Abstract
Acute esophageal variceal hemorrhage is one of the clinical events that define decompensated cirrhosis and is associated with high rates of morbidity and mortality. Although recent treatment strategies have led to improved outcomes, variceal hemorrhage still carries a 6-week mortality rate of 15-20%. Current standards in its treatment include antibiotic prophylaxis, infusion of a vasoactive drug and endoscopic variceal ligation. The placement of a transjugular intrahepatic portosystemic shunt (TIPS) is considered for patients that have treatment failure or recurrent bleeding. Recurrent hemorrhage is prevented with the combination of a non-selective beta-blocker and endoscopic variceal ligation. These recommendations however assume that all patients with cirrhosis are equal. Based on a review of recent evidence, a strategy in which patients are stratified by Child class, the main predictor of outcomes, is proposed.
Keywords: Cirrhosis, portal hypertension, variceal hemorrhage, risk stratification, hemorrhage control, prophylactic antibiotics, octreotide, esophageal variceal ligation, esophageal stent, transjugular intrahepatic portosystemic stent, recurrent hemorrhage, secondary prophylaxis, nonselective beta-blockers, carvedilol
Introduction
Variceal hemorrhage is a major consequence of cirrhosis and, together with ascites and encephalopathy, is one of the clinical complications that define decompensated cirrhosis given its high morbidity and mortality. Esophageal varices are present in nearly 50% of patients diagnosed with cirrhosis [1] but the rate of esophageal variceal hemorrhage (EVH) varies widely and depends on variceal characteristics (size and presence of red wale marks) and patient characteristics (severity of liver disease). With current prophylactic therapies (nonselective beta-blockers or ligation) the rate of first EVH in patients with medium/large varices is ∼8% per year. Mortality from EVH has decreased significantly over the last two decades with the implementation of intensive care management, including the use of antibiotic prophylaxis and endoscopic variceal band ligation [2;3]. However, acute EVH still carries a 6-week mortality rate of 15-20% [4].
Acute EVH usually occurs when the portosystemic pressure gradient exceeds 12 mmHg (normal gradient is 5mm Hg or less). However, a pressure gradient ≥20 mmHg is the best predictor of a poor outcome in patients presenting with EVH [5-7]. This finding has led to the concept of risk stratification among patients with EVH based on the identification of prognostic markers. The purpose of this paper is to review the current accepted practices in the management of esophageal EVH and to provide an update based on recent literature, placing special emphasis on risk stratification.
Risk stratification in patients with acute variceal hemorrhage
Measurement of hepatic venous pressure gradient (HVPG), performed within 24 hours of admission and after hemodynamic stabilization of patients with acute EVH, reveals that a HVPG ≥20 mmHg is a strong predictor of treatment failure defined as failure to control hemorrhage (23% vs. 0%), early rebleeding (50% vs. 12%) or death (1-year mortality, 64% vs. 20%) compared to those with an HVPG <20 mmHg. [5]. These findings have been independently validated [6;7].
However, recognizing that these measurements are unavailable at most centers, a study of 117 patients with acute EVH in whom HVPG was measured within 48 hours of admission, sought to define any clinical variables that might be of similar predictive accuracy to that of HVPG. The main endpoint was 5-day treatment failure (uncontrolled hemorrhage, early rebleeding or death within 5 days). Child class, systolic blood pressure at admission, and liver disease etiology were independent predictors of these outcomes. There was a strong relationship between the Child class and an HVPG ≥20mmHg, with more than 80% of Child C patients having an HVPG ≥20mmHg [7]. . Similarly, a more recent study evaluated 185 cirrhotic patients with EVH showing a five-day treatment failure rate of 17% and an overall 5-day mortality rate of 15%. Using multivariate analysis, the investigators determined that Child class C, white blood cell count over 10,000/mm3, and the presence of portal vein thrombosis were the only independent predictors for 5-day treatment failure. [8]. The predictive value of an elevated white blood cell count observed in this study is interesting and may indicate that other features such as the presence of infection or the degree of inflammation may modify outcomes among patients presenting with acute EVH as has been shown in hospitalized patients with cirrhosis [9]. Both studies coincide in their finding of Child class as a main predictor of outcomes in EVH.
General Management of the patient with acute variceal hemorrhage
a) Blood transfusion
Experimental studies had shown that complete blood volume restitution in rats with cirrhosis and portal hypertension subjected to hemorrhage would lead to an increase in portal pressure (higher than at baseline) [10] and to a higher mortality when compared to moderate volume of blood replacement (Castaneda Hepatology 2001). Based on these concerns, the AASLD guidelines recommend maintaining a hemoglobin ∼8 g/dL [4].
This recommendation has been recently validated in a large prospective randomized controlled trial performed in 849 patients presenting with upper gastrointestinal hemorrhage [11]. In the overall group, patients randomized to a restrictive transfusion strategy (transfusion threshold of 7 g/dL with a hemoglobin goal of 7-9 g/dL) had a significantly better 45-day survival than patients randomized to a liberal transfusion strategy (transfusion threshold of 9 g/dL with a hemoglobin goal of 9-11 g/dL). A separate analysis in a subgroup of patients with cirrhosis (277 or 31% of the total) confirms this survival benefit, with 5% mortality in the restrictive transfusion strategy group vs. 9% in the liberal transfusion group (p=0.02). However, the survival benefit was observed in Child class A and B patients (mortality 4% vs. 12% for restrictive and liberal transfusion strategies, respectively) but not in Child C patients in whom a high mortality was observed in both groups (38% and 41% for restrictive and liberal strategies, respectively, p=0.91). Similarly, rebleeding rates were significantly lower in Child A and B patients that received restrictive transfusion strategy (11%) compared to those randomized to liberal strategy (21%), but no significant differences in rebleeding rates were observed among Child C patients. In a subgroup of patients with cirrhosis, HVPG was measured within the first 48 hours and again 2-3 days later to assess the effect of transfusion on portal hypertension. Interestingly, HVPG increased significantly (from 20.5±3.1 mmHg to 21.4±4.3 mmHg, p= 0.03) in the liberal transfusion group but did not change in the restrictive strategy group [11].
This study further supports the recommendation of avoiding over-transfusing patients with acute EVH and could also indicate that volume expansion (with expanders other than blood) should also be used cautiously.
b) Antibiotics
Patients with cirrhosis and gastrointestinal hemorrhage are at a high risk of developing bacterial infections and these in turn are associated with a higher rate of variceal rebleeding and an increased mortality [12;13]. A meta-analysis of five randomized trials of prophylactic antibiotics (vs. no antibiotics) in patients with cirrhosis and GI hemorrhage with or without ascites demonstrates that antibiotic prophylaxis not only decreases the rate of infections but also improves survival [14]. Based on these results, the AASLD guideline recommends short-term(maximum 7 days) antibiotic prophylaxis with oral norfloxacin in all cirrhotic patients admitted with GI hemorrhage (variceal or nonvariceal). A more recent meta-analysis including 12 trials (1,241 patients with cirrhosis and GI hemorrhage) confirming that antibiotic prophylaxis is associated with a significant reduction in all-cause mortality and in the development of bacterial infections but also showing a significant reduction in mortality from bacterial infections, rebleeding and days of hospitalization [15]. Duration of antibiotic prophylaxis in these trials ranges from 3 to 10 days, with a majority using 7 days (thereby the AASLD recommendation) however antibiotic prophylaxis can be discontinued once hemorrhage is controlled and hospitalization should not be prolonged on the basis of completing a 7-day course of antibiotics. A randomized study comparing orally administered norfloxacin vs. intravenous ceftriaxone in patients with advanced liver disease (2 or more of the following: malnutrition, ascites, encephalopathy or jaundice) showed a lower rate of infections, including spontaneous bacterial peritonitis and spontaneous bacteremia, in patients randomized to ceftriaxone [16], however most of the difference was explained by a high rate of infections by quinolone-resistant organisms. Therefore, Baveno consensus continues to recommend oral quinolones for most patients, stating that intravenous ceftriaxone should be considered in patients with advanced cirrhosis, in hospital settings with a high prevalence of quinolone-resistant bacterial infections and in patients on previous quinolone prophylaxis [17].
The issue of infections due to antibiotic-resistant organisms has become an increasingly alarming problem among patients with cirrhosis [18;19] and therefore candidates for antibiotic prophylaxis must be chosen carefully. Although patients with severe liver disease (i.e., Child B and C) are at an increased risk of developing bacterial infections, this risk is not high in those with less severe liver disease (i.e., Child A). Only one of the randomized trials on antibiotic prophylaxis in patients with cirrhosis and GI hemorrhage stratified patients by Child class [20]. In this study, Child A/B patients who did not rebleed, were not enrolled in the study and therefore did not receive antibiotics. In these patients, the infection rate (18%) was not significantly different from the infection rate in patients enrolled in the study (Child C patients or Child A/B who had rebled) randomized to antibiotic prophylaxis (13%), while more than half of the patients randomized to not receive antibiotics developed an infection. This indicates that there is a role for patient stratification regarding antibiotic prophylaxis. In fact, a retrospective analysis of a prospectively collected database of patients with EVH demonstrated that the rate of infection in Child A patients who did not receive antibiotic prophylaxis was very low (2/42 or 5%) as was the 6-week mortality (only 1 patient died from hepatocellular carcinoma), suggesting that antibiotic prophylaxis is probably not necessary in these patients [21].
Specific therapies in the management of acute variceal hemorrhage
a) Vasoconstrictors
Vasoactive medications such as vasopressin and its analogue terlipressin and somatostatin and its analogues (vapreotide and octreotide) are recommended in the treatment of acute EVH, in combination with endoscopic therapy [1;4]. These medications act by producing splanchnic vasoconstriction, thereby reducing portal blood inflow and portal pressure. A recent meta-analysis of 30 randomized controlled trials (3,111 patients) shows that the use of vasoactive agents in acute EVH is associated with significantly lower 7-day all-cause mortality and lower transfusion requirements [22]. The meta-analysis, by type of vasoactive agent, did not demonstrate differences in efficacy, although the quality of evidence was very low. When looking at individual vasoactive agents, 11 studies evaluating octreotide and 7 evaluating terlipressin showed a significant benefit for each of them in the control of EVH, without differences in mortality.
In the United States, the only available vasoactive drug is octreotide, a somatostatin analogue that is administered as an initial 50 mcg bolus followed by a continuous infusion of 50 mcg/hour. Outside of the United States, somatostatin and terlipressin are more commonly used and drug choice is based mostly on cost, although adverse events associated with terlipressin are also taken into consideration. The recommended length of therapy is 3-5 days. One recent study examined whether this length could be shortened to 24 hours. The study included 130 patients who were randomized to receive either 24 hours vs. 72 hours of terlipressin in addition to endoscopic variceal ligation [23]. There were no differences regarding failure to control hemorrhage, 30-day rates of recurrent hemorrhage or mortality between the study groups. Notably, the investigators excluded a large percentage of patients (111 of 241; 46%) that they identified as being at high-risk of rebleeding, mostly patients with HCC and those with a Child score equal or greater to 12. This study suggests that a shorter duration of therapy may be reasonable in a selected population of patients without HCC and with reasonable liver function.
b) Endoscopic therapy
Endoscopic variceal ligation (EVL) is the endoscopic therapy of choice in the treatment of acute EVH and is recommended by both AASLD and the Baveno consensus [4;17]. Sclerotherapy is only indicated when EVL is not feasible. As specified above, it is recommended that EVL be used in combination with a vasoconstrictor [1;4]. Interestingly, one randomized study shows that EVL combined with proton pump inhibitors (PPI), used theoretically to reduce bleeding from post-EVL ulcers, is as effective as EVL combined with a vasoconstrictor (either somatostatin or terlipressin) with a higher rate of side effects in the latter group [24]. Patients included in the study were mostly Child A/B. Failure to control hemorrhage and early rebleeding occurred with the same frequency in both groups (2 in vasoconstrictor, 1 in PPI) and the only predictor of early rebleeding was the Child-score. Sources of hemorrhage were not different and bleeding from post-EVL ulcer occurred in only one patient in the vasoconstrictor group. Therefore, given that PPI does not appear to have an effect on bleeding, this study suggests that some patients, mostly those with low Child scores, may be able to receive EVL alone (without vasoconstrictors). An older meta-analysis suggests that vasoactive drugs alone are useful in controlling EVH [25] and further characterization of patients that may be candidates for single therapy (vasoactive drugs or EVL) would be highly desirable. Until more data becomes available, patients with acute EVH should be treated with EVL plus vasoactive drugs. During endoscopy on patients with acute EVH, blood in the stomach and/or esophagus can often obscure the endoscopic view and make endoscopic intervention difficult to perform. The administration of erythromycin, a prokinetic agent, 125 mg 30 minutes before endoscopy was recently shown to have a higher rate of achieving a stomach free of blood (49%) when compared to placebo (23%) and led to a significantly shorter endoscopy time (19 vs. 26 minutes) [26]
c) Transjugular intrahepatic portosystemic shunt (TIPS)
Current recommendation for those who fail combination endoscopic/pharmacological therapy is TIPS placement [4]. Control of hemorrhage with rescue TIPS exceeds 85% but is associated with a high mortality. This is not surprising given that patients that fail standard therapy usually have advanced disease (Child C) and with failure to control hemorrhage or with rebleeding, liver function deteriorates further so that they become unable to tolerate TIPS placement and, even though hemorrhage is controlled, death from liver insufficiency ensues.
This led to the novel idea of considering TIPS placement in patients at high risk of failing standard therapy, before failure occurs, that is, pre-emptive (or “early”) TIPS placement. In a preliminary study, in which uncoated TIPS stents were used, early placement of TIPS was associated with an improvement in survival [6]. A multicenter controlled trial further explored this concept and randomized high-risk patients with acute EVH to “early” TIPS (polytetrafluoroethylene-coated stents placed within 72 hours of the initial bleed) vs. standard therapy (EVL plus vasoconstrictors) with TIPS placement only in those that failed this therapy [27]. High-risk patients were defined as those with Child C (excluding those with the highest scores of 14 and 15) and Child B patients with active hemorrhage on initial endoscopy (that constituted a minority of patients enrolled). A total of 63 patients were randomized and, in a median followup of 16 months, rebleeding or failure to control hemorrhage occurred in 3% (1/32) of early TIPS group compared to 45% (14/31) of the standard therapy group (p=0.001). Importantly, mortality was significantly lower in the early TIPS group (12%) compared to standard therapy (39%)(p=0.01), without differences in hepatic encephalopathy. Seven patients in the standard therapy group received TIPS as rescue therapy, but four died. A higher than expected mortality rate in the standard therapy group has been noted and, in a retrospective analysis looking at patients treated with standard therapy that would have qualified for the early TIPS study, the mortality was noted to be of only 10% [28]. In order to confirm their findings in a real-life setting, investigators participating in the early TIPS trial performed a retrospective review of patients admitted to their centers after the trial was over and identified 30 eligible patients receiving standard medical therapy and 45 receiving early TIPS [29]. In a mean follow-up period of 13-14 months, failure occurred in 50% (15/30) of the standard therapy group and in 7% (3/45) of patients in whom early TIPS was performed (p<0.001). Regarding mortality, 33% (10/30) of the patients on standard therapy died compared to 13% (6/45) in the early TIPS group (p=0.048). Therefore, the beneficial effects of early TIPS in this high-risk population were confirmed and placement of early TIPS in Child C patients (Child score 10-13) is likely to become standard of care.
d) Balloon tamponade and esophageal stents
Up to 20% of EVH episodes can be refractory to standard therapy and are associated with a high mortality. A “bridge” therapy is necessary in order to acutely control hemorrhage while a more definitive therapy such as TIPS can be performed. Balloon tamponade is still a recommended as bridge therapy and provides hemostasis in up to 80% of patients but is associated with a high rate of severe adverse events and a mortality rate near 20% [4]. The endoscopic placement of self-expanding stents is a technique that has been recently explored in the management of refractory EVH. Since 2006 when it was first described in the literature [30], there have been six additional case series bringing together a total of 61 cases treated with these stents. They report 100% control of hemorrhage in patients with EVH without serious adverse events [31-36].
Esophageal stents in most studies have been removed after 9-11 days. In a recent series, all eight patients treated with the stent had complete and immediate hemostasis, however 3 of 5 patients who continued only on medical therapy had recurrent hemorrhage within 9 days after stent removal [35]. Thus, similar to balloon tamponade, these stents appear to be temporizing methods that will control hemorrhage until a more definitive therapy can be performed. However, in the most recent case series, longer term use of these stents was explored in five patients that were not candidates for TIPS. Stents were removed from two patients (one removed at 14 days and had liver transplantation and the other had their stent removed at 17 days after having TIPS placement) and the other three patients kept their stents until death (at 6, 11, and 214 days) (Holster, Kuipers et al. 2013). Results of an ongoing multicenter randomized trial of balloon tamponade vs. metal stents are eagerly awaited in order to elucidate how to bridge patients with uncontrolled bleeding.
Prevention of Recurrent Variceal Hemorrhage
If left untreated, patients in whom acute EVH is controlled will have a recurrence rate of 60% in the next 1-2 years. Therefore, an integral part of the management of acute EVH is the prevention of recurrent hemorrhage. AASLD guidelines and Baveno consensus recommend combination therapy with drugs plus EVL [4;17].
a) Combination drug therapy /EVL
Drug therapy consists of the oral administration of non-selective beta-blockers (NSBB), propranolol or nadolol, that should be initiated as soon as the parenteral vasoactive medication used during the acute episode (octreotide, terlipressin or somatostatin) is discontinued. AASLD guidelines recommend considering adding isosorbide mononitrate as a combination drug therapy in order to maximally reduce portal pressure and minimize rebleeding rates [4]. However, common practice in the U.S. is to use NSBB alone as patients better tolerate single drug therapy than combination drug therapy. Combination therapy would definitely be recommended in the rare patient that has contraindications to EVL. NSBB should be titrated to the maximum tolerated dose or to a heart rate of 50-55 beats/min. EVL should be performed every 2 weeks until varices are obliterated, after which surveillance endoscopies should be performed every 6 months with repeat EVL in case of variceal recurrence [4].
b) Drug therapy alone
A recent meta-analysis comparing dual therapy (drug therapy plus EVL) vs. monotherapy (either drug therapy or EVL) for the secondary prophylaxis of EVH, includes 9 randomized trials (n=955 total patients; 442 patients receiving combination therapy and 513 receiving monotherapy. The rate of rebleeding from varices was significantly lower among those who received combination therapy, whether compared to drug therapy alone or EVL alone, without differences in survival [37]. However, overall rebleeding rates (and this includes bleeding from post-EVL ulcers), were significantly lower with combination therapy when compared to EVL alone but not significantly different when comparing combination therapy vs. drug therapy alone (3 trials). The latter finding was confirmed in another smaller meta-analysis that examined 4 studies of NSBB alone vs. combination EVL plus NSBB, with lower rates of bleeding from varices in the combination therapy group but without differences in overall rebleeding rates or in survival [38]. This suggests that the benefit of dual therapy may be superseded by bleeding from post-EVL ulcers and methods to prevent this complication of EVL should be sought. It will also be important to further characterize patients who respond to monotherapy. Until more data becomes available, patients who have recovered from an episode of acute EVH should be treated with EVL plus vasoactive drugs
Carvedilol is a NSBB with added vasodilatory α-adrenergic blocking activity that, similar to the combination of NSBB+nitrates, would act by decreasing portal blood inflow (beta-blocker effect) and decreasing intrahepatic vascular resistance (vasodilatory effect). A recent study performed in 121 patients compared carvedilol to combination NSBB+nitrates in the prevention of recurrent EVH [39]. In a mean followup of 30 months, patients randomized to carvedilol (6.5- 12.5 mg /day) had a variceal rebleeding rate of 54% (33/61) that was not different from the 57% (34/60) observed in patients randomized to nadolol plus isosorbide mononitrate group (Lo, Chen et al. 2012). While carvedilol has been mostly studied in the setting of primary prophylaxis (i.e. patients that have not bled from varices) where low doses have a greater portal pressure reducing effect than NSBB, particularly in Child A patients [40], its vasodilatory effect in patients who have bled from varices (i.e. decompensated patients) could be deleterious leading to fluid retention and renal injury as has been shown to occur with higher doses [41]. Further data are needed in order to elucidate the potential use and safety of carvedilol in secondary prophylaxis and will likely depend on the severity of liver disease.
c) Patients with first variceal hemorrhage who had been on prophylaxis with NSBB
The recommendation of combined EVL+ drug therapy for secondary prophylaxis applies to patients who bled despite receiving primary prophylaxis with NSBB [1]. In this respect, a recent retrospective study analyzed 34 patients who had a first episode of variceal bleeding despite primary prophylaxis and compared them to 55 patients who bled without receiving primary prophylaxis [42]. All patients received secondary prophylaxis with a combination of EVL and NSBB and, after 2 years, a greater proportion of patients who had their first episode of bleeding while on NSBBs had recurrent hemorrhage, compared with controls (48% vs. 24%; P = .01), suggesting that these patients may require alternative treatment approaches.
d) Patients with hepatocellular carcinoma (HCC) who bleed from varices
An increasing number of patients with cirrhosis and HCC are presenting with EVH. Compared to patients without HCC, they have a significantly higher 6-week rebleeding (16% vs. 75%) and mortality (30% vs. 15%) rates [43]. In this case-control study, Child score, presence of HCC, portal vein thrombosis, and lack of secondary prophylaxis were independent predictors of death. The latter being the only “fixable” predictor. Interestingly, fewer patients with HCC received secondary prophylaxis after bleeding (77% vs. 89%), and standard combination therapy was used less frequently (58% vs. 70%). Lack of prophylaxis increased rebleeding and mortality. This indicates that patients with HCC should receive the same management guidelines for portal hypertension.
e) HVPG-guided therapy
HVPG-guided therapy would appear to be a rational approach in the treatment for portal hypertension. In a recent study, 48 patients with cirrhosis underwent HVPG measurements before and 14 days after initiation of combination drug therapy (NSBB + nitrates). Responders (HVPG ≤12 mm Hg or ≥20% decrease from baseline) were maintained on drugs and non-responders (n=24) had EVL added to drugs. Rebleeding rates were not significantly different between groups (16% in responders, 8% in non-responders, p=0.7), suggesting that adding EVL to drugs is an effective rescue strategy to prevent rebleeding in HVPG non-responders to drug therapy [44]. However, number of patients studied was small and lacked a control group of patients in whom HVPG-guided therapy was not performed. Although rational, there is not yet sufficient evidence to recommend this practice.
Summary and Conclusions
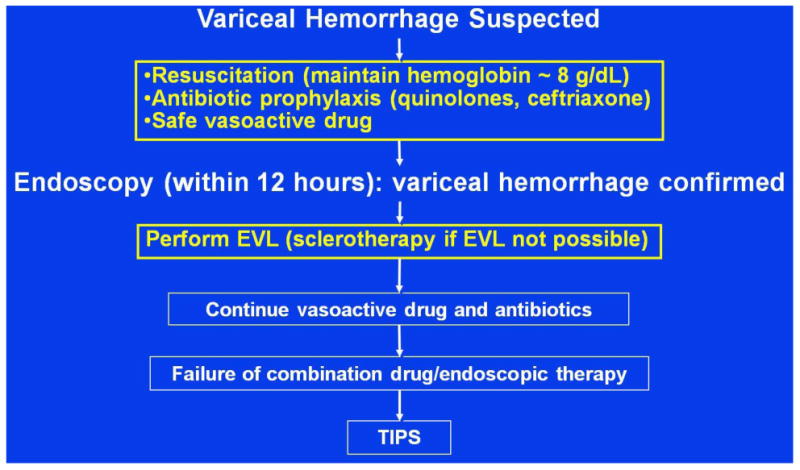
Current recommendations (Figure 1) indicate that when a patient is suspected of having a EVH, volume, particularly blood, should be replaced cautiously; blood transfusions should be given only when the hemoglobin has decreased to ∼ 7 g/dL with a goal of maintaining it around 8 g/dL. Antibiotics (either an oral quinolone or intravenous ceftriaxone if there is concern for quinolone-resistance or in the patient with severe liver disease) and an intravenous vasoactive drug (octreotide in the United States) should be initiated before diagnostic endoscopy, which should be performed within 12 hours of presentation. If a variceal source of hemorrhage is confirmed (by finding either a spurting varix, a clot or a “white nipple” overlying a varix or varices with no other lesion) variceal ligation is recommended. Antibiotics and the vasoactive drug are then continued for 3-5 days. If bleeding is controlled, and as the intravenous vasoactive drug is discontinued, an oral NSBB should be initiated and titrated to a heart rate of 50-55 beats/min. EVL is performed periodically until obliteration with followup surveillance every 6-12 months to check for variceal recurrence. If bleeding persists or recurs, rescue TIPS is recommended. If TIPS is successfully placed, assessment of patency should be performed every six months. Patients in whom TIPS is placed do not require NSBB or EVL.
Figure 1. Management of Acute Esophageal Variceal Hemorrhage – Standard of Care.
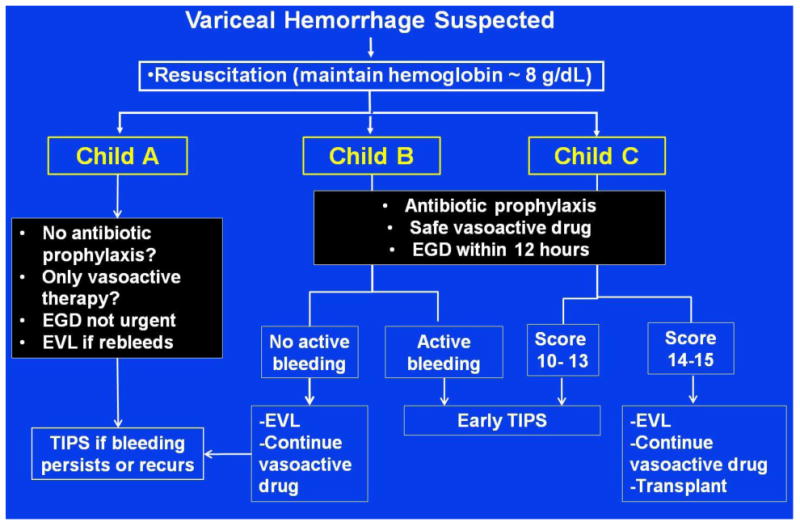
It is becoming obvious that treatment of EVH cannot be the same in all patients with cirrhosis. Treatment should be individualized and stratified according to different risk groups, with the Child class being the method that has been used in this stratification. Our proposed strategy is shown in Figure 2. While restrictive transfusion strategy should generally be recommended, this may not have a major impact in Child C patients. Child A patients may not require antibiotic prophylaxis and may be able to be treated only with vasoactive therapies (therefore diagnostic endoscopy could be delayed) for a shortened duration. If this treatment fails, then EVL would be performed. Child B/C patients would be started on antibiotic prophylaxis and a vasoactive drug, endoscopy would be required within 12 hours at which time EVL would be performed. Child B patients with active bleed or Child C patients with a score of 10-13 would be sent for TIPS within 24-48 hours. Child B patients without active bleeding would continue on a vasoactive drug and would only get TIPS if this treatment fails. Child C patients with a score of 14-15 are not candidates for TIPS and listing for transplant should be a priority. Regarding secondary prophylaxis in patients who did not receive TIPS (not shown in Figure), we would surmise that Child A patients would only require drug therapy, while all others would require combination drug therapy plus EVL. Elements of this strategy will require further validation from large studies before it can be recommended.
Figure 2. Management of acute esophageal variceal hemorrhage – Strategy for the future?
Acknowledgments
Grant support: NIH P-30DK 034989
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Brett Fortune and Guadalupe Garcia-Tsao declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by the authors.
References
- 1**.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–32. doi: 10.1056/NEJMra0901512. An updated look at guidelines for the management of varices and variceal hemorrhage. [DOI] [PubMed] [Google Scholar]
- 2.Carbonell N, Pauwels A, Serfaty L, et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 3.Stokkeland K, Brandt L, Ekbom A, et al. Improved prognosis for patients hospitalized with esophageal varices in Sweden 1969-2002. Hepatology. 2006;43:500–5. doi: 10.1002/hep.21089. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 5.Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626–31. doi: 10.1016/s0016-5085(99)70455-5. [DOI] [PubMed] [Google Scholar]
- 6.Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 7.Abraldes JG, Villanueva C, Banares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229–36. doi: 10.1016/j.jhep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Amitrano L, Guardascione MA, Manguso F, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107:1872–8. doi: 10.1038/ajg.2012.313. [DOI] [PubMed] [Google Scholar]
- 9.Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–82. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Kravetz D, Sikuler E, Groszmann RJ. Splanchnic and systemic hemodynamics in portal hypertensive rats during hemorrhage and blood volume restitution. Gastroenterology. 1986;90:1232–40. doi: 10.1016/0016-5085(86)90390-2. [DOI] [PubMed] [Google Scholar]
- 11**.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. Multicenter large randomized controlled trial that shows that a restrictive transfusion strategy is associated with improved survival, less rebleeding rates and a decrease in portal pressure in a subgroup of patients with cirrhosis. [DOI] [PubMed] [Google Scholar]
- 12.Bernard B, Cadranel JF, Valla D, et al. Prognostic significance of bacterial infection in bleeding cirrhotic patients: A prospective study. Gastroenterology. 1995;108:1828–34. doi: 10.1016/0016-5085(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 13.Vivas S, Rodriguez M, Palacio MA, et al. Presence of bacterial infection in bleeding cirrhotic patients is independently associated with early mortality and failure to control bleeding. Dig Dis Sci. 2001;46:2752–7. doi: 10.1023/a:1012739815892. [DOI] [PubMed] [Google Scholar]
- 14.Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–61. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 15.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509–18. doi: 10.1111/j.1365-2036.2011.04746.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez J, Ruiz DA, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049–56. doi: 10.1053/j.gastro.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17**.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–8. doi: 10.1016/j.jhep.2010.06.004. The most recent consensus conference among experts that led to modifications in the guidelines for the treatment of varices and variceal hemorrhage (reference 4) [DOI] [PubMed] [Google Scholar]
- 18*.Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–61. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 19*.Tandon P, Delisle A, Topal JE, et al. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291–8. doi: 10.1016/j.cgh.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauwels A, Mostefa-Kara N, Debenes B, et al. Systemic antibiotic prophylaxis after gastrointestinal hemorrhage in cirrhotic patients with a high risk of infection. Hepatology. 1996;24:802–6. doi: 10.1002/hep.510240408. [DOI] [PubMed] [Google Scholar]
- 21.Tandon P, Keough A, Bastiampillai RJ, et al. Child Pugh A patients with cirrhosis and acute variceal hemorrhage have low risk of bacterial infection. Hepatology; Presented at the Liver Meeting; Washington, DC. November 2013.2013. p. 993A. abstract. [Google Scholar]
- 22*.Wells M, Chande N, Adams P, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267–78. doi: 10.1111/j.1365-2036.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 23.Azam Z, Hamid S, Jafri W, et al. Short course adjuvant terlipressin in acute variceal bleeding: a randomized double blind dummy controlled trial. J Hepatol. 2012;56:819–24. doi: 10.1016/j.jhep.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Lo GH, Perng DS, Chang CY, et al. Controlled trial of ligation plus vasoconstrictor versus proton pump inhibitor in the control of acute esophageal variceal bleeding. J Gastroenterol Hepatol. 2013;28:684–9. doi: 10.1111/jgh.12107. [DOI] [PubMed] [Google Scholar]
- 25.D'Amico G, Pietrosi G, Tarantino I, et al. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124:1277–91. doi: 10.1016/s0016-5085(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 26.Altraif I, Handoo FA, Aljumah A, et al. Effect of erythromycin before endoscopy in patients presenting with variceal bleeding: a prospective, randomized, double-blind, placebo-controlled trial. Gastrointest Endosc. 2011;73:245–50. doi: 10.1016/j.gie.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 27**.Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–9. doi: 10.1056/NEJMoa0910102. Multicenter randomized controlled trial of “early” (preemptive) TIPS in patients at a high-risk of failing standard therapy (Child score 10-13, Child B with active hemorrhage) showing that this strategy is associated with an increased survival. [DOI] [PubMed] [Google Scholar]
- 28.Augustin S, Altamirano J, Gonzalez A, et al. Effectiveness of Combined Pharmacologic and Ligation Therapy in High-Risk Patients With Acute Esophageal Variceal Bleeding. Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.173. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Pagan JC, Di PM, Caca K, et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Hubmann R, Bodlaj G, Czompo M, et al. The use of self-expanding metal stents to treat acute esophageal variceal bleeding. Endoscopy. 2006;38:896–901. doi: 10.1055/s-2006-944662. [DOI] [PubMed] [Google Scholar]
- 31.Zehetner J, Shamiyeh A, Wayand W, et al. Results of a new method to stop acute bleeding from esophageal varices: implantation of a self-expanding stent. Surg Endosc. 2008;22:2149–52. doi: 10.1007/s00464-008-0009-7. [DOI] [PubMed] [Google Scholar]
- 32.Dechene A, Adamzik M, Gerken G, et al. Acute bronchial obstruction following esophageal stent implantation for variceal bleeding. Endoscopy. 2009;41(Suppl 2):E146–7. doi: 10.1055/s-0028-1119725. Epub;%2009 Jun;%19.: E146-E147. [DOI] [PubMed] [Google Scholar]
- 33.Wright G, Lewis H, Hogan B, et al. A self-expanding metal stent for complicated variceal hemorrhage: experience at a single center. Gastrointest Endosc. 2010;71:71–8. doi: 10.1016/j.gie.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Mishin I, Ghidirim G, Dolghii A, et al. Implantation of self-expanding metal stent in the treatment of severe bleeding from esophageal ulcer after endoscopic band ligation. Dis Esophagus. 2010;23:E35–E38. doi: 10.1111/j.1442-2050.2010.01090.x. [DOI] [PubMed] [Google Scholar]
- 35.Dechene A, El Fouly AH, Bechmann LP, et al. Acute management of refractory variceal bleeding in liver cirrhosis by self-expanding metal stents. Digestion. 2012;85:185–91. doi: 10.1159/000335081. [DOI] [PubMed] [Google Scholar]
- 36.Holster IL, Kuipers EJ, Van Buuren HR, et al. Self-expandable metal stents as definitive treatment for esophageal variceal bleeding. Endoscopy. 2013;45:485–8. doi: 10.1055/s-0032-1326227. [DOI] [PubMed] [Google Scholar]
- 37.Thiele M, Krag A, Rohde U, et al. Meta-analysis: banding ligation and medical interventions for the prevention of rebleeding from oesophageal varices. Aliment Pharmacol Ther. 2012;35:1155–65. doi: 10.1111/j.1365-2036.2012.05074.x. [DOI] [PubMed] [Google Scholar]
- 38.Ko SY, Kim JH, Choe WH, et al. Pharmacotherapy alone vs endoscopic variceal ligation combination for secondary prevention of oesophageal variceal bleeding: meta-analysis. Liver Int. 2012;32:867–9. doi: 10.1111/j.1478-3231.2011.02681.x. [DOI] [PubMed] [Google Scholar]
- 39.Lo GH, Chen WC, Wang HM, et al. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. J Gastroenterol Hepatol. 2012;27:1681–7. doi: 10.1111/j.1440-1746.2012.07244.x. [DOI] [PubMed] [Google Scholar]
- 40*.Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634–41. doi: 10.1136/gutjnl-2012-304038. [DOI] [PubMed] [Google Scholar]
- 41.Minano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39:681–95. doi: 10.1016/j.gtc.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Souza AR, La M V, Reverter E, et al. Patients whose first episode of bleeding occurs while taking a beta-blocker have high long-term risks of rebleeding and death. Clin Gastroenterol Hepatol. 2012;10:670–6. doi: 10.1016/j.cgh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 43**.Ripoll C, Genesca J, Araujo IK, et al. Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case-control study. Hepatology. 2013;58:2079–88. doi: 10.1002/hep.26629. Important case-control study showing that patients with HCC and variceal hemorrhage are less likely to receive secondary prophylactic therapy and the lack of this therapy is associated with a higher mortality. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez A, Augustin S, Dot J, et al. Adding banding ligation is effective as rescue therapy to prevent variceal rebleeding in haemodynamic non-responders to pharmacological therapy. Dig Liver Dis. 2012;44:55–60. doi: 10.1016/j.dld.2011.07.019. [DOI] [PubMed] [Google Scholar]


