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694–699. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- 65.Heaf PJ, Prime FJ. The compliance of the thorax in normal human subjects. Clin Sci (Lond) 1956;15:319–327. [PubMed] [Google Scholar]
- 66.Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20:248–254. doi: 10.1111/j.1399-6576.1976.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 67.Hedenstierna G, Santesson J, Norlander O. Airway closure and distribution of inspired gas in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20:334–342. doi: 10.1111/j.1399-6576.1976.tb05047.x. [DOI] [PubMed] [Google Scholar]
- 68.Hida W, Shindoh C, Satoh J, Sagara M, Kikuchi Y, Toyota T, Shirato K. N-acetylcysteine inhibits loss of diaphragm function in streptozotocin-treated rats. Am J Respir Crit Care Med. 1996;153:1875–1879. doi: 10.1164/ajrccm.153.6.8665049. [DOI] [PubMed] [Google Scholar]
- 69.Holguin F, Cribbs S, Fitzpatrick AM, Ingram RH, Jr., Jackson AC. A deep breath bronchoconstricts obese asthmatics. J Asthma. 47:55–60. doi: 10.3109/02770900903318330. [DOI] [PubMed] [Google Scholar]
- 70.Holley HS, Milic-Emili J, Becklake MR, Bates DV. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46:475–481. doi: 10.1172/JCI105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 72.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 73.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 74.Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135:419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- 75.Jackson MB, Ahima RS. Neuroendocrine and metabolic effects of adipocyte-derived hormones. Clin Sci (Lond) 2006;110:143–152. doi: 10.1042/CS20050243. [DOI] [PubMed] [Google Scholar]
- 76.Jarvis D, Chinn S, Potts J, Burney P. Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. Clin Exp Allergy. 2002;32:831–837. doi: 10.1046/j.1365-2222.2002.01380.x. [DOI] [PubMed] [Google Scholar]
- 77.Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. 1991;85:309–311. doi: 10.1016/s0954-6111(06)80102-2. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 80.Johnston RA, Theman TA, Shore SA. Augmented Responses to Ozone in Obese Carboxypeptidase E-Deficient Mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R126–R133. doi: 10.1152/ajpregu.00306.2005. [DOI] [PubMed] [Google Scholar]
- 81.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 82.Karason K, Lindroos AK, Stenlof K, Sjostrom L. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. doi: 10.1001/archinte.160.12.1797. [DOI] [PubMed] [Google Scholar]
- 83.Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 84.Kaufman BJ, Ferguson MH, Cherniack RM. Hypoventilation in obesity. J Clin Invest. 1959;38:500–507. doi: 10.1172/JCI103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 86.King GG, Brown NJ, Diba C, Thorpe CW, Munoz P, Marks GB, Toelle B, Ng K, Berend N, Salome CM. The effects of body weight on airway calibre. Eur Respir J. 2005;25:896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- 87.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, Brown L, Teague GW, Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8(32) doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kronander UN, Falkenberg M, Zetterstrom O. Prevalence and incidence of asthma related to waist circumference and BMI in a Swedish community sample. Respir Med. 2004;98:1108–1116. doi: 10.1016/j.rmed.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Lazarus R, Colditz G, Berkey CS, Speizer FE. Effects of body fat on ventilatory function in children and adolescents: cross-sectional findings from a random population sample of school children. Pediatr Pulmonol. 1997;24:187–194. doi: 10.1002/(sici)1099-0496(199709)24:3<187::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 90.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111:891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 91.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr., Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 92.Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 94.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 95.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 97.Lin CC, Wu KM, Chou CS, Liaw SF. Oral airway resistance during wakefulness in eucapnic and hypercapnic sleep apnea syndrome. Respir Physiol Neurobiol. 2004;139:215–224. doi: 10.1016/j.resp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008;121:1075–1084. doi: 10.1016/j.jaci.2008.03.005. quiz 1085-1076. [DOI] [PubMed] [Google Scholar]
- 99.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 100.Lopata M, Onal E. Mass loading, sleep apnea, and the pathogenesis of obesity hypoventilation. Am Rev Respir Dis. 1982;126:640–645. doi: 10.1164/arrd.1982.126.4.640. [DOI] [PubMed] [Google Scholar]
- 101.Lotti P, Gigliotti F, Tesi F, Stendardi L, Grazzini M, Duranti R, Scano G. Respiratory muscles and dyspnea in obese nonsmoking subjects. Lung. 2005;183:311–323. doi: 10.1007/s00408-005-2544-5. [DOI] [PubMed] [Google Scholar]
- 102.Lourenco RV. Diaphragm activity in obesity. J Clin Invest. 1969;48:1609–1614. doi: 10.1172/JCI106126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 104.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maeda S, Jesmin S, Iemitsu M, Otsuki T, Matsuo T, Ohkawara K, Nakata Y, Tanaka K, Goto K, Miyauchi T. Weight loss reduces plasma endothelin-1 concentration in obese men. Exp Biol Med (Maywood) 2006;231:1044–1047. [PubMed] [Google Scholar]
- 106.Magnani KL, Cataneo AJ. Respiratory muscle strength in obese individuals and influence of upper-body fat distribution. Sao Paulo Med J. 2007;125:215–219. doi: 10.1590/S1516-31802007000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, Sofia M. Weight loss and asthma control in severely obese asthmatic females. Respir Med. 2008;102:102–108. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 108.Milic-Emili J, Torchio R, D'Angelo E. Closing volume: a reappraisal (1967–2007) Eur J Appl Physiol. 2007;99:567–583. doi: 10.1007/s00421-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 109.Mohamed EI, Maiolo C, Iacopino L, Pepe M, Di Daniele N, De Lorenzo A. The impact of body-weight components on forced spirometry in healthy italians. Lung. 2002;180:149–159. doi: 10.1007/s004080000089. [DOI] [PubMed] [Google Scholar]
- 110.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5:218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007;132:1322–1336. doi: 10.1378/chest.07-0027. [DOI] [PubMed] [Google Scholar]
- 112.Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. 2004;82:940–946. [PMC free article] [PubMed] [Google Scholar]
- 113.Moon YS, Kim DH, Song DK. Serum tumor necrosis factor-alpha levels and components of the metabolic syndrome in obese adolescents. Metabolism. 2004;53:863–867. doi: 10.1016/j.metabol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Murr MM, Siadati MR, Sarr MG. Results of Bariatric Surgery for Morbid Obesity in Patients Older than 50 Years. Obes Surg. 1995;5:399–402. doi: 10.1381/096089295765557494. [DOI] [PubMed] [Google Scholar]
- 115.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 117.O'Brien PE, Dixon JB, Brown W, Schachter LM, Chapman L, Burn AJ, Dixon ME, Scheinkestel C, Halket C, Sutherland LJ, Korin A, Baquie P. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652–660. doi: 10.1381/096089202321019639. [DOI] [PubMed] [Google Scholar]
- 118.O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AF, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- 119.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Trevisan M, Cassano PA, Iacoviello L, Schunemann HJ. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129:853–862. doi: 10.1378/chest.129.4.853. [DOI] [PubMed] [Google Scholar]
- 120.Ofir D, Laveneziana P, Webb KA, O'Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007;102:2217–2226. doi: 10.1152/japplphysiol.00898.2006. [DOI] [PubMed] [Google Scholar]
- 121.Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948–956. doi: 10.1016/j.amjmed.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 122.Paek YJ, Jung KS, Hwang YI, Lee KS, Lee DR, Lee JU. Association between low pulmonary function and metabolic risk factors in Korean adults: the Korean National Health and Nutrition Survey. Metabolism. 2009 doi: 10.1016/j.metabol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 1998;85:1236–1243. doi: 10.1152/jappl.1998.85.4.1236. [DOI] [PubMed] [Google Scholar]
- 124.Pelosi P, Croci M, Calappi E, Mulazzi D, Cerisara M, Vercesi P, Vicardi P, Gattinoni L. Prone positioning improves pulmonary function in obese patients during general anesthesia. Anesth Analg. 1996;83:578–583. doi: 10.1097/00000539-199609000-00025. [DOI] [PubMed] [Google Scholar]
- 125.Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 126.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 127.Pistelli F, Bottai M, Carrozzi L, Pede FD, Baldacci S, Maio S, Brusasco V, Pellegrino R, Viegi G. Changes in obesity status and lung function decline in a general population sample. Respir Med. 2008;102:674–680. doi: 10.1016/j.rmed.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 128.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;3(28 Suppl 3):S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 129.Rasmussen F, Mikkelsen D, Hancox RJ, Lambrechtsen J, Nybo M, Hansen HS, Siersted HC. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33:382–388. doi: 10.1183/09031936.00040708. [DOI] [PubMed] [Google Scholar]
- 130.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 131.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 132.Refsum HE, Holter PH, Lovig T, Haffner JF, Stadaas JO. Pulmonary function and energy expenditure after marked weight loss in obese women: observations before and one year after gastric banding. Int J Obes. 1990;14:175–183. [PubMed] [Google Scholar]
- 133.Ri-Li G, Chase PJ, Witkowski S, Wyrick BL, Stone JA, Levine BD, Babb TG. Obesity: associations with acute mountain sickness. Ann Intern Med. 2003;139:253–257. doi: 10.7326/0003-4819-139-4-200308190-00007. [DOI] [PubMed] [Google Scholar]
- 134.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol. 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- 135.Rochester DF, Enson Y. Current concepts in the pathogenesis of the obesity-hypoventilation syndrome. Mechanical and circulatory factors. Am J Med. 1974;57:402–420. doi: 10.1016/0002-9343(74)90135-1. [DOI] [PubMed] [Google Scholar]
- 136.Romagnoli I, Laveneziana P, Clini EM, Palange P, Valli G, de Blasio F, Gigliotti F, Scano G. Role of hyperinflation vs. deflation on dyspnoea in severely to extremely obese subjects. Acta Physiol (Oxf) 2008;193:393–402. doi: 10.1111/j.1748-1716.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 137.Rorvik S, Bo G. Lung volumes and arterial blood gases in obesity. Scand J Respir Dis Suppl. 1976;95:60–64. [PubMed] [Google Scholar]
- 138.Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112:828–832. doi: 10.7326/0003-4819-112-11-828. [DOI] [PubMed] [Google Scholar]
- 139.Sahebjami H. Dyspnea in obese healthy men. Chest. 1998;114:1373–1377. doi: 10.1378/chest.114.5.1373. [DOI] [PubMed] [Google Scholar]
- 140.Sahebjami H, Gartside PS. Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest. 1996;110:1425–1429. doi: 10.1378/chest.110.6.1425. [DOI] [PubMed] [Google Scholar]
- 141.Said SI. Abnormalities of pulmonary gas exchange in obesity. Ann Intern Med. 1960;53:1121–1129. doi: 10.7326/0003-4819-53-6-1121. [DOI] [PubMed] [Google Scholar]
- 142.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 143.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes (Lond) 2008;32:502–509. doi: 10.1038/sj.ijo.0803752. [DOI] [PubMed] [Google Scholar]
- 144.Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- 145.Sampson MG, Grassino K. Neuromechanical properties in obese patients during carbon dioxide rebreathing. Am J Med. 1983;75:81–90. doi: 10.1016/0002-9343(83)91171-3. [DOI] [PubMed] [Google Scholar]
- 146.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58:1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 149.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54:396–402. doi: 10.1136/thx.54.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharp JT, Druz WS, Kondragunta VR. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am Rev Respir Dis. 1986;133:32–37. doi: 10.1164/arrd.1986.133.1.32. [DOI] [PubMed] [Google Scholar]
- 151.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of Mass Loading the Respiratory System in Man. J Appl Physiol. 1964;19:959–966. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- 152.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. The Total Work of Breathing in Normal and Obese Men. J Clin Invest. 1964;43:728–739. doi: 10.1172/JCI104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shore SA. Obesity and asthma: cause for concern. Curr Opin Pharmacol. 2006;6:230–236. doi: 10.1016/j.coph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 154.Shore SA. Obesity and asthma: implications for treatment. Curr Opin Pulm Med. 2007;13:56–62. doi: 10.1097/MCP.0b013e3280110196. [DOI] [PubMed] [Google Scholar]
- 155.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 156.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol. 108:735–743. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 158.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- 159.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 160.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 161.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 162.Sjostrom L, Larsson B, Backman L, Bengtsson C, Bouchard C, Dahlgren S, Hallgren P, Jonsson E, Karlsson J, Lapidus L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992;16:465–479. [PubMed] [Google Scholar]
- 163.Sood A, Verhulst SJ, Varma A, Eagleton LE, Henkle JQ, Hopkins-Price P. Association of excess weight and degree of airway responsiveness in asthmatics and non-asthmatics. J Asthma. 2006;43:447–452. doi: 10.1080/02770900600758309. [DOI] [PubMed] [Google Scholar]
- 164.Stanescu D, Veriter C. A normal FEV1/VC ratio does not exclude airway obstruction. Respiration. 2004;71:348–352. doi: 10.1159/000079638. [DOI] [PubMed] [Google Scholar]
- 165.Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity (Silver Spring) 2009;17:578–584. doi: 10.1038/oby.2008.584. [DOI] [PubMed] [Google Scholar]
- 166.Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64:719–725. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- 167.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. Bmj. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 169.Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71–79. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- 170.Suratt PM, Wilhoit SC, Hsiao HS, Atkinson RL, Rochester DF. Compliance of chest wall in obese subjects. J Appl Physiol. 1984;57:403–407. doi: 10.1152/jappl.1984.57.2.403. [DOI] [PubMed] [Google Scholar]
- 171.Sutherland TJ, Cowan JO, Taylor DR. Dynamic hyperinflation with bronchoconstriction: differences between obese and nonobese women with asthma. Am J Respir Crit Care Med. 2008;177:970–975. doi: 10.1164/rccm.200711-1738OC. [DOI] [PubMed] [Google Scholar]
- 172.Sutherland TJ, Goulding A, Grant AM, Cowan JO, Williamson A, Williams SM, Skinner MA, Taylor DR. The effect of adiposity measured by dual-energy X-ray absorptiometry on lung function. Eur Respir J. 2008;32:85–91. doi: 10.1183/09031936.00112407. [DOI] [PubMed] [Google Scholar]
- 173.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b–positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 174.Tankersley C, Kleeberger S, Russ B, Schwartz A, Smith P. Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol. 1996;81:716–723. doi: 10.1152/jappl.1996.81.2.716. [DOI] [PubMed] [Google Scholar]
- 175.Tankersley CG, O'Donnell C, Daood MJ, Watchko JF, Mitzner W, Schwartz A, Smith P. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol. 1998;85:2261–2269. doi: 10.1152/jappl.1998.85.6.2261. [DOI] [PubMed] [Google Scholar]
- 176.Teixeira CA, Dos Santos JE, Silva GA, de Souza ES, Martinez JA. Prevalence of and the potential physiopathological mechanisms involved in dyspnea in individuals with class II or III obesity. J Bras Pneumol. 2007;33:28–35. doi: 10.1590/s1806-37132007000100008. [DOI] [PubMed] [Google Scholar]
- 177.Thomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989;44:382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Uchida Y, Ninomiya H, Saotome M, Nomura A, Ohtsuka M, Yanagisawa M, Goto K, Masaki T, Hasegawa S. Endothelin, a novel vasoconstrictor peptide, as potent bronchoconstrictor. Eur J Pharmacol. 1988;154:227–228. doi: 10.1016/0014-2999(88)90106-9. [DOI] [PubMed] [Google Scholar]
- 179.Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- 180.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 181.Van Lith P, Johnson FN, Sharp JT. Respiratory elastances in relaxed and paralyzed states in normal and abnormal men. J Appl Physiol. 1967;23:475–486. doi: 10.1152/jappl.1967.23.4.475. [DOI] [PubMed] [Google Scholar]
- 182.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 183.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and Obesity. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 184.Vaughan RW, Cork RC, Hollander D. The effect of massive weight loss on arterial oxygenation and pulmonary function tests. Anesthesiology. 1981;54:325–328. doi: 10.1097/00000542-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 185.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 186.Von Behren J, Lipsett M, Horn-Ross PL, Delfino RJ, Gilliland F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, waist size and prevalence of current asthma in the California Teachers Study cohort. Thorax. 2009;64:889–893. doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.von Kries R, Hermann M, Grunert VP, von Mutius E. Is obesity a risk factor for childhood asthma? Allergy. 2001;56:318–322. doi: 10.1034/j.1398-9995.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 188.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 190.Waltemath CL, Bergman NA. Respiratory compliance in obese patients. Anesthesiology. 1974;41:84–85. doi: 10.1097/00000542-197407000-00022. [DOI] [PubMed] [Google Scholar]
- 191.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98:512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 192.Weil JV, Byrne-Quinn E, Sodal IE, Filley GF, Grover RF. Acquired attenuation of chemoreceptor function in chronically hypoxic man at high altitude. J Clin Invest. 1971;50:186–195. doi: 10.1172/JCI106472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weiner P, Waizman J, Weiner M, Rabner M, Magadle R, Zamir D. Influence of excessive weight loss after gastroplasty for morbid obesity on respiratory muscle performance. Thorax. 1998;53:39–42. doi: 10.1136/thx.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Winkler G, Lakatos P, Salamon F, Nagy Z, Speer G, Kovacs M, Harmos G, Dworak O, Cseh K. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med. 1999;16:207–211. doi: 10.1046/j.1464-5491.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 196.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol. 2007;103:655–663. doi: 10.1152/japplphysiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 197.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yamakawa T, Tanaka S, Yamakawa Y, Kiuchi Y, Isoda F, Kawamoto S, Okuda K, Sekihara H. Augmented production of tumor necrosis factor-alpha in obese mice. Clin Immunol Immunopathol. 1995;75:51–56. doi: 10.1006/clin.1995.1052. [DOI] [PubMed] [Google Scholar]
- 199.Yamane T, Date T, Tokuda M, Aramaki Y, Inada K, Matsuo S, Shibayama K, Miyanaga S, Miyazaki H, Sugimoto K, Yoshimura M. Hypoxemia in inferior pulmonary veins in supine position is dependent on obesity. Am J Respir Crit Care Med. 2008;178:295–299. doi: 10.1164/rccm.200801-113OC. [DOI] [PubMed] [Google Scholar]
- 200.Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79:1199–1205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- 201.Ybarra J, Lehmann TN, Golay A, Juge-Aubry CE, Roux-Lombard P, Dayer JM, Meier CA. Gender-based dimorphic pattern for interleukin-1 receptor antagonist in type 2 diabetes mellitus. Diabetes Metab. 2008;34:75–81. doi: 10.1016/j.diabet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 202.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 203.Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM. Body fat and circulating leukocytes in children. Int J Obes (Lond) 2006;30:906–911. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- 204.Zavorsky GS, Hoffman SL. Pulmonary gas exchange in the morbidly obese. Obes Rev. 2008;9:326–339. doi: 10.1111/j.1467-789X.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 205.Zavorsky GS, Murias JM, Kim do J, Gow J, Sylvestre JL, Christou NV. Waist-to-hip ratio is associated with pulmonary gas exchange in the morbidly obese. Chest. 2007;131:362–367. doi: 10.1378/chest.06-1513. [DOI] [PubMed] [Google Scholar]
- 206.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 207.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
Further Reading
- Malhotra A, Hillman D. Obesity and the lung: Obesity, respiration, and intensive care. Thorax. 2008;63:925–931. doi: 10.1136/thx.2007.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Rev Immunol. 2006;10:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Paramesawaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–210. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handbook of Obesity. Etiology and Pathophysiology. 2nd edition. New York: Marcel Dekker, Inc; 2004. [Google Scholar]
- The Obesity Society website. www.obesity.org.