Abstract
Background
Hypertrophic cardiomyopathy (HCM) is a common genetic disorder caused mainly by mutations in sarcomeric proteins and is characterized by maladaptive myocardial hypertrophy, diastolic heart failure, increased myofilament Ca2+ sensitivity and high susceptibility to sudden death. We tested the following hypothesis: correction of the increased myofilament sensitivity can delay or prevent the development of the HCM phenotype.
Methods and Results
We used an HCM mouse model with an E180G mutation in α-tropomyosin (Tm180) that demonstrates increased myofilament Ca2+ sensitivity, severe hypertrophy and diastolic dysfunction. To test our hypothesis, we reduced myofilament Ca2+ sensitivity in Tm180 mice by generating a double transgenic (DTG) mouse line. We crossed Tm180 mice with mice expressing a pseudo-phosphorylated cardiac troponin I (cTnI) (S23D and S24D; TnI-PP). TnI-PP mice demonstrated a reduced myofilament Ca2+ sensitivity compared to wild-type mice. The development of pathological hypertrophy did not occur in mice expressing both Tm180 and TnI-PP. Left ventricle performance was improved in DTG compared to their Tm180 littermates, which express wild-type cTnI. Hearts of DTG mice demonstrated no changes in expression of phospholamban (PLN) and Serca2a, increased levels of PLN and TnT phosphorylation, and reduced phosphorylation of TnI compared to Tm180 mice. Moreover, expression of TnI-PP in Tm180 hearts inhibited modifications in the activity of ERK1/2 and GATA-4 in Tm180 hearts.
Conclusions
Our data strongly indicate that reduction of myofilament sensitivity to Ca2+ and associated correction of abnormal relaxation can delay or prevent development of HCM and should be considered as a therapeutic target for HCM.
Keywords: cardiomyopathy, cardiac remodeling, murine model, treatment, HCM, myofilament Ca sensitivity, new therapy
Introduction
Hypertrophic cardiomyopathy (HCM) is currently defined as a genetic cardiovascular disease with a primary defect in the sarcomeric proteins1. It is characterized by the presence of myocardial hypertrophy, interstitial fibrosis, myofibrillar disarray, diastolic dysfunction and increased susceptibility to arrhythmias2, 3. HCM shows autosomal dominant inheritance in the vast majority of the cases and has been linked to more than a thousand mutations in at least 9 genes encoding components of the sarcomere4-7. The affected proteins include the thick filament proteins; myosin heavy chain (MyHC), myosin binding protein C (MyBP-C), regulatory (LC2) and essential myosin light chain, as well as the thin filament proteins; α-actin, troponin complex protein, troponin T (TnT), troponin I (TnI), troponin C, α-tropomyosin (αTm), and titin along with other Z-disk related proteins8-10.
Although the first known HCM mutation in MyHC was discovered by Seidman’s group more than 20 years ago11 and was followed by a great number of studies in the field, disease-specific therapies are lacking, with the current pharmacological treatment for patients with HCM being traditional therapies used for heart failure (HF) and arrhythmias including β-blockers, Ca2+-channel blockers, anti-arrhythmic drugs, diuretics, cardiac implantable defibrillators and heart transplants12. More recent experimentation in animal models of HCM have provided potential new therapies which include statins, renin-angiotensin-aldosterone system inhibitors and N-acetylcysteine13-19, however, further animal studies are required to develop and validate more specific treatments for individual cases. We and others previously reported that improving the rate of relaxation from the levels dictated by thin filament protein mutations in HCM mouse models, by either interventions in the rate of sarcoplasmic reticulum (SR) Ca2+ uptake20,21, or by increasing the expression of parvalbumin, a cytoplasmic Ca2+-binding protein22, improved the cardiac function of HCM animals. These data strongly support the idea that improving relaxation rates may be a potential new therapeutic target for HCM.
In addition to interventions in Ca2+ flux regulation or buffering, another, more direct way to improve relaxation in HCM is to directly target the myofilaments and reduce their sensitivity to Ca2+. Although currently there are no approved drugs that specifically desensitize myofilament to Ca2+, our and others previous work has demonstrated that one way to reduce sensitivity is by PKA-mediated cTnI phosphorylation at Ser23 and 24, which decreases the myofilament Ca2+ response and increases the kinetics of myocardial relaxation23-28. To prove this concept we used mice that express TnI pseudo-phosphorylated at the PKA phosphorylation sites (Ser23 and Ser24 were mutated to Asp; TnI-PP) and showed decreased myofilament Ca2+ sensitivity28. While pseudo-phosphorylation does not always mimic phosphate incorporation, we and others have demonstrated the mutation of TnI Ser23/24 residues to the negatively charged Asp residues function both structurally and functionally identical to the effects of PKA-mediated negative charged phosphate incorporation into TnI Ser-23/24 of cardiac muscle28-31. For the current study, we crossed TnI-PP mice28 with mice that expressed mutated Tm at position 180 (E180G or Tm180) linked to HCM32. The Tm180 mice exhibit increased myofilament Ca2+ sensitivity, diastolic dysfunction and cardiac hypertrophy in accordance with the main aspects of the human disease20,21,32. We found that Tm180 mice expressing TnI-PP (DTG mice) were rescued from pathological cardiac remodeling and further displayed improved LV performance when compared to Tm180 littermates expressing wild-type TnI. Our results strongly suggest that myofilament desensitization to Ca2+ is a viable novel therapeutic strategy for treatment of HCM linked to thin filament protein mutations.
Methods
For more detailed methods see the on-line Supplemental Material.
Generation of new TG mice
New TG mouse lines were generated by crossbreeding existing lines of mice, Tm18032 and TnI-S23,24D (TnI-PP)28 mice. All mice used in this work were in the FVBN genetic background. Four groups of mice were used for experiments: 1) NTG, which express wild-type Tm and TnI; 2) TnI-PP, which express wild-type Tm and TnI-PP; 3) Tm180, which express Tm180 and wild-type TnI; 4) Tm180/TnI-PP (DTG), which express Tm180 and TnI-PP.
All animal procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Review Board of the University of Illinois at Chicago.
pCa-Force relationship in skinned fiber preparation
Measurements of pCa-force relations were performed as previously described33.
Whole troponin complex exchange in skinned fiber preparation
Cloning and whole troponin exchange in skinned fiber preparation were done as previously described34. For skinned fiber experiments, the bundles from NTG or Tm180 hearts were obtained as described above.
Echocardiography
Echocardiography was performed using a Vevo 770 High-Resolution In Vivo Imaging System and RMVTM 707B scan head with a center frequency of 30 MHz (VisualSonics, Toronto, ON, Canada) as previously described20. Echocardiographic studies were performed in each animal at 1, 2, 8 and 14 weeks after birth.
Langendorff perfused hearts
Left ventricular function was measured in 6-week old animals as previously described35.
In situ hemodynamic
In situ pressure-volume measurements were performed in 14-week old mice as previously described20.
Histology
The histology was done in hearts section from 14-week old mice as previously described20.
Western blots
The western blots were performed as previously described20,36,37 with slight modifications.
Assessment of myofilament phosphorylation by Pro-Q Diamond Stain
Assessment of myofilament phosphorylation was performed as previously described38 with slight modifications.
Hydroxyproline assay
Hydroxyproline (HOP) content was determined as previously described21.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 6. Data in the manuscript are presented as mean±SE, n=number of samples. In addition to graphical representation of data, numerical mean±SD are presented in Tables in the Supplemental Material. Differences among four groups were analyzed by one-way ANOVA followed by post-hoc analysis, employing Tukey’s or Fisher’s LSD tests. The name of the post-hoc test used is specified either in figure legends, table legends or in the text. Explicit values for significance are given in the Supplemental Material. When only two isolated groups were compared, Student’s t-test was used. Differences were considered significant when p<0.05.
Results
TnI pseudo-phosphorylation reduces myofilament sensitivity to Ca2+
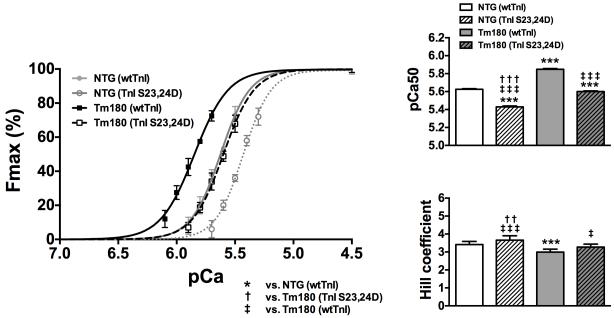
To determine the potential level of myofilament desensitization caused by pseudo-phosphorylation of TnI at Ser23,24, we performed exchange experiments in fiber bundles prepared from NTG and Tm180 hearts. This method allows about 70% exchange of endogenous with exogenous troponin complex39. We exchanged native Tn in detergent skinned fibers from Tm180 and NTG hearts with a Tn complex reconstituted with either unphosphorylated TnI (wtTnI) or pseudo-phosphorylated TnI (TnI-S23,24D). Figure 1 shows that NTG and Tm180 myofilaments reconstituted with TnI-S23,24D were less sensitive to Ca2+ (rightward shift) compared to myofilaments reconstituted with wtTnI (pCa50 =5.62±0.01 NTG(wtTnI), pCa50 =5.43±0.01 NTG(TnI-S23,24D), pCa50 =5.84±0.01 Tm180(wtTnI) and pCa50 =5.60±0.01 Tm180(TnI-S23,24D); n=8). Interestingly, Tm180 myofilaments reconstituted with TnI-S23,24D showed only a slightly smaller sensitivity and a similar cooperativity (Hill coefficient) as NTG myofilaments reconstituted with wtTnI.
Figure 1.
Effects of TnI-S23/24 pseudo-phosphorylation (TnI-S23,24D) on Ca2+-activated tension in skinned fiber preparations. pCa-force relations in NTG and Tm180 skinned fiber bundles exchanged with Tn complex containing either wtTnI or TnI -S23,24D. Exchange with TnI-S23,24D in both NTG and Tm180 fiber bundles resulted in a significant rightward shift (decrease in pCa50) and increase in cooperativity (Hill coefficient) compared to fibers exchanged with Tn complex containing wtTnI. Data are presented as mean±SE. *significantly different vs. NTG(wtTnI); †significantly different vs. Tm180(TnI-S23,24D); ‡significantly different vs. Tm180(wtTnI). ***, ††† or ‡‡‡ p<0.001; †† p<0.01; ‡ p<0.05 based on post-hoc multiple comparison analysis (Tukey’s test). n=8 per group.
Expression of TnI-PP prevents Tm180 hearts from pathological remodeling
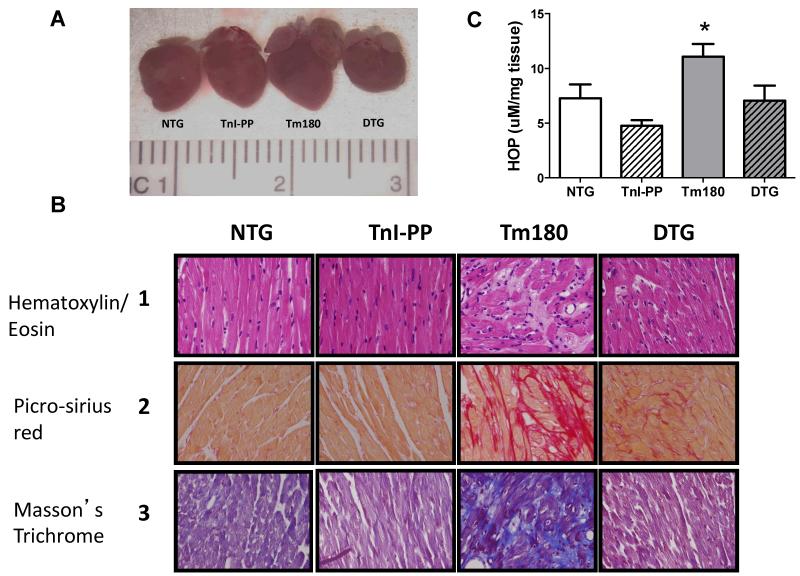
High resolution echocardiography and gross pathology evaluation demonstrated that Tm180 hearts developed cardiac chamber remodeling that was rescued by expression of TnI-PP (Figures 2 and 3). Figure 2A-B shows that Tm180 hearts displayed significantly enlarged left atria (LA) as early as 2 weeks of age and atrial size was lower or normal in Tm180 hearts expressing TnI-PP. Figure 2C-F show that Tm180 hearts displayed severe LV concentric hypertrophy (increased septal wall thickness (SWT) with unaltered LV internal diastolic dimension (LVDd), higher LV mass and higher heart weight to tibia length (HW/TL)) ratio that was not seen in DTG hearts. Histological studies of Tm180 heart sections demonstrated marked myocardial disarray, myocyte hypertrophy and higher collagen deposition in the extracellular matrix compared to all other groups (Figure 3). When compared to NTG, DTG hearts also displayed higher collagen content in the extracellular matrix, but this was seen to a lesser degree than in Tm180 hearts (Figure 3B). Collagen content was also determined by hydroxyproline assay. Figure 3C shows that hydroxyproline content was significantly higher in Tm180 hearts compared to NTG (11.07±1.17 μmol/mg (n=5) vs. 7.27±1.28 μmol/mg (n=5)), but was normal in DTG hearts (7.07±1.38 μmol/mg (n=5)).
Figure 2.
Evaluation of cardiac morphology by two-dimentional (2-D) and M-mode of high resolution echocardiography. Panel A. 2-D short axis views of NTG, TnI-PP, Tm180 and DTG 8 week-old mouse hearts. Left atrium (LA) size is increased only in Tm180 mouse (white arrow). No morphological abnormalities were detected in TnI-PP or DTG hearts. Panels B-E. Time dependent changes in morphological parameters in NTG, TnI-PP, Tm180 and DTG mice: LA anterio-posterior internal dimension (B), LV internal diastolic dimension (LVDd) (C), septal wall thickness (SWT) (D), LV calculated mass (E). Panel F. HW/TL ratio in 14 week-old mice. Data are presented as mean±SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm180 based on post-hoc multiple comparison analysis (Tukey’s test). Numerical data and p values for significance are presented in Table 1 in the Supplemental Material. n=4-8 per group.
Figure 3.
Morphology and histology of 14-week old mouse heart. Panel A. Comparative gross morphology. Panel B. 40x magnification of heart sections (septal wall) stained with Hematoxylin and Eosin (HE, line 1), Picro-sirius red (PSR, line 2) and Masson’s Trichrome (line 3). The intense myocyte disarray is depicted in HE-stained sections and the extensive fibrosis is observed in PSR-(red stain) and Masson’s Trichrome-stained sections (blue stain) in Tm180 hearts. DTG hearts show less disarray and fibrosis than Tm180. Panel C. Hydroxyproline content in NTG, TnI-PP, Tm180 and DTG mice. Data are presented as mean±SE. *Significantly different from all other groups based on post-hoc multiple comparison analysis (Fisher’s test; p<0.05). n=4-5 per group.
TnI-PP prevents the LV dysfunction seen in Tm180 hearts
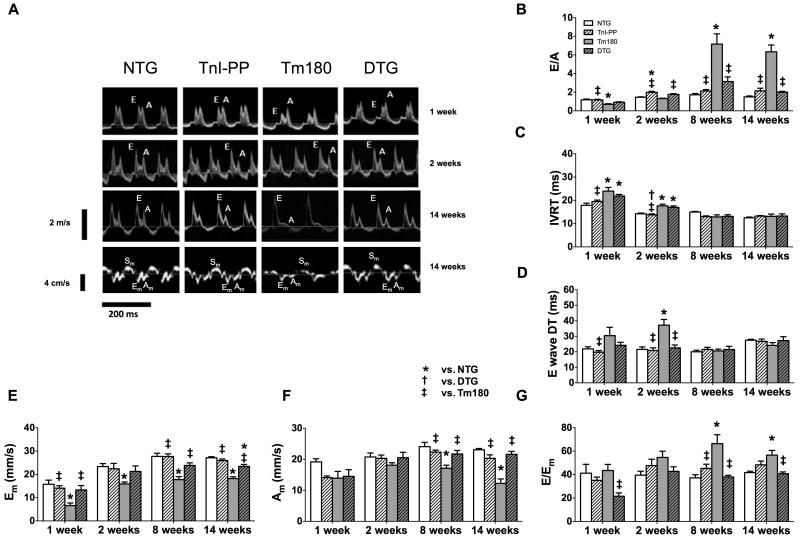
Serial echocardiography and Doppler studies showed that Tm180 mice developed severe diastolic dysfunction. Figure 4 summarizes the evaluation of the diastolic function by pulsed and tissue Doppler (TDI) studies in all four groups of mice at different ages. The mitral inflow pattern showed impaired relaxation of the LV with lower E/A ratio and prolonged isovolumic relaxation time (IVRT) in 1-week old Tm180 mice, which progressed to a restrictive pattern of LV filling with high E/A ratio, normal IVRT and E wave DT in 8-week old mice (Figure 4B-D). Peak myocardial velocity in the early phase of diastole (Em), assessed by TDI, was lower in Tm180 mice compared to NTG and TnI-PP mice, which confirmed early LV impaired relaxation (Figure 4E). Peak myocardial velocity in late diastole (Am) was lower and the E/Em ratio was higher in Tm180 hearts after 8 weeks when compared to NTG, suggesting a progressive decrease of the LV compliance and increased filling pressure (Figure 4F-G). Importantly, the mitral inflow pattern and TDI-derived indexes demonstrated that the diastolic function was normal in DTG mice.
Figure 4.
Serial evaluation of diastolic function by echocardiography. Panel A. Representative images of pulsed Doppler of mitral inflow (top 3 panels) and TDI recordings of the mitral annulus (bottom panel) in NTG, TnI-PP, Tm180 and DTG mice at 1, 2 and 14 weeks of age. Panels B-D. Diastolic function parameters measured by pulsed Doppler: peak velocity of mitral blood inflow in early diastole (E) to peak velocity of mitral blood inflow in late diastole (A) (E/A ratio) (B), isovolumic relaxation time (IVRT) (C), E wave deceleration time (DT) (D) in NTG, TnI-PP, Tm180 and DTG mice. Tm180 hearts show progression from a mild form of diastolic dysfunction (1 week) to a severe form (8 weeks). Panels E-G. Diastolic function parameters measured by TDI: peak myocardial velocity in early diastole (Em) (E), peak myocardial velocity in late diastole (Am) (F) and E to Em ratio (E/Em) (G). Tm180 hearts showed decreased Em in all ages, decreased Am and increased E/Em ratio after 2 weeks, suggesting early impaired relaxation and progressive decrease in LV compliance. DTG hearts showed mitral inflow and TDI patterns similar to NTG controls. Data are presented as mean±SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm180 based on post-hoc multiple comparison analysis (Tukey’s test). Numerical data and p values for significance are presented in Table 2 in the Supplemental Material. n=4-8 per group.
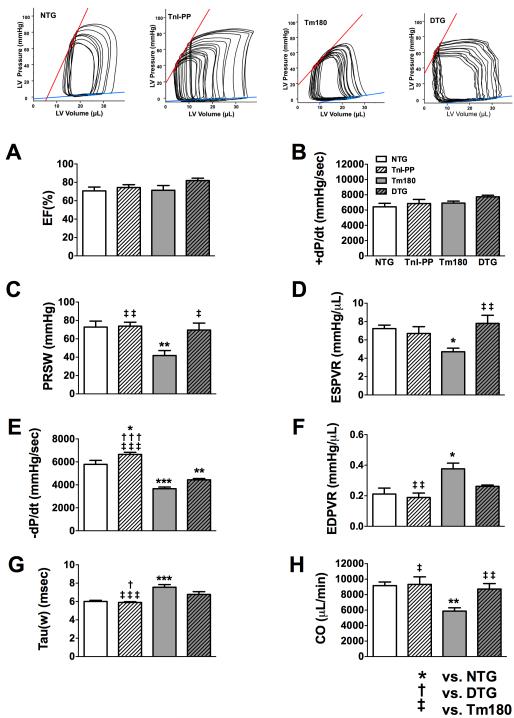
Systolic function, as assessed by ejection fraction, was preserved in Tm180 mice until 14 weeks of age (NTG=62.4±2.5% (n=8), TnI-PP=62.0±3.8% (n=8), Tm180=63.9±2.5% (n=8) and DTG=70.9±2.7% (n=6)). However, at this age systolic peak myocardial velocity (Sm) was lower in Tm180 mice when compared to NTG (17.6±0.7 cm/s vs. 21.7±0.3 cm/s, respectively), suggesting impaired myocardial contractility. This finding was not observed in DTG mice (Sm=21.8±0.6 cm/s), which was not significantly different from NTG.
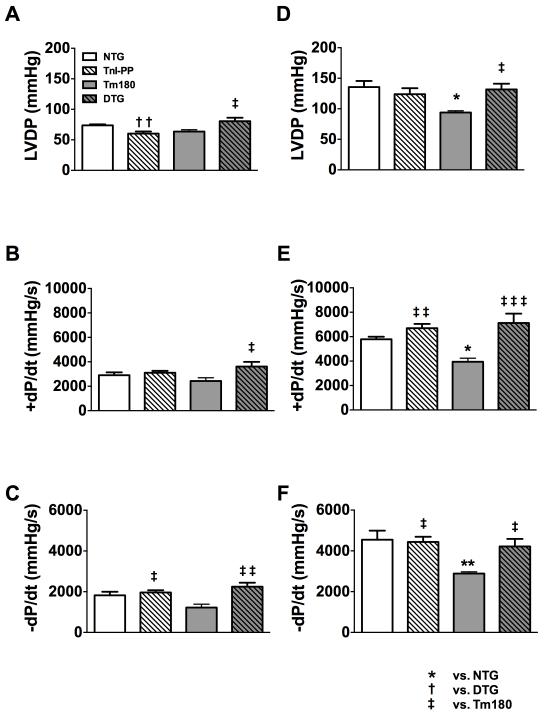
We also evaluated the LV performance of isolated hearts from 6-week old mice in Langendorff preparations (Figure 5 and Table 3 in the Supplemental Material). The LV performance was studied at baseline conditions and after stimulation with 25 nmol/L of isoproterenol (ISO). Although at this age the basal contractile parameters were not significantly different in Tm180 hearts compared to NTG (Figure 5A-C), after stimulation with ISO, LVDP and +dP/dt were significantly lower in Tm180 hearts compared to NTG (Figure 5D-E), indicating a reduced capacity of the Tm180 hearts to adapt to increased contractile demand. Moreover, the -dP/dt was lower in 6-week old Tm180 mice compared to NTG (Figure 5F), which denotes an impaired lusitropic response to stress. On the other hand, age-matched DTG mice showed normal LV performance at rest and adequate inotropic and lusitropic responses to stress, as denoted by LVDP, +dP/dt and -dP/dt, both at rest and after stimulation with ISO.
Figure 5.
Inotropic and lusitropic responses to β-adrenergic stimulation in isolated 6 weeks old Langendorff perfused NTG, TnI-PP, Tm180 and DTG hearts. A. LV developed pressure (LVDP), B. the maximal rate of contraction (+dP/dt) and C. the maximal rate of relaxation (-dP/dt) at baseline. D. LVDP, E. +dP/dt and F. -dP/dt during perfusion with 25 nmol/L isoproterenol. Data are presented as mean±SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm180 based on post-hoc multiple comparison analysis (Tukey’s test). ‡‡‡ p<0.001; ** or †† p<0.01; ‡ or * p<0.05. Numerical data are presented in Table 3 in the Supplemental Material. n=4-5 per group.
We also used a P-V conductance catheter to perform hemodynamic studies in 14-week old mice (Figure 6 and Table 4 in the Supplemental Material). Tm180 mice showed normal values for ejection fraction and +dP/dt, but lower preload recruited stroke work (PRSW) and end systolic pressure-volume relation (ESPVR), a load-independent parameter of contractility. The diastolic function was impaired in Tm180 mice as assessed by the load-dependent parameters - dP/dt and Tau and by the load-independent parameter end diastolic pressure volume relation (EDPVR). However, there was no significant difference in end diastolic pressure between groups. These abnormalities of myocardial contractility and relaxation resulted in reduced hemodynamic performance in Tm180 hearts, as demonstrated by the lower cardiac output in Tm180 mice compared to NTG. Importantly, myocardial contractility was normal in age-matched DTG mice, with the mean values of +dP/dt, ESPVR and PRSW showing no differences from NTG mice. Despite the fact that DTG mice showed lower relaxation rate (lower -dP/dt compared to NTG), this diastolic abnormality was less pronounced than in Tm180. Furthermore, the load-independent parameter EDPVR in DTG mice was not significantly different from NTG. Overall, cardiac performance was normal in DTG mice, as can be seen by the normal values of cardiac output.
Figure 6.
In situ cardiac function in 14-week old NTG, TnI-PP, Tm180 and DTG mice. A. Ejection fraction (EF), B. the maximal rate of contraction (+dP/dt), C. Preload recruited stoke work (PRSW), D. end systolic pressure-volume relation slope (ESPVR), E. the maximal rate of relaxation (-dP/dt), F. end diastolic pressure-volume relation (EDPVR), G. relaxation time constant calculated by Weiss method (tau), H. cardiac output (CO). Data are presented as mean±SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm180 based on post-hoc multiple comparison analysis (Tukey’s test). *** or ‡‡‡ p<0.001; ** or ‡‡ p<0.01; ‡, * or † p<0.05. Numerical data are presented in Table 4 in the Supplemental Material. n=6-8 per group.
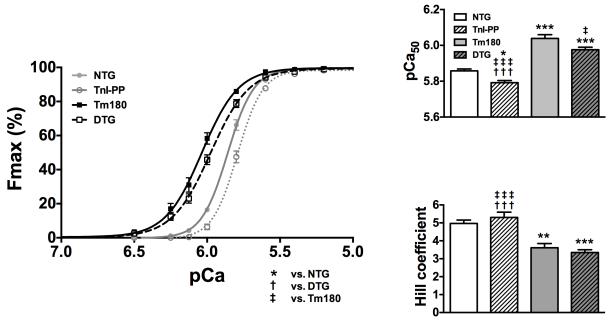
Expression of TnI-PP in Tm180 mice reduces myofilament Ca2+ sensitivity
In the exchange experiments we observed that myofilament sensitivity to Ca2+ is significantly lower when fibers were exchanged with TnIS23,24D compared to fibers exchanged with wtTnI, independent of whether the fibers were prepared from 14-week old NTG or Tm180 hearts. To test the degree to which these findings are similar in a more physiologically-relevant system, we also evaluated the degree of desensitization in skinned fibers prepared from NTG and Tm180 mouse hearts expressing either wild-type TnI or TnI-PP (Figure 7). The pCa-force relations showed that myofilaments from TnI-PP hearts had a lower Ca2+ sensitivity (pCa50=5.79±0.01; n=6) than NTG (pCa50=5.85±0.01, n=6), while Tm180 hearts had higher Ca2+ sensitivity (pCa50=6.04±0.02, n=6). The Ca2+ sensitivity of fibers from DTG hearts (pCa50=5.97±0.01, n=6) was significantly lower than Tm180 and was observed somewhere between the sensitivities for Tm180 and NTG fibers. The Hill coefficient, an indicator of cooperativity or steepness of the pCa-force relationship, was lower in Tm180 and DTG fibers when compared to their matched controls, NTG and TnI-PP, but there were no differences in the maximal generated tensions (data not shown).
Figure 7.
pCa-force relations in skinned fiber bundles prepared from papillary muscles of NTG, Tm180, TnI-PP and DTG mice. Expression of TnI-PP in the presence of Tm180 mutations (DTG) caused a decrease in the myofilament Ca2+ (pCa50) sensitivity and cooperativity of activation (Hill coefficient). Data are presented as mean±SE; *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm180 based on post-hoc multiple comparison analysis (Tukey’s test). ***, ††† or ‡‡‡ p<0.001; ** p<0.01; * or ‡ p<0.05. n=6-8 per group.
Since Tm180 and TnI-PP were co-expressed in DTG mice, we assessed the levels of expression of Tm180 in Tm180 and DTG hearts. There were no significant differences in Tm180 expression between hearts from Tm180 and DTG mice (63.08±4.79% (n=4) vs. 62.56±3.69% (n=4), respectively). Also, the total Tm expression was not different among the four groups of animals (normalized to actin, in arbitrary units: NTG = 0.68±0.07, TnI-PP=0.70±0.06, Tm180=0.78±0.08 and DTG=0.79±0.06, n=5 for each group). Since the reduction in myofilament Ca2+ sensitivity caused by expression of TnI-PP in Tm180 mice was smaller than the decreased myofilament sensitivity caused by exchange of wtTnI by TnI-PP (Figure 1), we also assessed the level of expression of TnI-PP in TnI-PP and DTG mice, as well as the levels of myofilament phosphorylation. There were no significant differences in TnI-PP expression between hearts from TnI-PP and DTG mice (85.5±2.26% (n=4) and 86.0±3.4% (n=4) respectively). Furthermore, DTG mice demonstrated increased TnT phosphorylation and a trend towards increased phosphorylation of LC2 compared to Tm180 mice. The level of TnI phosphorylation was reduced in TnI-PP and DTG mice, however no changes in MyBP-C were observed (see also the Supplemental Material).
Total expression of Serca2 and phospholamban (PLN) is unaltered, but the phosphorylation level of PLN is higher in DTG compared to Tm180
Since the rate of cardiac relaxation depends on the Ca2+ uptake by the SR, we assessed expression of Serca2 and PLN at 14 weeks of age. There were no changes in expression of either protein between the four groups of mice (data not shown), but we found that the level of PLN phosphorylation at the PKA site, Ser-16, was higher in DTG mice compared to Tm180 (see the Supplemental Material).
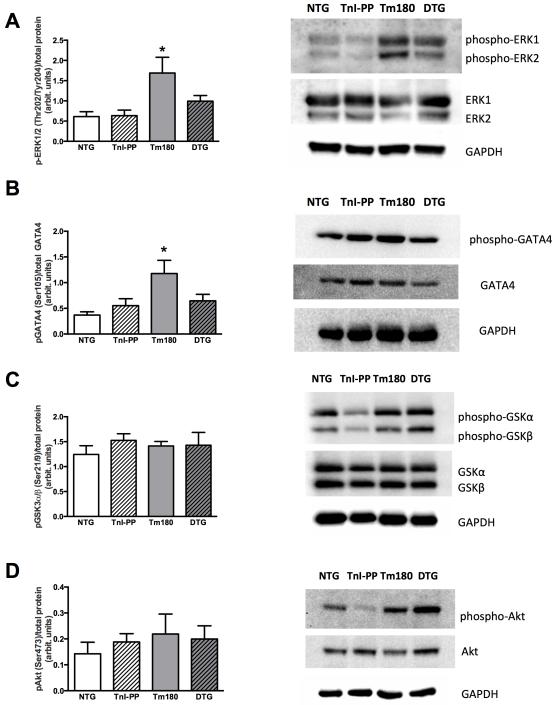
Expression of TnI-PP in Tm180 hearts results in normal activity of ERK1/2 and GATA4
Figure 8A shows that the level of phosphorylation of ERK1/2 was higher in Tm180 hearts compared to control groups and that phosphorylation was normal in DTG mice (NTG =0.61±0.12; TnI-PP=0.63±0.14; Tm180=1.69±0.39; DTG=0.99±0.14, n=7). The level of GATA4 phosphorylation was also higher in Tm180 compared to control groups, along with normal level in DTG hearts (NTG=0.37±0.06; TnI-PP=0.55±0.14; Tm180=1.18±0.26; DTG=0.65±0.13, n=6). There were no observed differences in the activation of Akt or GSK3α/β between groups.
Figure 8.
Phosphorylation of ERK1/2, GATA4, GSK3α/β and Akt. A. phosphorylation of ERK1/2, B. phosphorylation of GATA4, C. phosphorylation of GSK3α/β and D. phosphorylation of Akt. Data are presented as mean±SE. Phosphorylated protein bands were normalized to total protein. No significant differences in total protein expression were observed. *significantly different from all other groups based on post-hoc multiple comparison analysis (Fisher’s test; p<0.05). n=6-7 per group.
Discussion
Experiments reported here are the first to show that a partial and sustained decrease of myofilament Ca2+ sensitivity, by expression of pseudo-phosphorylated TnI, in an HCM mouse model linked to a Tm mutation can completely prevent the development of the hypertrophic phenotype and improve cardiac function.
Most of the mutations in thin filament proteins linked to HCM consistently show increased myofilament sensitivity to Ca2+ resulting in diastolic dysfunction and eventual late progression to systolic dysfunction9,40,41. Therefore, correcting the relaxation properties in HCM through desensitization of the myofilaments seems to be a straightforward and logical approach. We have previously shown that decreasing myofilament Ca2+ sensitivity in Tm180 hearts by expression of chimeric α/β-Tm rescued the hypertrophic phenotype and improved cardiac function42. The major limitation of these studies was that by crossing two TG mouse models with changes in Tm, we generated double-TG mice that not only had a decreased myofilament Ca2+ sensitivity compared to Tm180 mice, but also expressed lower levels of mutated Tm180. Therefore, the prevention of the hypertrophic phenotype could have been attributed to both a decreased myofilament Ca2+ sensitivity and to a lower level of expression of mutated Tm180. This was not the case in the present study, as the reduction in Ca2+ sensitivity occurred without changes in the expression level of Tm180. Similar studies have also been performed by Li et al43. The authors corrected diastolic dysfunction caused by a mutation in TnI by expressing a truncated TnI. However, Li et al43 did not use an HCM, but rather a restrictive cardiomyopathy (RCM) mouse model that showed only mild diastolic dysfunction without hypertrophy and only a slight increase in LA size. In our studies we used a mouse model with severe hypertrophy, fibrosis and diastolic dysfunction present as early as 1 week of age that progressed to severe diastolic dysfunction as early as 2 weeks of age (Figure 4). Moreover, our goal was to desensitize the myofilaments without altering the expression of mutated Tm to make the studies more clinically relevant and as a proof of concept that treatment of HCM with desensitizers should be considered as a potential new treatment. TnI has been recognized as an important regulatory protein for Ca2+-mediated thin filament activation44,45 and furthermore, phosphorylation of TnI at Ser-23/24 reduces the myofilament Ca2+ sensitivity46,47. This makes TnI an excellent potential target for the development of Ca2+ desensitizers. Moreover, the mechanism of action for some Ca2+ sensitizers is through TnC-TnI48 suggesting the possibility that development of Ca2+ desensitizers could also target TnI. To test the hypothesis that expression of pseudo-phosphorylated TnI at Ser23/24 can successfully desensitize Tm180 myofilaments to Ca2+, we performed exchange experiments in which native TnI was replaced by either TnI-S23,24D or wtTnI in skinned fibers from NTG and Tm180 hearts (Figure 1). We found that replacement of Tm180 myofilaments with a Tn complex possessing TnI-S23,24D successfully desensitized them to Ca2+. This would suggest that our DTG mice, which express Tm180 and TnI-PP, should also show normalized myofilament Ca2+ sensitivity. However, skinned fiber data from DTG mice showed only partial restoration of myofilament Ca2+ sensitivity (Figure 7). This can be due to differences in posttranslational modifications of myofilaments between different groups of mice. For example, we found that there was a trend toward higher LC2 phosphorylation in DTG compared to Tm180 mice (Supplemental Material). It is well documented that phosphorylation of LC2 results in increased myofilament Ca2+ sensitivity and can balance the decrease caused by increasing TnI phosphorylation at PKA sites49. Overall our data indicate that either small desensitization is sufficient to prevent the development of a hypertrophic phenotype or that additional compensatory mechanisms are present in DTG mice and contribute to the observed rescue of the Tm180 phenotype. We have previously shown that increasing SR Ca2+ uptake can rescue Tm180 hearts21, therefore we tested whether expression of Serca2a and PLN proteins or phosphorylation of PLN are altered in DTG mice compared to other groups. We found no changes in Serca2a and PLN expression or PLN phosphorylation at Thr17 between groups. However, Ser16 phosphorylation of PLN was increased in DTG mice compared to the other groups, suggesting that the increase in SR Ca2+ uptake may also contribute to the prevention of the hypertrophic phenotype.
Based on these findings, we postulate that there is a range of myofilament sensitivity to Ca2+ that one could consider as a “homeostatic safe zone”. Our data suggest that if the use of Ca2+ desensitizers could bring the myofilament Ca2+ sensitivity into this homeostatic range, then this would be of therapeutic benefit in preventing the development of the hypertrophic phenotype in HCM caused by mutations in thin filament proteins. It is important to note, however, that exacerbated desensitization of the myofilament has the potential to cause a reduction in systolic function and may ultimately lead to systolic HF, although this was not observed in our mouse model. This idea of a “safe zone” of myofilament Ca2+ sensitivity is also indirectly supported by other studies. It is known that the myofilament Ca2+ sensitivity changes as a result of phosphorylation of the myofilament proteins, not only in pathological conditions as a result of adaptation or maladaptation, but also during physiological conditions such as exercise50. Several in vitro studies have shown that phosphorylation of TnI by PKA can shift the myofilament Ca2+ sensitivity as much as 0.2 pCa units46,51, suggesting that similar changes occur in vivo. Also, we have previously shown that another TG mouse model of HCM with the mutation D175N in Tm, which shows a small relative increase of myofilament sensitivity to Ca2+, also demonstrates only a small relative degree of diastolic dysfunction and almost no hypertrophy52. This suggests that the myofilaments and hearts from TmD175N mice operate near this “safe zone” of Ca2+ sensitivity.
The reduction in myofilament Ca2+ sensitivity in Tm180 mice resulted not only in improvement of heart morphology and function, but also prevented the altered activity of ERK1/2 and GATA4. The increased activity of ERK1/2 in an HCM transgenic rabbit model caused by a mutation in β-MHC has been previously reported18. We have also previously shown this in the Tm180 mouse model in which the increased phosphorylation of ERK1/2 seen could be prevented by PLN knockout (PLNKO)20. This finding suggests that either an intervention in Ca2+ regulation and/or correction of relaxation contributes to the observed restoration of ERK1/2 activity. However, our current data show that correction of relaxation by desensitization of the myofilaments, without major changes in SERCA or PLN expression, also prevented the ERK1/2 modifications. The involvement of ERK1/2 signaling along with JNK1 and p38 kinases in the development of hypertrophy is well documented53. Recently, Kehat et al.54 reported that ERK1/2 uniquely regulates the balance between eccentric and concentric growth of the heart, suggesting that elevations in ERK1/2 activity simultaneously inhibits addition of new sarcomeres in series while promoting addition of new sarcomeres in parallel. It is possible that ERK1/2 activation depends on both the kind of stimulus (pressure vs. volume overload or genetically linked HCM) as well as the changes that occur during the course of disease development.
In addition to the improvement in morphology and cardiac function in Tm180 mice, desensitization of myofilaments to Ca2+ in HCM could have an additional beneficial effect – reduction of the susceptibility to arrhythmia. Increased myofilament sensitivity to Ca2+ increases the risk of arrhythmia and, therefore, reduction of Ca2+ sensitivity would be anti-arrhythmic55. In as much as DTG hearts mice show decreased fibrosis and reduced myofilament Ca2+ sensitivity, they also should be less sensitive to arrhythmia. More experiments, which are beyond the scope of the present experiments, are required.
In summary, we have demonstrated that desensitization of myofilaments to Ca2+ should be considered as a new and promising therapeutic intervention for HCM caused by a mutation in thin filament proteins and that TnI may be an important target for the development of myofilament Ca2+ desensitizers. A similar approach may be also adequate in cases of HCM caused by gene mutations in thick filament or Z-disc proteins but more studies would be required when specific myofilament Ca2+ desensitizers become available.
Supplementary Material
Acknowledgments
Funding Sources: This research was supported by NIH research grants PO1 HL-62426 (Project 1, RJS and BMW, and Core C, CMW), RO1 HL-64035 (RJS and BMW), RO1 HL-81680 (DFW), RO1 HL-91056 (BJB), P01 HL-077101 (JR), P01 HL-49058 (JR), R01 HL-105924 (JR) and The Transatlantic Network of Excellence Program grant from Le Fondation Leducq (J.R.). RDG, JNS, ACH, RTD and BJB were supported by T32 HL-07692.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:e783–831. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: An american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. doi: 10.1111/j.1365-2559.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- 4.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 5.Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201–211. doi: 10.1016/j.jacc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer WP, van Dijk SJ, Stienen GJ, van Rossum AC, van der Velden J, Germans T. The development of familial hypertrophic cardiomyopathy: From mutation to bedside. Eur Journal Clin Invest. 2011;41:568–578. doi: 10.1111/j.1365-2362.2010.02439.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. 2011;109:86–96. doi: 10.1161/CIRCRESAHA.111.242974. [DOI] [PubMed] [Google Scholar]
- 8.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 9.Tardiff JC. Thin filament mutations: Developing an integrative approach to a complex disorder. Circ Res. 2011;108:765–782. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 11.Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, et al. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 12.Marian AJ. Contemporary treatment of hypertrophic cardiomyopathy. Tex Heart Inst J. 2009;36:194–204. [PMC free article] [PubMed] [Google Scholar]
- 13.Nagueh SF, Lombardi R, Tan Y, Wang J, Willerson JT, Marian AJ. Atorvastatin and cardiac hypertrophy and function in hypertrophic cardiomyopathy: A pilot study. Eur Journal Clin Invest. 2010;40:976–983. doi: 10.1111/j.1365-2362.2010.02349.x. [DOI] [PubMed] [Google Scholar]
- 14.Araujo AQ, Arteaga E, Ianni BM, Buck PC, Rabello R, Mady C. Effect of losartan on left ventricular diastolic function in patients with nonobstructive hypertrophic cardiomyopathy. Am J Cardiol. 2005;96:1563–1567. doi: 10.1016/j.amjcard.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 15.Tsybouleva N, Zhang LF, Chen SN, Patel R, Lutucuta S, Nemoto S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284–1291. doi: 10.1161/01.CIR.0000121426.43044.2B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827–834. doi: 10.1016/j.jacc.2005.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, et al. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2005;97:285–292. doi: 10.1161/01.RES.0000177090.07296.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, et al. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation. 2009;119:1398–1407. doi: 10.1161/CIRCULATIONAHA.108.790501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaffin RD, Pena JR, Alves MS, Dias FA, Chowdhury SA, Heinrich LS, et al. Long-term rescue of a familial hypertrophic cardiomyopathy caused by a mutation in the thin filament protein, tropomyosin, via modulation of a calcium cycling protein. J Mol Cell Cardiol. 2011;51:812–820. doi: 10.1016/j.yjmcc.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pena JR, Szkudlarek AC, Warren CM, Heinrich LS, Gaffin RD, Jagatheesan G, et al. Neonatal gene transfer of Serca2a delays onset of hypertrophic remodeling and improves function in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2010;49:993–1002. doi: 10.1016/j.yjmcc.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 24.Wolska BM, Arteaga GM, Pena JR, Nowak G, Phillips RM, Sahai S, et al. Expression of slow skeletal troponin I in hearts of phospholamban knockout mice alters the relaxant effect of beta-adrenergic stimulation. Circ Res. 2002;90:882–888. doi: 10.1161/01.res.0000016962.36404.04. [DOI] [PubMed] [Google Scholar]
- 25.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: Evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circ Res. 2002;90:649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 26.Pena JR, Wolska BM. Troponin i phosphorylation plays an important role in the relaxant effect of beta-adrenergic stimulation in mouse hearts. Cardiovasc Res. 2004;61:756–763. doi: 10.1016/j.cardiores.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, et al. Phosphorylation of troponin I by protein kinase a accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 28.Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, et al. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 29.Finley N, Abbott MB, Abusamhadneh E, Gaponenko V, Dong W, Gasmi-Seabrook G, et al. NMR analysis of cardiac troponin C-troponin I complexes: Effects of phosphorylation. FEBS Lett. 1999;453:107–112. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- 30.Ward DG, Brewer SM, Gallon CE, Gao Y, Levine BA, Trayer IP. NMR and mutagenesis studies on the phosphorylation region of human cardiac troponin I. Biochemistry. 2004;43:5772–5781. doi: 10.1021/bi036310m. [DOI] [PubMed] [Google Scholar]
- 31.Dohet C, al-Hillawi E, Trayer IP, Ruegg JC. Reconstitution of skinned cardiac fibres with human recombinant cardiac troponin-I mutants and troponin-c. FEBS Lett. 1995;377:131–134. doi: 10.1016/0014-5793(95)01319-9. [DOI] [PubMed] [Google Scholar]
- 32.Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, et al. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–1828. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- 33.Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, et al. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H2414–2423. doi: 10.1152/ajpheart.2000.279.5.H2414. [DOI] [PubMed] [Google Scholar]
- 34.Engel PL, Kobayashi T, Biesiadecki B, Davis J, Tikunova S, Wu S, et al. Identification of a region of troponin I important in signaling cross-bridge-dependent activation of cardiac myofilaments. J Biol Chem. 2007;282:183–193. doi: 10.1074/jbc.M512337200. [DOI] [PubMed] [Google Scholar]
- 35.Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Pena JR, et al. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Warren CM, Arteaga GM, Rajan S, Ahmed RPH, Wieczorek DF, Solaro RJ. Use of 2-D dige analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics. 2008;8:100–105. doi: 10.1002/pmic.200700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaffin RD, Chowdhury SA, Alves MS, Dias FA, Ribeiro CT, Fogaca RT, et al. Effects of nicotine administration in a mouse model of familial hypertrophic cardiomyopathy, alpha-tropomyosin D175N. Am J Physiol Heart Circ Physiol. 2011;301:H1646–1655. doi: 10.1152/ajpheart.00277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19:1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- 39.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves ML, Gaffin RD, Wolska BM. Rescue of familial cardiomyopathies by modifications at the level of sarcomere and Ca2+ fluxes. J Mol Cell Cardiol. 2010;48:834–842. doi: 10.1016/j.yjmcc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in troponin that cause HCM, DCM and RCM: What can we learn about thin filament function? J Mol Cell Cardiol. 2010;48:882–892. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, et al. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol. 2007;293:H949–958. doi: 10.1152/ajpheart.01341.2006. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Charles PY, Nan C, Pinto JR, Wang Y, Liang J, et al. Correcting diastolic dysfunction by ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol. 2010;49:402–411. doi: 10.1016/j.yjmcc.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solaro RJ, Rarick HM. Troponin and tropomyosin – proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998;83:471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 45.Solaro RJ, Rosevear P, Kobayashi T. The unique functions of cardiac troponin i in the control of cardiac muscle contraction and relaxation. Biochem Biophys Res Commun. 2008;369:82–87. doi: 10.1016/j.bbrc.2007.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, et al. Troponin i in the murine myocardium: Influence on length-dependent activation and interfilament spacing. J Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- 48.Sorsa T, Heikkinen S, Abbott MB, Abusamhadneh E, Laakso T, Tilgmann C, et al. Binding of levosimendan, a calcium sensitizer, to cardiac troponin C. J Biol Chem. 2001;276:9337–9343. doi: 10.1074/jbc.M007484200. [DOI] [PubMed] [Google Scholar]
- 49.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 50.Frey N, Luedde M, Katus HA. Mechanisms of disease: Hypertrophic cardiomyopathy. Nat Rev Cardiol. 2012;9:91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- 51.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, et al. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin i in the heart. J Physiol. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, et al. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha-tropomyosin manifests cardiac dysfunction. Circ Res. 1999;85:47–56. doi: 10.1161/01.res.85.1.47. [DOI] [PubMed] [Google Scholar]
- 53.Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–280. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.