Abstract
Maintaining human alertness and behavioral capability under conditions of sleep loss and circadian misalignment requires fatigue management technologies due to: (1) dynamic nonlinear modulation of performance capability by the interaction of sleep homeostatic drive and circadian regulation; (2) large differences among people in neurobehavioral vulnerability to sleep loss; (3) error in subjective estimates of fatigue on performance; and (4) to inform people of the need for recovery sleep. Two promising areas of technology have emerged for managing fatigue risk in safety-sensitive occupations. The first involves preventing fatigue by optimizing work schedules using biomathematical models of performance changes associated with sleep homeostatic and circadian dynamics. Increasingly these mathematical models account for individual differences to achieve a more accurate estimate of the timing and magnitude of fatigue effects on individuals. The second area involves technologies for detecting transient fatigue from drowsiness. The Psychomotor Vigilance Test (PVT), which has been extensively validated to be sensitive to deficits in attention from sleep loss and circadian misalignment, is an example in this category. Two shorter-duration versions of the PVT recently have been developed for evaluating whether operators have sufficient behavioral alertness prior to or during work. Another example is online tracking the percent of slow eyelid closures (PERCLOS), which has been shown to reflect momentary fluctuations of vigilance. Technologies for predicting and detecting sleepiness/fatigue have the potential to predict and prevent operator errors and accidents in safety-sensitive occupations, as well as physiological and mental diseases due to inadequate sleep and circadian misalignment.
Keywords: Sleepiness, fatigue, vigilance, drowsiness, biomathematical models, Psychomotor Vigilance Test (PVT), PERCLOS, safety
INTRODUCTION
There are extensive data documenting that acute and chronic partial sleep loss, prolonged wakefulness, and waking performance at night when humans are biologically programmed to sleep, are risk factors for performance errors and accidents in a wide range of occupational settings.1–3 In addition, short sleep duration, sleep disorders and circadian misalignment have been found to associate with several physiological and mental disorders including hypertension, diabetes, obesity, depression, or cancer.4–11 Recently, two new promising technologies for managing sleepiness/fatigue risk in human systems have emerged. These include preventing fatigue by optimizing work schedules using biomathematical models of performance changes associated with sleep and circadian dynamics,12–13 and technologies for detecting drowsy and fatigued operators on the job.14 A recent review of technologies for managing fatigue and sleepiness identified that there are significant challenges related to these and other fatigue mitigation technologies.14 There is need to establish their validity, safety value, acceptance, use adherence, and abuse potential.13–16
Fatigue is the word used throughout government, industry, labor, and the public to indicate the effects of working too long, following too little rest, and/or being unable to sustain a certain level of performance on a task.1 These issues overlap extensively with those that relate to sleepiness and its performance effects, and consequently, sleepiness and fatigue are used interchangeably in this review.
OPERATORS' INCAPACITATION FROM FATIGUE REQUIRES NOVEL SOLUTIONS
Human neurobehavioral functions (e.g., alertness, attention, working memory, problem solving, reaction time, situational awareness, risk taking, etc.) are dynamically controlled by the interaction of sleep homeostatic drive and circadian regulation.17–19 When total sleep deprivation is continued for several days, the detrimental effects from sleep homeostatic drive on alertness and performance continue (nearly linear) to increase, however the circadian process modulates the changes daily and can mitigate some of the effects of sleep loss during times of the circadian peak.20 For example, when remaining awake for 40 hours, it is a counterintuitive fact that fatigue and performance deficits are worse at 24 hours than at 40 hours awake. Dependence on these processes makes the prediction of neurobehavioral performance nonlinear. The nonlinearity means that performance predictions based on simple linear fatigue models, which are widely used by industry and regulatory bodies, are often grossly inaccurate. These historical limits on work time are all based on the assumption that the longer one works the more fatigued one will become. In contrast, there is extensive scientific evidence that work-related fatigue limits should be based on the amount of sleep obtained and on circadian phase as they dynamically interact over time modulating performance capability and therefore safety. This dynamic nonlinearity in the brain’s performance capability is the reason that developing and validating mathematical models that predict performance is increasingly regarded as essential. These models have increasingly assumed a critical role in fatigue risk management.12–13
A second area of technology development concerns the development and validation of technologies for detecting fatigued operators on the job.14 There are three scientifically-based reasons why objective sleepiness-detection technologies are needed in safety-sensitive operations. One reason is to inform people of when recovery sleep is essential and if possible, how much sleep is needed. There are extensive data documenting that performance deficits from sleep loss accumulate over days to high levels when daily recovery sleep is chronically inadequate.20–26 Two seminal experimental studies documented precise dose-related effects of chronic sleep restriction on neurobehavioral performance measures in healthy adults.21–22 In both experiments, performance deficits increased steadily across consecutive days of sleep restriction, and the less sleep chronically provided each night below 7h, the more rapidly the performance deficits increased across days of restriction. Within 5 – 6 days of sleep restricted to less that 7 h, decrements in behavioral alertness increased to levels equivalent to having had no sleep at all for 24 – 48 hours. In addition, there is scientific evidence that one night of 10h sleep is not sufficient to recover from neurobehavioral deficits after five consecutive nights of 4h sleep restriction.26
Another justification for technologies that detect fatigued operators stems from the fact that humans are often unable to subjectively estimate the degree of impairment of their alertness and performance due to inadequate sleep, working at night, or a sleep disorder.22, 27 A classic finding from experiments on chronic partial sleep deprivation is that people overestimated their subjective alertness and underestimated the severity of their reduced behavioral alertness and the likelihood of having performance lapses or sudden sleep onsets under conditions of chronic partial sleep loss.22 That is, people tend to believe they can overcome sleepiness either by force of will or by engaging in certain behaviors (e.g., listening to music, etc.), but these alerting stimuli have only small and short-lived effects.28–29 In addition, fatigue-risk management programs that rely largely on self-reported fatiguesleepiness are likely to miss at-risk chronically sleep-deprived individuals, and those at greatest risk for a performance lapse that could have serious consequences for safety (e.g., drift off of road crash).
A third reason for technologies that detect fatigued operators relates to the large and stable differences among people in the rate at which they are neurobehaviorally vulnerable to sleep loss and night work. While everyone will ultimately experience neurobehavioral deficits from sleep loss if it is sustained long enough, some individuals are highly vulnerable to performance deficits early in sleep deprivation (we labeled these Type 3 responses), while others take much longer to show deficits or manifest only moderate deficits until sleep loss is severe (labeled Type 1 responses). Still others show deficits intermediate to these two extremes (labeled Type 2 responses).30–31 These individual differences in response to sleep loss may depend on the cognitive domain studied--an area requiring further research. Regardless of the cognitive area, they appear to be stable and trait-like, indicative of a phenotypic response.32–34 For example, in experiments involving repeated exposure to sleep deprivation in the same subjects, the intraclass correlation (ICC) coefficient, which expresses the proportion of variance that is explained by systematic inter-individual variability, revealed that stable responses within individuals accounted for between 58 to 68 percent of the overall variance in degradation of vigilant attention measured by the Psychomotor Vigilance Test (PVT: see details in fagigue detection technologies' section) between multiple sleep-deprivation exposures.22, 31–36 Thus, healthy adults who had high lapse rates during sleep deprivation after one exposure also had high lapse rates during a second exposure (separated by weeks or months), and similarly, those with low lapse rates during one exposure had low lapse rates during a second exposure.30, 32, 34 These results strongly suggest a genetic component of different vulnerability to sleep loss.33, 37–40 A recent study by Kuna and colleagues33 of monozygotic (MZ) and dizygotic (DZ) twin pairs confirmed the genetic component of neurobehavioral vulnerability to sleep loss. They found that the ICC for PVT lapses over 38h of sleep deprivation in MZ twin pairs was 56.2 % whereas it was 14.5 % for DZ twins, showing that behavioral impairment produced by sleep deprivation is a highly heritable trait. These discoveries have resulted in a search for biomarkers that would predict the vulnerability of individuals to the neurobehavioral effects of sleep loss. Several recent studies have investigated the effects of genetic polymorphisms33, 37–39, 41–44 and neuroimaging biomarkers45–51 on inter-individual differences in neurobehavioral vulnerability to sleep loss. It is not known if other effects of sleep loss (e.g., weight gain8) demonstrate phenotypic vulnerability. Although prediction of sleepiness is a critical goal for fatigue management, the latter also requires detection of sleepiness in real time in order to prevent imminent risk of errors and accidents. Short term fluctuation between alertness and drowsiness can occur even among individuals who are less vulnerable to sleep loss. Therefore, in addition to biomarkers for trait-like performance vulnerability to sleep loss,52 fatigue detection technologies offer the ability to detect the immediate state of an operator.
SLEEPNESS/FATIGUE PREDICTION TECHNOLOGIES
Mathematical models predicting sleepiness/fatigue over multiple days have received significant attention in the past two decades.12, 17, 53–54 The two-process model of sleep regulation17 can predict sleep timing and duration, however, this simple model failed to predict neurobehavioral effects of chronic sleep restriction.55–56 Recent biomathematical models of neurobehavioral performance have been developed to predict behavioral alertness to both total sleep deprivation and chronic sleep restriction.54, 57 An important prediction from the model54 is that deterioration of the neurobehavioral performance converged to an asymptotically stable equilibrium when daily wake duration was below 20.2h (3.8h TIB), but performance deficits increased markedly when daily wake duration was above 20.2h (i.e., less than 3.8h of sleep in 24h). Another important prediction from this model is that a single night of recovery sleep is inadequate to recover from chronic sleep restriction. This prediction has been confirmed by the recent experimental findings.26
Another limitation of the previous mathematical models is that they failed to accurately predict behavioral alertness of individuals with the different phenotypic vulnerabilities to sleep loss. To address this limitation, Van Dongen et al.53 developed an adaptive Bayesian forecasting performance prediction method that uses the results of an individual's past performance to identify the values of his/her traits, and then predicts future performance, updated by a fatigue detection technology. As the number of past data points increases, the model increases the accuracy with which the trait parameters are estimated (Figure 1). The individualized predictions more accurately predict actual future performance of each individual than does the population average prediction. The mathematical model accounting for individual differences achieves more accurate estimate of the timing and magnitude of fatigue effects on individuals,53 which should facilitate a use of individualized countermeasures (e.g., naps, recovery sleep, caffeine intake).
Figure 1.
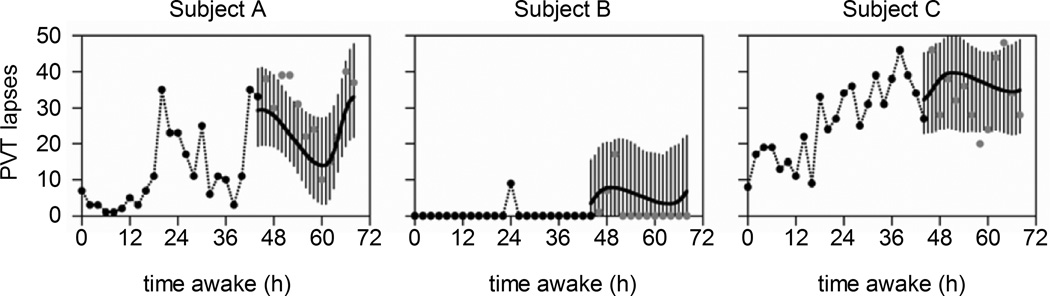
Simulation using the Bayesian forecasting procedure to predict future performance of three individuals, measured with the 10-minute PVT, during total sleep deprivation. Performance is predicted starting from t = 44h of wakefulness, with mean (black line) and 95% confidence intervals (vertical lines). Individual predictions are based on traits identified from prior performance measurements up to 44h (black dots). The gray circles show the actual performance measurements during the 24h prediction period. Figure reprinted with permission from Van Dongen and colleagues.53
Mathematical modeling is currently being used to identify work schedules that pose a sleep deprivation risk and to estimate the magnitude of the risk.13 There is recognition, however, that mathematical models developed to predict and prevent fatigue risks from sleep loss and circadian interactions have limitations. For example, they need feedback from actual values of neurobehavioral performance to improve their accuracy.53 In addition, no model can predict a momentary change of fatigue/sleepiness. Therefore, they may be only one of the important elements in a fatigue risk management system. Integrated use of sleepiness-prediction and detection technologies holds promise as technologies that could be used to mitigate accidents and the risk of errors more effectively.
SLEEPNESS/FATIGUE DETECTION TECHNOLOGIES
Fitness-for-duty tests
Vigilant attention is a requirement for a great many safety-sensitive tasks, from operating moving conveyances, to performing many kinds of work, to detecting anomalies and threats. Reviews of cognitive performance tests have consistently found that vigilant attention tasks are among the most sensitive measures of sleep loss and circadian periodicity.58 A recent meta-analysis investigated 70 published studies of the effects of a night of acute total sleep loss on a total of 147 cognitive tests including simple attention, complex attention, working memory, processing speed, short-term memory and reasoning.58 This study revealed that effect sizes were largest for lapses in attention and smallest for reasoning accuracy.58 Thus, deficits in the ability to sustain attention and respond quickly are among the primary adverse effects of inadequate sleep on performance.58
PVT performance, in particular, has proven to be very sensitive to all types of sleep loss, while also having the advantage of virtually no learning curve or aptitude variance.59 The PVT is an example of a probed-performance fitness-for-duty test.59 It is based on probing the ability of the brain to sustain attention and respond quickly (i.e., behavioral alertness) and relies on very precise measurements of repeated reaction times (RT) to a simple visual (or auditory) stimulus occurring at a predetermined inter-stimulus-interval (ISI) range. The PVT typically requires a button press to the onset of a visual millisecond counter. Stimuli are presented with a random inter-stimulus interval of 2 to 10 s. The digital counter showing reaction time to the light stimulus remains visible and stops counting immediately at the subject's response. All responses are displayed digitally in milliseconds (ms), with incorrect responses (i.e. false start, incorrect key press, or keeping the button pressed) coded into the recording as errors. Basner and Dinges reported criteria for method and variables for the 10 min-PVT.59 They also recommended that reciprocal mean 1/RT (i.e., response speed) and number of lapses should be considered as primary outcomes for the 10-min PVT due to their superior conceptual and statistical properties and high sensitivity to sleep deprivation.59 Both the standard 10-minute PVT59 and the briefer 3-minute PVT (the Brief PVT: PVT-B)60, which is based on a modified performance algorithm, have been extensively validated to be sensitive to both acute total and chronic partial sleep deprivation, revealing the temporal dynamics of sleep homeostatic and circadian interactions. The 10-minute PVT has become perhaps the most widely used measure of behavioral alertness owing in large part to the combination of its brevity, its high sensitivity to both acute total sleep deprivation and chronic sleep restriction, and its psychometric advantages over other neurobehavioral tests of sleepiness.59 It has also been validated as a reliable measure to identify fatigue in occupational settings61 and clinical settings62 as well as to screen for sleep apnea patients who have higher risks of fatigue-related accidents.63–64
However, the standard 10-minute PVT is often considered impractical for operational or clinical settings because of its duration. Neurobehavioral tests for fatigue assessment and fitness for duty not only need to be operationally and conceptually valid, reliable, sensitive, specific, generalizable, and easy to use, but also brief enough to be acceptable for the target population and to allow for repeated administration in operational environments. To meet these criteria, two shorter-duration versions of the PVT (with modified algorithms for performance evaluation) have been developed with extensive validation for their sensitivity to both acute total and chronic partial sleep deprivation. These are the Brief PVT (PVT-B)60 and the Adaptive-Duration Version of the PVT (PVT-A).65
The PVT-B has ISIs decreased from the standard 2-10 s of the 10-min PVT, to 1-4 s and reduced lapse threshold from 500 to 355 ms.60 The PVT-B has been shown to track the standard 10-min PVT closely over time in experiments on both total sleep deprivation and chronic sleep restriction.60 PVT-B test duration was decreased 70% relative to the 10-min PVT, its effect size for sensitivity to sleep loss was decreased by only 22.7%.60 This is an acceptable trade-off between task duration and sensitivity. In a laboratory study of work performance to determine if the PVT-B had potential as a fitness-for-duty test, it was demonstrated that PVT-B closely tracked fatigue-related threat-detection performance decrements on a simulated luggage-screening task.66 Performance on the PVT-B and the simulated luggage-screening task covaried over a 34h period of total sleep deprivation. This is a particularly important finding because the threat-detection task has high fidelity to what operators must do while screening luggage through x-ray machines. Thus, the PVT-B has the potential to predict operationally-relevant performance relative to vigilance work.
Although PVT-B may be a useful tool for assessing behavioral alertness in settings where the duration of the 10-min PVT is considered impractical, the shorter PVT versions seem to be too short to detect relevant deterioration in vigilant attention in subjects with moderate impairment whose performances deteriorate only later during the test, whereas the longer versions may be unnecessarily long for other subjects who are apparently fully alert or severely impaired.65 The adaptive PVT (i.e., PVT-A) is a modified PVT with a duration dependent on the subject's performance.65 Thus, in contrast to the fixed durations of the PVT and PVT-B, the PVT-A65 duration is variable. It stops sampling once it has gathered enough information to correctly classify performance as high, medium, or low, according to the number of lapses and false starts. In a validation experiment, test duration of the PVT-A averaged less than 6.5 minutes (SD 2.4) for a training data set and 6.4 min (SD 1.7) for a validation data set. In addition, the PVT-A was shown to be highly accurate, sensitive, and specific relative to 10-minute PVT performance. Thus, the adaptive-duration strategy of the PVT-A may be superior to a simple reduction of PVT duration. Future studies are needed to show its feasibility and usefulness in professional screeners and operational environments as a fitness-for-duty test.
Online operator monitoring
Fitness-for duty tests hold the promise of detecting the state of sleep-related fatigue in populations at risk for accidents and errors due to fatigue-inducing work schedules. However, as noted above, the neurobehavioral effects of sleep loss and circadian periodicity follow a non-linear time course within and between days, as well as more transient evoked effects on alertness from body posture, social interaction, caffeine, etc. Therefore, using biomathematical models augmented with online operator monitoring may be more comprehensive way to detect fatigue relative to work. The following is an example of one type of continuous monitoring of operator fatigue based in sleepiness, using a measure of slow eyelid closures (i.e., slow blinks) referred to as PERCLOS (proportion of time that the eyes are closed over a certain interval).16, 67–72 This example illustrates the criticality of the validation science that must be undertaken as an initial first step toward developing a truly reliable unobtrusive measure of sleepiness.
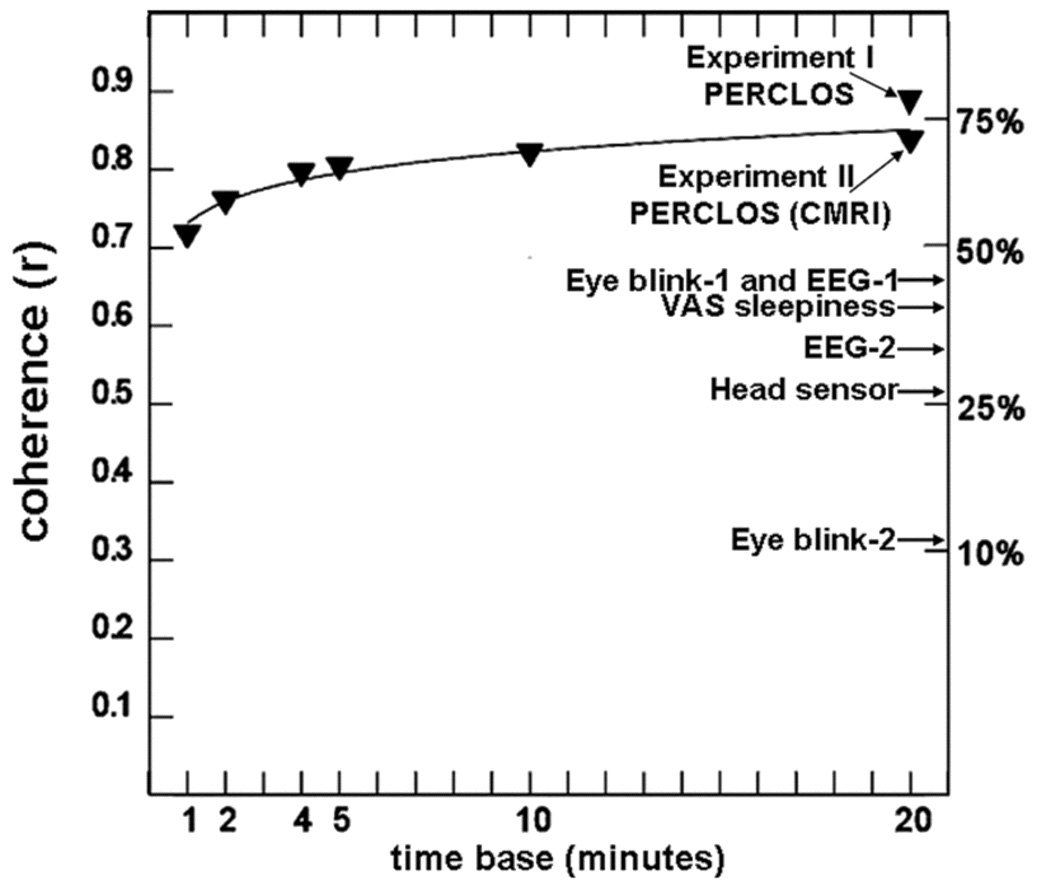
To validate PERCLOS and several other approaches, Dinges and colleagues16, 67 systematically evaluated the validity of a number of putative sleepiness-detection technologies. These included brain wave (EEG) algorithms, eye-blink rate devices, a measure of slow eyelid closures (i.e., PERCLOS), and a head position sensor, as well as individuals’ own ratings of their sleepiness. In a series of tightly controlled, double-blind experiments, they evaluated the extent to which each technology detected the alertness of subjects over a 40h period of wakefulness, as measured by PVT lapses of attention—a well-validated measure of behavioral alertness.16, 67 Each putative fatigue-detection technology was time-locked to PVT performance in a manner that permitted precise determination of whether a given technology could reliably track minute-by-minute (across a normal day and during nocturnal and diurnal periods of sleep deprivation), the waxing and waning of alertness as evident in PVT lapses of attention. The evaluation for the minute-by-minute recordings from each technology was done by the respective developer of each technology, blind to PVT performance (and the latter was scored for performance lapses blind to each technology score of alertness for each minute). A biostatistician then fit the prediction of alertness from each technology to the PVT performance data for each subject across a 42h period of evaluation. This resulted in a measure of statistical coherence for each technology for each study participant evaluated. Human-scored PERCLOS proved superior to all other detection technologies in blindly predicting when PVT lapses of attention were occurring across the 42h awake each subject underwent. The initial results reported in Dinges et al.16 were subsequently replicated for a retinal reflectance measure of PERCLOS.67 As shown in Figure 2, only PERCLOS reliably and accurately tracked PVT lapses of attention in all subjects, outperforming not only all the other technologies, but also subjects’ own ratings of their fatigue and alertness in both validation trials.16, 67
Figure 2.
Mean percent time of slow eyelid closures (PERCLOS) coherence for PVT lapse frequency across 42h of waking (triangles), as a function of the time base used to define an epoch. A distance-weighted least squares function was fit to the data. PERCLOS was measured by a human scoring videos of slow eyelid closures (Experiment 1) and by infrared retinal reflectance (Experiment 2, CMRL). In both experiments it had much higher coherence with PVT performance lapses of attention (i.e., high sensitivity to behavioral alertness) than any other technology evaluated in the experiments (i.e., two different EEG algorithms [EEG-1, EEG-2), two different eye blink technologies [Eye blink-1, Eye blink-2], and head movement sensor technology [Head sensor]). PERCLOS was also a better predictor of alertness than subjects’ self-reports of sleepiness by a visual analogue scale (i.e., VAS sleepiness). The accuracy of PERCLOS predictions of PVT performance increased as the time base for integrated assessments increased from 1 to 20 minutes. More recent work also supports the accuracy of PERCLOS for unobtrusive detection of sleepiness while performing a behavioral maintenance of wakefulness test 70 and the PVT 71. Figure reprinted from Dinges and colleagues.16, 67.
More recent studies have compared accuracies for predicting vigilance deterioration among several measurements including EEG frequency band activities, heart rate variability, and ocular variables (saccade, slow eye movement, pupil, blink, or eyelid closure).70–71 The experiments also found that PERCLOS was the most effective indicator of sleepiness-based fatigue among the variables evaluated.70–71 Dinges et al.73–76 are now developing a new technique that involves precise and completely unobtrusive tracking of PERCLOS in real time, using optical computer recognition.
Another example of online operator monitoring technology is the Johns Drowsiness Scale (JDS; scores ranging from 0 to 10, where 0 = very alert and 10 = very drowsy) based on a weighted combination of several sleepiness indicators derived from ocular measures such as blink duration and amplitude-velocity ratios during the closing and reopening phase of blinks measured by infrared reflectance oculography.77–78 The JDS score was shown to track performance levels during vigilance attention tasks, and a driving simulator task as well as alertness levels after caffeine ingestion.77–82 In addition, higher JDS scores (≥ 4.5) were associated with self-reported inattention during on-road driving events in nurses commuting to and from night and rotating shifts.83 However, the JDS requires wearing special glasses, which may be a deterrent to its use in certain settings.
Two independent studies using 40h of continuous wakefulness under constant routine have investigated the accuracies (area under the receiver-operating characteristic curve: AUC ranging from 0.5 to 1.0; higher value is better) of PERCLOS or JDS to identify a threshold increase (> 25%, > 50%, and > 75%) in the number of PVT lapses, measured relative to each subject's performance during baseline (first 16h of wakefulness).71, 84 The results showed that AUC for the PERCLOS and JDS are 0.89-0.91 and 0.74-0.76, respectively.71, 84 Although the procedures for measuring the vigilance were not identical (auditory or visual PVT, 1h or 2h intervals of test bout, etc), these results indicate that the accuracy of PERCLOS was higher than that of JDS. Future studies will be needed to compare their accuracies in the same protocol.
One study showed that PVT lapses occur during eyes open,85 meaning deteriorated vigilance can occur even during no sign of PERCLOS.70 Missing detection of deteriorated vigilance (false negatives) potentially causes accidents, and inappropriate warning of decreased vigilance in alert persons (false positives) may decrease compliance for use of the technologies. There need to be continued improvements of the accuracy of online operator fatigue monitoring.
Field study of fatigue-detection technologies
In one of the relatively few fatigue-monitoring studies in over-the-road commercial truck drivers, Dinges and colleagues investigated whether feedback from fatigue-detection technologies would help truck drivers maintain their alertness in actual driving conditions.86 The technologies included driving performance variables (e.g., lane tracking variability), PERCLOS, head sensor, wrist actiwatch and the 10-minute PVT test. The results from this study revealed that the drivers felt the fatigue detection devices informed them of their fatigue levels and prompted them to acquire more sleep on their days off duty. In fact, the wrist actigraphy data confirmed that when receiving feedback on their alertness levels, drivers increased their sleep by an average of 45 minutes on days off duty.86 This is a remarkable and unexpected outcome, and it suggests another purpose for fatigue detection technologies in the workplace—namely to urge operators to obtain longer recovery sleep. If we could use fatigue management technologies to warn drivers when they are getting sleepy and to encourage them to get off the roadway, it may be possible to reduce the risk of sleepiness-related accidents and errors.
Another example of fatigue detection technologies used in real-world operational environments is vigilance monitoring of astronauts who stay long term in the International Space Station (ISS). Spaceflight Cognitive Assessment Tool for Windows (WinSCAT) has been used to evaluate neurobehavioral performance levels of astronauts in the ISS,87 however, the WinSCAT requires about 30 minutes of crew times. Therefore, the test is not suitable to evaluate astronauts' neurobehavioral performance within a day or every day. The PVT-B also has been studied on ISS to evaluate astronauts' vigilance levels.88 Importantly, a comprehensive yet brief performance test (now referred to as COGNITION) has been developed to evaluate several neurocognitive and emotional domains of astronauts' performance on board the ISS.89 These approaches offer a way to quickly and reliably assess not only behavioral alertness, but also a range of cognitive functions that may be affected by sleep loss.
CONCLUSION
Technology for predicting and evaluating whether operators have sufficient performance capability (relative to sleep need and circadian timing) prior to beginning or during their work is important to prevent accidents and errors due to the presence of fatigue-related neurobehavioral deficits. In addition, complementary use of these technologies including mathematical models, fitness-for duty tests, or online operator monitoring might be more effective to find the risk of accidents and errors quickly and accurately. Such technologies allow operators to employ countermeasures to mitigate sleepiness and fatigue before starting or continuing their work, contributing to reduce operational errors and accidents due to neurobehavioral deficits from sleep loss and circadian misalignment. Furthermore, considering that sleep loss is a risk factor for several physiological and mental disorders,4–11 the urging effect of fatigue-management technologies to inform people of the need for recovery sleep might also contribute to prevent developing various diseases associated with inadequate sleep.
ACKNOWLEDGMENTS
The time and effort required to write the review were supported by JSPS Postdoctoral Fellowships for Research Abroad and KAKENHI Grant Number 22730598 (T. Abe); NIH grant R01 NR004281 (D.F. Dinges); National Space Biomedical Research Institute through NASA NCC 9-58 (D.F. Dinges); and by the Office of Naval Research and the Navy BUMED Advanced Medical Development Program through contracts N65236-09-D-3809, N00014-10-C-0392, N00014-11-C-0592, and N62645-12-C-4004 (D. Mollicone).
Footnotes
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Abe and Dr. Basner have no financial conflicts of interest. Dr. Mollicone is president and CEO of Pulsar Informatics. Dr. Dinges is compensated by the Associated Professional Sleep Societies, LLC, for serving as Editor in Chief of SLEEP and has received compensation for serving on a scientific advisory council for Mars, Inc.
REFERENCES
- 1.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 2.Dinges DF, Abe T. International Association of Traffic and Safety Sciences (eds). IATSS Booklet No4, Traffic Safety in the Future, Session by IATSS Symposium Department. Tokyo: International Association of Traffic and Safety Sciences; 2013. Transportation safety and operator sleepiness: Where biology needs technology; pp. 88–120. [Google Scholar]
- 3.Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public-policy – Consensus report. Sleep. 1988;11:100–109. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 5.Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis. 2008;196:727–732. doi: 10.1016/j.atherosclerosis.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36:981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okajima I, Komada Y, Nomura T, Nakashima K, Inoue Y. Insomnia as a risk for depression: a longitudinal epidemiologic study on a Japanese rural cohort. J Clin Psychiatry. 2012;73:377–383. doi: 10.4088/JCP.10m06286. [DOI] [PubMed] [Google Scholar]
- 10.Abe T, Inoue Y, Komada Y, Nakamura M, Asaoka S, Kanno M, Shibui K, Hayashida K, Usui A, Takahashi K. Relation between morningness-eveningness score and depressive symptoms among patients with delayed sleep phase syndrome. Sleep Med. 2011;12:680–684. doi: 10.1016/j.sleep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, Rea MS, Reinlib L. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallis MM, Mejdal S, Nguyen TT, Dinges DF. Summary of the key features of seven biomathematical models of human fatigue and performance. Aviat. Space Environ. Med. 2004;75:A4–A14. [PubMed] [Google Scholar]
- 13.Dawson D, Ian Noy Y, Harma M, Akerstedt T, Belenky G. Modelling fatigue and the use of fatigue models in work settings. Accid Anal Prev. 2011;43:549–564. doi: 10.1016/j.aap.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Balkin TJ, Horrey WJ, Graeber RC, Czeisler CA, Dinges DF. The challenges and opportunities of technological approaches to fatigue management. Accid Anal Prev. 2011;43:565–572. doi: 10.1016/j.aap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Dinges DF. Critical research issues in development of biomathematical models of fatigue and performance. Aviat Space Environ Med. 2004;75:A181–A191. [PubMed] [Google Scholar]
- 16.Dinges DF, Mallis MM, Maislin G, Powell JW. Evaluation of techniques for ocular measurement as an index of fatigue and as the basis for alertness management. Report no. DOT HS 808 762. 1998 [Google Scholar]
- 17.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 18.Åkerstedt T, Gillberg M, Wetterberg L. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 19.Goel N, Van Dongen HPA, Dinges DF. Circadian Rhythm in Sleepiness, Alertness and Performance. In: Kryger MH, T R, Dement W, editors. Principles and Practice of Seep Medicine. Philadelphia: Elsevier; 2011. pp. 4445–4455. [Google Scholar]
- 20.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 21.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 23.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 24.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- 25.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 26.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–1026. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth T, Hartse KM, Zorick F, Conway W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airway sleep apnea. Sleep. 1980;3:425–439. [PubMed] [Google Scholar]
- 28.Reyner LA, Horne JA. Evaluation "in-car" countermeasures to sleepiness: cold air and radio. Sleep. 1998;21:46–50. [PubMed] [Google Scholar]
- 29.Schwarz JFA, Ingre M, Fors C, Anund A, Kecklund G, Taillard J, Phillip P, Åkerstedt T. In-car countermeasures open window and music revisited on the real road: popular but hardly effective against driver sleepiness. J Sleep Res. 2012;21:595–599. doi: 10.1111/j.1365-2869.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 30.Goel N, Dinges DF. Behavioral and genetic markers of sleepiness. J Clin Sleep Med. 2011;7:S19–S21. doi: 10.5664/JCSM.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ. Med. 2004;75:A147–A154. [PubMed] [Google Scholar]
- 32.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 33.Kuna ST, Maislin G, Pack FM, Staley B, Hachadoorian R, Coccaro EF, Pack AI. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–1233. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35:1163–1172. doi: 10.5665/sleep.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijkman M, Sachs N, Levine E, Mallis M, Carlin MM, Gillen KA, Powell JW, Samuel S, Mullington J, Rosekind MR, Dinges DF. Effects of reduced stimulation on neurobehavioral alertness depend on circadian phase during human sleep deprivation. Sleep Res Online. 1997;26:265. [Google Scholar]
- 36.Van Dongen HPA, Dijkman MV, Maislin G, Dinges DF. Phenotypic aspect of vigilance decrement during sleep deprivation. Physiologist. 1999;42:A-5. [Google Scholar]
- 37.Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, von Schantz M, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 38.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS ONE. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–1519. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS ONE. 2011;6:e29283. doi: 10.1371/journal.pone.0029283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groeger JA, Viola AU, Lo JCY, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 42.Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, Maquet P, Dijk DJ. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J Neurosci. 2009;29:7948–7956. doi: 10.1523/JNEUROSCI.0229-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, von Schantz M, Archer SN, Dijk DJ. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS ONE. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rupp TL, Wesensten NJ, Newman R, Balkin TJ. PER3 and ADORA2A polymorphisms impact neurobehavioral performance during sleep restriction. J Sleep Res. 2013;22:160–165. doi: 10.1111/j.1365-2869.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 45.Mu Q, Mishory A, Johnson KA, Nahas Z, Kozel FA, Yamanaka K, Bohning DE, George MS. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–446. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 46.Caldwell JA, Mu Q, Smith JK, Mishory A, Caldwell JL, Peters G, Brown DL, George MS. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci. 2005;119:694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]
- 47.Chee MWL, Chuah LYM, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. NeuroImage. 2006;31:419–428. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Chee MWL, Tan JC. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. NeuroImage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poudel GR, Innes CR, Jones RD. Cerebral perfusion differences between drowsy and nondrowsy individuals after acute sleep restriction. Sleep. 2012;35:1085–1096. doi: 10.5665/sleep.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J, Wu WC, Wang JJ, Detre JA, Dinges DF, Rao HY. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. NeuroImage. 2010;49:3426–3435. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goel N, Dinges DF. Predicting risk in space: Genetic markers for differential vulnerability to sleep restriction. Acta Astronaut. 2012;77:207–213. doi: 10.1016/j.actaastro.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dongen HPA, Mott CG, Huang JK, Mollicone DJ, McKenzie FD, Dinges DF. Optimization of biomathematical model predictions for cognitive performance impairment in individuals: accounting for unknown traits and uncertain states in homeostatic and circadian processes. Sleep. 2007;30:1129–1143. doi: 10.1093/sleep/30.9.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HP. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dongen HPA. Comparison of mathematical model predictions to experimental data of fatigue and performance. Aviat Space Environ Med. 2004;75:A15–A36. [PubMed] [Google Scholar]
- 56.Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 57.McCauley P, Kalachev LV, Mollicone DJ, Banks S, Dinges DF, Van Dongen HPA. Dynamic circadian modulation in a mathematical model for the effects of sleep and sleep loss on waking neurobehavioral performance. Sleep. 2013;36:1989–1999. doi: 10.5665/sleep.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim J, Dinges DF. A Meta-Analysis of the Impact of Short-Term Sleep Deprivation on Cognitive Variables. Psychol Bull. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basner M, Mollicone D, Dinges DF. Validity and Sensitivity of a Brief Psychomotor Vigilance Test (PVT-B) to Total and Partial Sleep Deprivation. Acta Astronaut. 2011;69:949–959. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gander P, Millar M, Webster C, Merry A. Sleep Loss and Performance of Anaesthesia Trainees and Specialists. Chronobiol Int. 2008;25:1077–1091. doi: 10.1080/07420520802551428. [DOI] [PubMed] [Google Scholar]
- 62.Sunwoo BY, Jackson N, Maislin G, Gurubhagavatula I, George CF, Pack AI. Reliability of a Single Objective Measure in Assessing Sleepiness. Sleep. 2012;35:149–158. doi: 10.5665/sleep.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–1316. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang CB, Varvarigou V, Parks PD, Gautam S, Bueno AV, Malhotra A, Kales SN. Psychomotor Vigilance Testing of Professional Drivers in the Occupational Health Clinic A Potential Objective Screen for Daytime Sleepiness. J Occup Environ Med. 2012;54:296–302. doi: 10.1097/JOM.0b013e318223d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basner M, Dinges DF. An Adaptive-Duration Version of the PVT Accurately Tracks Changes in Psychomotor Vigilance Induced by Sleep Restriction. Sleep. 2012;35:193–202. doi: 10.5665/sleep.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basner M, Rubinstein J. Fitness for duty: a 3-minute version of the Psychomotor Vigilance Test predicts fatigue-related declines in luggage-screening performance. J Occup Environ Med. 2011;53:1146–1154. doi: 10.1097/JOM.0b013e31822b8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinges DF, Price NJ, Maislin G, Powell JW, Ecker AJ, Mallis MM, Szuba MP. Prospective laboratory revalidation of ocular-based drowsiness detection technologies and countermeasures. NHTSA Drowsy driver detection and interface projects. 2002 DTNH-22-00-D-07007. [Google Scholar]
- 68.Mallis MM, Dinges DF. Monitoring Alertness by Eyelid Closure. In: Stanton N, Hedge A, Brookhuis K, Salas E, Hendrick H, editors. The Handbook of Human Factors and Ergonomics Methods. New York: CRC Press; 2005. pp. 25.1–25.6. [Google Scholar]
- 69.Mallis MM, Banks S, Dinges DF. Sleep and circadian control of neurobehavioral functions. In: Parasuraman R, Rizzo M, editors. Neuroergonomics: The Brain at Work. Oxford: Oxford University Press; 2007. pp. 207–220. [Google Scholar]
- 70.Abe T, Nonomura T, Komada Y, Asaoka S, Sasai T, Ueno A, Inoue Y. Detecting deteriorated vigilance using percentage of eyelid closure time during behavioral maintenance of wakefulness tests. Int J Psychophysiol. 2011;82:269–274. doi: 10.1016/j.ijpsycho.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Chua EC, Tan WQ, Yeo SC, Lau P, Lee I, Mien IH, Puvanendran K, Gooley JJ. Heart Rate Variability Can Be Used to Estimate Sleepiness-related Decrements in Psychomotor Vigilance during Total Sleep Deprivation. Sleep. 2012;35:325–334. doi: 10.5665/sleep.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong JL, Asplund CL, Chia TT, Chee MW. Now you hear me, now you don't: eyelid closures as an indicator of auditory task disengagement. Sleep. 2013;36:1867–1874. doi: 10.5665/sleep.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinges DF, Rider RL, Dorrian J, McGlinchey EL, Rogers NL, Cizman Z, Goldenstein SK, Vogler C, Venkataraman S, Metaxas DN. Optical computer recognition of facial expressions associated with stress induced by performance demands. Aviat Space Environ Med. 2005;76:B172–B182. [PubMed] [Google Scholar]
- 74.Dinges DF, Venkataraman S, McGlinchey EL, Metaxas DN. Monitoring of facial stress during space flight: Optical computer recognition combining discriminative and generative methods. Acta Astronaut. 2007;60:341–350. [Google Scholar]
- 75.Basner M, Dinges DF, Mollicone D, Ecker A, Jones CW, Hyder EC, Di Antonio A, Savelev I, Kan K, Goel N, Morukov BV, Sutton JP. Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. Proc Natl Acad Sci USA. 2013;110:2635–2640. doi: 10.1073/pnas.1212646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones CW, Basner M, Yu X, Yang G, Goel N, Metaxas D, Dinges DF. Unobtrusive tracking of slow eyelid closures as a measure of fatigue from sleep loss. Sleep. 2013;35:A110. [Google Scholar]
- 77.Johns MW, Chapman R, Crowley K, Tucker A. A new method for assessing the risks of drowsiness while driving. Somnologie. 2008;12:66–74. [Google Scholar]
- 78.Johns MW, Tucker A, Chapman R, Crowley K, Michael N. Monitoring eye and eyelid movements by infrared reflectance oculography to measure drowsiness in drivers. Somnologie. 2007;11:234–242. [Google Scholar]
- 79.Wilkinson VE, Jackson ML, Westlake J, Stevens B, Barnes M, Swann P, Rajaratnam SM, Howard ME. The accuracy of eyelid movement parameters for drowsiness detection. J Clin Sleep Med. 2013;9:1315–1324. doi: 10.5664/jcsm.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ftouni S, Rahman SA, Crowley KE, Anderson C, Rajaratnam SM, Lockley SW. Temporal dynamics of ocular indicators of sleepiness across sleep restriction. J Biol Rhythms. 2013;28:412–424. doi: 10.1177/0748730413512257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson C, Chang AM, Sullivan JP, Ronda JM, Czeisler CA. Assessment of drowsiness based on ocular parameters detected by infrared reflectance oculography. J Clin Sleep Med. 2013;9:907–920. doi: 10.5664/jcsm.2992. 20A-20B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michael N, Johns M, Owen C, Patterson J. Effects of caffeine on alertness as measured by infrared reflectance oculography. Psychopharmacology. 2008;200:255–260. doi: 10.1007/s00213-008-1202-z. [DOI] [PubMed] [Google Scholar]
- 83.Ftouni S, Sletten TL, Howard M, Anderson C, Lenne MG, Lockley SW, Rajaratnam SMW. Objective and subjective measures of sleepiness, and their associations with on-road driving events in shift workers. J Sleep Res. 2013;22:58–69. doi: 10.1111/j.1365-2869.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 84.Ftouni S, Rahman SA, Crowley KE, Anderson C, Rajaratnam SM, Lockley SW. Temporal dynamics of ocular indicators of sleepiness across sleep restriction. J Biol Rhythms. 2013;28:412–424. doi: 10.1177/0748730413512257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anderson C, Wales AW, Horne JA. PVT lapses differ according to eyes open, closed, or looking away. Sleep. 2010;33:197–204. doi: 10.1093/sleep/33.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinges DF, Maislin G, Brewster RM, Krueger GP, Carroll RJ. Safety: Older Drivers; Traffic Law Enforcement; Management; School Transportation; Emergency Evacuation; Truck and Bus; and Motorcycles. Washington: Transportation Research; 2005. Pilot test of fatigue management technologies; pp. 175–182. [Google Scholar]
- 87.Kane RL, Short P, Sipes W, Flynn CF. Development and validation of the spaceflight cognitive assessment tool for windows (WinSCAT) Aviat Space Environ Med. 2005;76:B183–B191. [PubMed] [Google Scholar]
- 88.Dinges DF, Basner M, Mollicone DJ, Goel N, Braun M, Jones CW, Ecker A, Bartels R, Mott C, Studna M. ISS Missions: Elevated Workload and Reduced Sleep Duration; Paper presented at: 2013 NASA Human Research Program Investigators' Workshop; Feb 11-14; Galveston, Texas. 2013. [Google Scholar]
- 89.Schneiderman JS, Gur RC, Dinges DF, Mollicone DJ, Mott CG, McCann T, Roberts ZT, Hansen J, Savitt AP, Basner M. Individualized Real-Time Neurocognitive Assessment Toolkit For Space Flight Fatigue (NeuroCATS); Paper presented at: 2013 NASA Human Research Program Investigators' Workshop; Feb 11-14; Galveston, Texas. 2013. [Google Scholar]