Abstract
Clostridium difficile infection, the most frequent cause of nosocomial diarrhea, disproportionately affects older adults. The two most important risk factors for developing C. difficile infection are antimicrobial exposure and age >65 years old. Risk factors specific to older adults are frequent interactions with healthcare systems and age-related changes in physiology, including immune senescence and changes to the gut microbiome. Metronidazole and oral vancomcyin are the mainstays of conventional treatment for C. difficile infection. Alternative therapies include fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and fectal bacteriotherapy, which offers an excellent therapeutic outcome. Strategies to prevent C. difficile infections include enhanced infection control measures and reducing inappropriate antimicrobial use through stewardship.
Keywords: aging, antimicrobial stewardship, Clostridium difficile infection, fecal bacteriotherapy, fidaxomicin, infection control, long-term care facilities, metronidzole, older adults, vancomycin
Clostridium difficile infection, the most common cause of nosocomial diarrhea, remains among the most serious of healthcare-acquired infections [101]. Older adults, frequent users of healthcare, experience the greatest morbidity and mortality from C. difficile infection. Presented here is a discussion regarding C. difficile infection that highlights aspects distinct to older adults, including their increased susceptibility, treatment challenges and opportunities for prevention.
Microbiology & pathogensis
C. difficile is a Gram-positive, spore-forming bacillus. Individuals acquire C. difficile through accidental ingestion of spores acquired from the environment. The human gut microbiome, a frequently overlooked form of host defense, protects most individuals from enteric pathogens, such as C. difficile through colonization resistance [1]. Simply stated, colonization resistance is the means through which the host gut microbiota prevents C. difficile from gaining a foothold in the intestine, causing it to pass through the body without causing disease. Systemic antibiotics temporarily change the gut microbiome, disrupt colonization resistance and render individuals vulnerable to C. difficile infection. Accordingly, systemic antibiotics are the principal risk factor for C. difficile infection. Spores ingested by individuals without intact colonization resistance, germinate into vegetative forms that reproduce and secrete the toxins that mediate disease; manifestations range from watery diarrhea to fulminant colitis and death. Most C. difficile strains produce two exotoxins, A and B, both of which translocate into the cytosol of target cells, cause active depolymeraization and subsequent cell death. Specifically, the toxins bind and glucosylate a family of Rho GTPase, locking them into an inactive form and blocking downstream signaling pathways, including those required to organize and maintain the actin cytoskeleton [2]. While toxin A was previously thought to be more pathogenic, reports indicate that naturally occurring toxin A−B+ C. difficile strains cause infection with typical clinical manifestations [3]. Two independent publications confirm that an A−B+ mutant causes disease in an animal model, but they differ on whether an A+B− mutant causes disease [4,5]. The disparity between their findings may be due to differences in the development of the mutant strains and highlights that experimental manipulation of C. difficile is indeed difficult [2].
In the last 10–15 years, reports emerged describing a dramatic increase in the incidence and severity of C. difficile infections. These were eventually linked to an epidemic C. difficile strain, characterized as toxinotype III, restriction endonuclease group BI, North American pulsed field gel electrophoresis type 1 (NAP1) and ribotype 027 [6,7]. There is also evidence indicating that among Europeans, an age >65 years correlates with an increased risk for C. difficile infection due to ribotype 027 [8,9]. The BI/NAP1/027 epidemic strain produces a binary toxin that may cause or be associated with more severe diarrhea and higher fatality rates [10]. It was also found to secrete 16- and 23-fold greater concentrations of toxins A and B, respectively, compared with nonepidemic strains [11]. Initially, these differences were attributed to an 18-base pair deletion in tcdC, a gene believed to encode a negative regulator of toxin A and B production. More recent work, however, disputes these findings. Using precise genetic manipulation of C. difficile, Cartman et al. restored the tcdC gene to a ribotype 027 strain with a naturally occurring deletion but found no associated decrease in toxin production. The authors suggest that tcdC may act as a ‘safety catch’ for toxin production, rather than affect the amount of toxin produced [12].
Another notable feature of the BI/NAP1/027 epidemic strain is its resistance to fluoroquinolone antibiotics [13]. Widespread use of fluoroquinolones may have conferred a selective advantage for the BI/NAP1/027 epidemic C. difficile strain, further contributing to the increased incidence and severity of resulting infections, as well as its global dissemination [14–16]. While more recent evaluations suggests that the BI/NAP1/027 strain may not cause severe disease in a nonoutbreak setting, its emergence and the resulting epidemic continues to have a significant impact on the epidemiology of C. difficile infection [17,18].
Epidemiology
Older adults are disproportionately affected by C. difficile infections, including those caused by the BI/NAP1/027 epidemic strain. In 2009, nearly 1% of all hospitalizations in the USA involved C. difficile infection [19]. The average age of those patients was 67.9 years compared with 48.1 years for all other hospital stays. The oldest patients (>85 years) experienced the highest rates of C. difficile infection-related stays (Figure 1). Just as with hospitalizations, deaths due to C. difficile infection have also increased in the USA, rising from 793 in 1999 to 7251 in 2009 [102]. Mortality due to C. difficile infection also increases with age, rising from 5% for people 61–70 years to >10% for people >80 years [6]. In 2010, 91% of deaths due to C. difficile infection occurred in people >65 years, making it the 18th leading cause of death among USA citizens in this age group [102].
Figure 1. The risk of hospitalization associated with a Clostridium difficile infection increases with age.
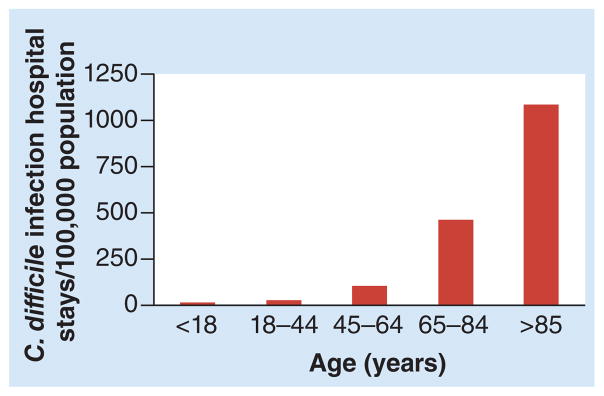
Data taken from [19].
C. difficile infection may be broadly categorized as healthcare-acquired or community-acquired, with the former being far more common [20]. Community-acquired is defined as C. difficile infection in persons who have not been discharged from a healthcare facility in the previous 12 weeks [21]. On average, patients who develop community-acquired infection are significantly younger than those with healthcare-acquired infection, yet the incidence of community-acquired C. difficile infection also increases with age [22–24]. The age difference between community- and healthcare-acquired C. difficile infection may reflect that older adults experience more frequent admissions to healthcare facilities.
C. difficile infection places a significant financial burden on healthcare systems. In 2009, the aggregate cost associated with hospitalizations related to C. difficile infection was an estimated US$8.2 billion, approximately 2.3% of all US hospital costs [19]. Similarly, the European CDC projects costs of 3 million per year for the EU [25]. Given that these figures do not account for C. difficile infection in long-term care facilities (LTCFs) [26], they probably underestimate the true financial impact of C. difficile infection. Thankfully, recent data indicate stabilization and perhaps a decline in the C. difficile infections. Hospitalization rates for C. difficile infection in the USA reached a plateau between 2008 and 2009 [19]. In England, C. difficile infections have declined by 44% since 2009, with a concurrent reduction in mortality, which may be explained by a decrease in the prevalence of the ribotype 027 strain [27,28].
General risk factors
Aside from exposure to systemic antibiotics, advanced age, followed by gastric acid-suppressive medications are the two most notable risk factors for developing C. difficile infection [29–31]. Advanced age and possible reasons for increased vulnerability to C. difficile are discussed in detail below. Proton-pump inhibitors were first implicated as a risk factor for hospitalized patients in 2004 [32]. Subsequent research demonstrated that gastric acid suppression, whether due to histamine-2 receptor agonists or proton-pump inhibitors, increased the risk for developing C. difficile infection in community-dwelling adults [30]. These findings were significant because they convincingly identified gastric acid suppression as a risk factor distinct from hospitalization and from persons with multiple comorbidities. Interestingly, gastric acid suppression does not appear to increase the risk of severe or recurrent C. difficile infection [33]. In the nearly 10 years that have elapsed since identification of gastric acid suppression as a risk factor, the mechanisms for this association remain unknown [34,35].
Other risk factors for C. difficile infection are an indication of patients’ underlying susceptibility (Table 1). Less expected is the recent description of smoking as a risk factor. Current smokers are 80% more likely to develop C. difficile infection compared with those who have never smoked [36]. Interestingly, the authors suggest that the presence of Clostridial species in cigarette filters may be a route for oral inoculation with spores. Also of interest are recent descriptions of reduced risk for C. difficile infection among those on statins [37,38]. Finally, some reports describe culturing C. difficile from retail meat products [39,40]. A large-scale study using a consensus method for C. difficile culture, however, did not recover the organism from 1755 meat products sampled from nine centers across the USA over 12 months [41]. Coupled with the lack of reports of restaurants or food-associated outbreaks, it is unclear that foodborne C. difficile represents a significant clinical risk factor for C. difficile infection.
Table 1.
Nonantibiotic risk factors for acquiring Clostridium difficile infection since the advent of the BI/NAP1/027 epidemic strain.
| Risk factor | Comments |
|---|---|
| Well-established | |
| Advanced age | CDI risk increases ~2% each year for every year >18 years of age [6,91] Age >70 years associates with severe CDI [61] Predicts death due to CDI [42] Predicts initial and recurrent disease [92,93] |
| Gastric acid suppression | Some studies specifically identify proton-pump inhibitors that are more frequently used than histamine-2 blockers [4,30,94] Does not appear to affect disease severity or outcomes [24] May [91] or may not [24] predict disease recurrence Probably a risk factor for community-acquired CDI [30], although some disparate findings [37] |
| Low albumin (<3.5 g/dl) | This likely reflects diminished health status [93] Predicts death due to CDI [42] <2.5 g/dl associates with severe disease [61] |
| Underlying disease severity | Determined by physicians upon patient admission using a modified Horn’s index. A score of ≥3 predicts nosocomial CDI [65] |
| Previous hospital admission | Depending on the study, the time frame extends up to the previous 12 weeks. Suggestive of diminished health and also furnish opportunities for exposure to C. diffiicile spores in the environment and for receipt of systemic antimicrobials [20,45,92] Predicts disease recurrence [37] |
| Residence in a LTCF | LTCFs present opportunities for exposure to C. difficile spores and for receipt of systemic antimicrobials [20,95] |
| Less well-established | |
| NSAIDs | A recent population-based case–control study specifically implicates diclofenac [96] |
| Smoking | Both former smokers and current smokers are at an increased risk to acquire CDI compared to never smokers [36] |
| CDI pressure | Defined as a patient’s daily exposure to other patients with CDI on the same ward divided divided by the patient’s at risk length of stay [93] |
| Mechanical ventilation | Predicts CDI [93,97] Predicts disease recurrence [91] |
| Statins/HMG-CoA reductase inhibitors | Statins are protective against developing CDI while hospitalized [38] and against community-acquired CDI [37] |
CDI: Clostridium difficile infection; LTCF: Long-term care facility.
Age-related risk factors
Advanced age is an established risk factor for C. difficile infection. Using a large, prospective cohort, Loo et al. quantified this risk, determining that for every additional year of age after 18 years, the risk of healthcare-associated C. difficile infection increases by approximately 2% [29]. The reasons for this are multifactorial and may relate, in part, to older adults’ frequent interactions with healthcare systems and to age-related changes in physiology. Among older adults specifically, longer courses of antibiotics (>4 weeks) or treatment with more than four agents increased the risk of death due to C. difficile infection, as did a low serum albumin and history of coronary artery disease [42].
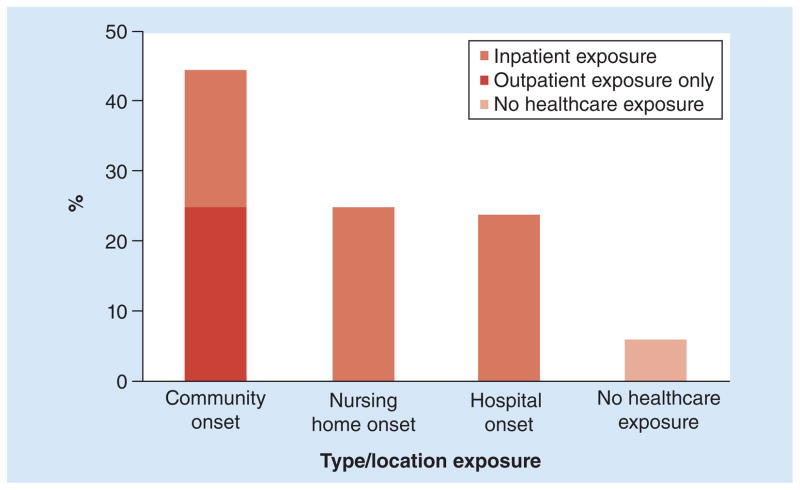
Frequent interaction with healthcare systems increases the opportunity for exposure to antimicrobials and for contact with physical environments contaminated with C. difficile spores. Both symptomatic and asymptomatic carriers of C. difficile shed spores onto their skin and into their environment, creating a risk for acquisition from other patients [43–45]. Data from the CDC’s Emerging Infectious Program revealed that, in 2010, exposure to healthcare preceded 94% of C. difficile infections. Of those, 75% were inpatient exposures, implicating that the remaining 25% of infections were associated with LTCFs and outpatient care settings (Figure 2) [20].
Figure 2. Percentage of Clostridium difficile infection cases by inpatient or outpatient status at the time of stool collection and type/location of exposures.
Data taken from [20].
C. difficile infection is endemic in LTCFs, where 3.6% of Americans >65 years old reside [46]. The Ohio Department of Public Health (USA) demonstrated that, in 2006, over half of new cases and three-quarters of recurrent C. difficile infections were diagnosed at nursing homes [19]. Compared with those not infected, hospitalized patients infected with C. difficile are more likely to be discharged to a LTCF [43]. This reflects the major loss of function reported in 60 and 93% of patients with a primary or secondary C. difficile infection, respectively [19]. These patients become a reservoir for C. difficile spores, which helps to explain why simply residing in a LTCF poses a risk for developing C. difficile infection, particularly within the first several weeks after admission [47]. Furthermore, in the USA, more than 75% of LTCF residents require assistance with at least four activities of daily living [46]. The frequent close contact between residents and healthcare workers presents abundant opportunities for transfer of C. difficile spores. Healthcare workers, particularly when understaffed, may unintentionally contribute to transmission of infectious diseases through poor infection control practices [48,49]. Finally, LTCF residents receive 2.9–13.9 courses of antimicrobials per 1000 days of care, and many of them are inappropriate or unnecessary [50–52].
Beyond their interactions with the healthcare system, older adults incur age-related physiologic changes that also contribute to their increased risk of C. difficile infection. Aging is accompanied by immune senescence. Diminished antibodies against C. difficile toxins may permit the development of symptomatic disease, rather than asymptomatic carriage, which may also increase the risk of recurrent infection [53]. Additionally, the gut microbiome of older adults differs to that of younger individuals. Using an in vitro assay that tests C. difficile growth in fecal emulsions as a functional measure of colonization resistance, Borriello and Barclay found that fecal emulsions derived from geriatric patients were less inhibitory for in vitro growth of C. difficile, compared with healthy adult volunteers [54]. This suggests that older adults’ gut microbiomes have less robust colonization resistance at baseline, which may reflect differences in the relative proportion and diversity of member species. Using microbiological cultivation, Hopkins and Macfarlane found decreased bacterial diversity among the feces of a small cohort of older adults, especially those with C. difficile infection, compared with younger adults [55]. Furthermore, Rea et al. used pyrosequencing of ribosomal RNA amplicons to describe a similar decrease in microbial diversity observed among older adults colonized or infected with C. difficile [56]. Comparison of older and younger adults’ gut microbiomes (average ages 73 and 31 years old, respectively) using whole-genome sequencing demonstrated a relative decrease among bacteria from the phylum Bacteroidetes and Clostridium cluster IX (includes Veillonella species) and an increase in those from Clostridium cluster XIVa (includes Eubacterium, Lachnospira, Ruminococcus and Roseburia species) [57]. While the clinical implications of these data are still unclear, changes in composition of the human gut microbiome in health and disease states is an active and exciting area of investigation.
Disease manifestations & treatment
C. difficile infection may manifest with a range of symptoms including asymptomatic colonization, frequent watery diarrhea (>3 episodes/day) and fulminant colitis requiring colectomy. Prior to the emergence of the epidemic strain, while older adults were at increased risk for developing C. difficile infection, the clinical features of the disease were not substantially different between younger and older adults [58,59]. This changed, however, following the outbreak of the BI/NAP1/027 strain, which began in Canada in around 2003 and spread to the USA by 2005 [6,60]. Infection with the BI/NAP1/027 strain was a risk factor for death [6]. Furthermore, in a retrospective chart review conducted between June 2005 and May 2006, Henrich et al. determined that age >70 years was a risk factor for severe C. difficile infection (odds ratio: 3.24; 95% CI: 1.42–7.38) [61].
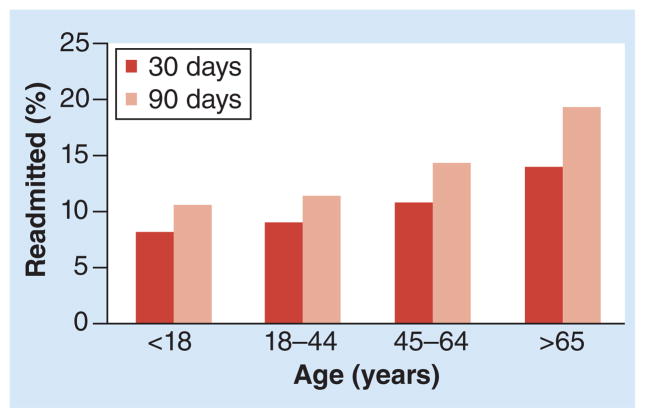
Older adults are also at greater risk for recurrent C. difficile infection. In 2009, among individuals discharged from hospitals in the USA with a diagnosis of C. difficile infection, the risk of readmission due to recurrent disease increased with age (Figure 3) [62]. A recently described model based on a clinical trial with nearly 1000 adults with C. difficile infection predicts that the risk of recurrent disease rises from <20% for those <40 years to just over 30% for those >80 years [63]. Furthermore, the first episode of disease recurrence becomes a predictor of additional episodes, regardless of whether they are due to relapse or due to reinfection with the same or a different strain [53]. Currently, identifying those at risk for recurrent infection may be achieved through a clinical prediction rule that assigns one point each for age >65 years, severe underlying disease and additional antibiotic use [64]. This scoring system considers patients with a score >2 as high risk for recurrent disease with an accuracy of approximately 72%. Older adults’ increased risk of recurrent C. difficile infections probably reflects immune senescence and age-related changes in the gut microbiome, as discussed above. Aside from age, other risk factors for recurrent disease include continued exposure to non-C. difficile-specific antibiotics after diagnosis, as well as administration of gastric acid-suppressive medications [65].
Figure 3. Following a Clostridium difficile infection-related hospital stay, the risk of readmission at 30 and 90 days due to C. difficile infection increases with age.
Data taken from [62].
Treatment recommendations do not vary with the age of the patient. Cessation of the inciting antibiotic remains an important aspect of management. The Society for Healthcare Epidemiology of America/Infectious Disease Society of America (SHEA/IDSA) and European Society of Clinical Microbiology and Infectious Disease (ESCMID) guidelines recommend similar medical treatments for initial episodes and first recurrence episodes of C. difficile infection (Table 2) [21,66]. Subsequent recurrences of C. difficile infection are challenging and may, as detailed in both the SHEA/IDSA and ESCMID guidelines, respond to tapered vancomycin therapy. While some studies have raised concerns for an increased rate of failure among older adults treated with oral metronidazole, these findings seem to indicate treatment failure for individuals with severe disease rather than an age-related deficit [53,67]. A novel medication, fidaxomicin, has potent activity against C. difficile but spares most other bacterial groups in the gut microbiome [68]. Compared with oral vancomycin, fidaxomicin leads to fewer episodes of first recurrences of C. difficile infection [63]. Interestingly, the BI/NAP1/027 epidemic strain may obviate the benefits of fidaxomicin compared with oral vancomycin; the reasons for different outcomes depending on strain are unclear [69,70].
Table 2.
Recommendations for the treatment of Clostridium difficile infection†.
| Clinical situation | Description | Recommended treatments |
|---|---|---|
| All | Stop inciting antibiotics whenever possible | |
| Initial episode: nonsevere | >3 unformed or watery stools per day and a stool test result positive for toxigenic C. difficile or its toxins, or pseudomembranous colitis on colonoscopic or histopathologic examination | Metronidazole, 500 mg by mouth three-times a day for 10–14 days |
| Initial episode: severe | Leukocytosis with white blood cell count >15,000 cell/ml and a serum creatinine >1.5-times the premorbid level |
Vancomcyin 125 mg by mouth four-times a day for 10–14 days‡ |
| Initial episode: severe and complicated | Hemodynamic instability, ileus or toxic megacolon§ Fever (>38.5°C), rigors, peritonitis, band neutropihils >20% of leukocytes; elevated serum lactate, pseudomembranous colitis, colonic wall thickening, pericolonic fat stranding, ascitics without another cause¶ |
Vancomycin 500 mg four-times a day by mouth or through nasogastric tube; metronidazole 500 mg intravenously If complete ileus, consider adding rectal installation of vancomycin Surgical consultation |
| Indications for colectomy | Colonic perforation Failure to respond to antibiotic treatment with a deteriorating clinical status, including toxic megacolon or severe ileus |
|
| Recurrent disease: first episode | Same as for initial episode | |
| Recurrent disease: subsequent episodes | Vancomycin taper or pulse therapy# |
Based on both the Society for Healthcare Epidemiology of America/Infectious Disease Society of America and European Society of Clinical Microbiology and Infectious Disease treatment guidelines.
Where available, teicoplanin 100 mg by mouth twice a day may replace oral vancomycin.
Per the Society for Healthcare Epidemiology of America/Infectious Disease Society of America Guidelines.
Per the European Society of Clinical Microbiology and Infectious Disease Guidelines.
Vancomycin 125 mg by mouth four-times a day for 10–14 days followed by vancomycin 125 mg by mouth twice a day for 1 week, 125 mg by mouth once daily for 1 week and then 125 mg by mouth every 2–3 days for 2–8 weeks.
Fecal bacteriotherapy, also termed fecal transplant, is a therapeutic option that is gaining wider acceptance in both Europe and the USA. The premise is that instillation of normal fecal bacteria into the intestine of a person afflicted with C. difficile infection will permit rapid recovery of the gut microbiome and colonization resistance. The procedure relies upon obtaining healthy stool and administering it to the patient via nasogastric tube, enema or via colonoscopy. Donor feces usually comes from a close contact of the patient, including an intimate partner, relative or household contact. Beyond convenience, obtaining stool from a close contact of the patient minimizes the risk of introducing a new pathogens and is associated with lower relapse rate compared with feces obtained from unrelated volunteers [71,72]. A recent randomized controlled trial, however, used a prescreened pool of healthy donors with excellent results [73]. In total, 15 of 16 patients (93%) in the fecal bacteriotherapy arm were cured of their C. difficile infection with one to two instillations of donor feces. Analysis of the microbiome recovered from patients following successful treatment revealed an increase in bacteria from the phylum Bacteroidetes, Clostridium cluster IV (includes Clostridium leptum spp.), Clostridium cluster XIVa (includes Eubacterium, Lachnospira, Ruminococcus and Roseburia spp.) and a decrease in bacteria from Proteobacteria. Fecal bacteriotherapy seems to be a safe and well-tolerated therapy to treat patients with C. difficile infection, including older adults with recurrent disease [74].
Prevention
Preventing C. difficile infection involves both minimizing transmission and reducing patients’ vulnerability. The longevity and tenacity of C. difficile spores in the environment contributes to the risk of acquisition during institutionalization [21]. C. difficile spores may remain dormant on environmental surfaces for months, far longer than any other nosocomial pathogen [21]. Furthermore, the spores contaminate a variety of environmental surfaces in hospitals and LTCFs, including toilets, bedrails, equipment used to obtain vital signs and bedside curtains, with some indications that they may even be airborne [75]. Given that C. difficile spores are resistant to most surface disinfectants and that environmental staff may not appropriately clean many surfaces, it is not surprising that occupying a room in which the previous patient had C. difficile infection is a significant risk factor for acquiring a new C. difficile infection [76].
A variety of strategies have been employed to combat the persistence of C. difficile spores in the environment, ranging from improved cleaning methods to new disinfectant technologies. Chlorine-containing cleaning agents are an effective means to reduce the environmental burden and, at least to some extent, the incidence of C. difficile infection [21]. In a hospital with a high rate of C. difficile infection (24.2 cases/10,000 patient days), daily cleaning of all rooms with bleach wipes reduced the incidence of hospital-acquired C. difficile infection to 3.6 cases/10,000 patient days [77]. The drawbacks to using chlorine-containing agents include the strong odor, possible hypersensitivity, a corrosive effect on equipment over time, achieving sufficient contact time on surfaces and that its application is operator dependent [21]. Among new technologies, adding hydrogen peroxide vapor to terminal cleaning of hospital rooms appears to have reduced facility-wide C. difficile infection rates from 0.88 to 0.55 cases/1000 patient days in one retrospective quasiexperimental study [78]. While effective, drawbacks associated with using hydrogen peroxide vapor include the expense of purchasing specialized equipment and associated consumables, as well as the need to seal rooms [21]. Ultraviolet radiation may also hold promise as a means to reduce environmental contamination, although it has not yet been shown to reduce C. difficile infection rates. Also relatively expensive, ultraviolet radiation has the distinct advantage that it requires the patient to be absent from the room for only a short period, making it a practical means to reduce the environmental pathogens during a patient’s stay. While this may not confer an advantage for hospitals, in which the length of stay is relatively short, it may hold promise as a means to reduce the burden of C. difficile spores in LTCFs and similar facilities [44].
Besides direct contact with contaminated environmental surfaces, people may also acquire C. difficile infection through contact with healthcare workers. While alcohol hand rub effectively kills most nosocomial pathogens, C. difficile spores are resistant to alcohol. The best means to remove spores from hands is the mechanical action associated with using soap and water. In response to low rates of hand hygiene employed by healthcare workers, several organizations, including the CDC and the WHO, have launched campaigns to improve hand hygiene at healthcare facilities. The ‘Cleanyourhands’ campaign in 187 acute trusts in England and Wales found that, in the setting of a high-profile political drive, increased procurement of soap and alcohol rub correlated with decreased rates of C. difficile infection and bacteremia due to methicillin-resistant Staphylococcus aureus [79]. Routine use of gloves may also be an effective means to reduce nosocomial transmission of C. difficile spores [21].
In addition to reducing the burden of spores in the environment, a key aspect of preventing C. difficile infection in older adults is to minimize their vulnerability by avoiding unnecessary antibiotic exposure. In the USA, >50% of antimicrobials prescribed in hospitals and 25–75% of those prescribed in LTCFs may be inappropriate or unnecessary [51,80,81]. Beyond C. difficile infection, adverse consequences of inappropriate antimicrobial use include selection for resistant pathogens, increased risk for drug–drug interactions and greater costs. Improving antimicrobial use through antimicrobial stewardship reduces C. difficile infections in both acute and long-term care settings. Prior to the emergence of the BI/NAP1/027 epidemic C. difficile strain, a teaching hospital in the UK reduced the incidence of C. difficile infection on a single geriatric ward by nearly 50% through restrictions on intravenous cephalosporins [82]. When enhanced infection-control measures proved ineffective at reducing the incidence of the BI/NAP1/027 epidemic strain, two Canadian hospitals implemented an educational initiative to direct the choice of empiric antimicrobial therapy away from specific agents associated with C. difficile infection. Comparisons of the same 4-week period over 3 years showed a reduction in total antimicrobial use by over 20% and in the incidence of C. difficile infection by 60% [83]. Three hospitals in Northern Ireland achieved similar results during an outbreak of the BI/NAP1/027 epidemic strain by restricting only fluoroquinolones [84]. Antimicrobial stewardship in a LTCF that reduced total antibiotic use by 30% and fluoroquinolones by 28% led to a decline in the rate of positive C. difficile tests [85]. These and similar studies concur with the SHEA/IDSA recommendations to use antimicrobial stewardship as a means to reduce C. difficile infection [21].
Conclusion & future perspective
Among the causes of morbidity and mortality among older adults, C. difficile infection stands out because most instances are iatrogenic. Not only do older adults experience increased disease severity, they are also more likely to have recurrent infection with courses of treatment that may last for months. Developing a diagnostic test to identify patients at high risk for recurrent disease may augment medical management of these individuals. For example, notifications through the electronic medical record may alert healthcare providers of patients with a high risk for recurrence, perhaps reducing the likelihood of further antimicrobial prescriptions, apart from those used for treating C. difficile infection.
Fecal bacteriotherapy is an effective option to treat both initial and recurrent C. difficile infection. While it has yet to receive an endorsement from the SHEA, IDSA or ESCMID, fecal bacteriotherapy is gaining acceptance by healthcare providers in the USA [86]. The American College of Gastroenterology’s C. difficile Infection guidelines offer a conditional recommendation to consider fecal bacteriotherapy among patients who failed pulsed-oral vancomycin (i.e., more than three recurrences) [87]. Over the next few years, larger hospitals and referral centers may develop fecal bacteriotherapy centers, coordinating the efforts of infectious disease physicians and gastroenterologists. Furthermore, as fecal bacteriotherapy centers become more common, we may also see the advent of clinical studies comparing the source of the sample (household contact, genetically related family member, prescreened donors), preparation of the sample (blender, filtration) and recipient, (bowel lavage, duration without antibiotics) and method of administration (capsule, instillation via nasogastric, duodenal or jejunal tube, enema or colonoscopy).
Further investigations into the gut microbiome will continue to identity specific bacteria that confer colonization resistance. Using a mouse model, Lawley et al. have identified six bacterial species, three of which are novel, that together are able to restore colonization resistance to the gut microbiome of mice experimentally infected with C. difficile [88]. Coming from distinct branches of the phylogenetic tree that comprises the gut microbiome, these bacteria are Staphylococcus warneri, Enterococcus hirae, Lactobacillus reuteri, Anaerotspies species nov., Bacteroidetes species nov. and Enterohabdus species nov. [88]. Translating this work into clinical trials may eventually foster development of an evidence-based probiotic formulation targeted specifically for people with C. difficile infection. Ideally, administration of live microorganisms as a medical therapy would be approved and regulated by the US FDA with the organisms that can be quantified and identified by genus, species and strain.
There are ongoing efforts to develop a vaccine effective against C. difficile infection. A candidate vaccine against toxoids A and B administered as a series of four intramuscular injections over 8 weeks cured recurrent infection in patients who had previously been oral vancomcyin for 7, 9 and 22 months [89]. Some hurdles to vaccine development include whether to target toxin A, B or both, and to determine if systemic (IgG) or mucosal (IgA) antibodies are most effective at disease prevention. For an excellent review of vaccines against C. difficile that also addresses immunologic and biotherapeutic approaches, please see [90].
Further research and clinical studies will continue to bring advances in the treatment and eventually, prevention of C. difficile infection. Currently, however, our best efforts to reduce the risk of this disease must focus on enhanced infection-control measures, specifically rigorous hand hygiene and stringent environmental decontamination, coupled with reducing older adults’ vulnerability through good antimicrobial stewardship practices in all healthcare settings.
Executive summary.
Pathology
Clostridium difficile is a Gram-positive, spore-forming bacillus that causes disease following ingestion by a susceptible host.
The gut microbiome prevents C. difficile from gaining a foothold in the intestine through colonization resistance.
Systemic antibiotics disrupt the gut microbiome, rendering hosts vulnerable to C. difficile infection.
An epidemic strain of C. difficile emerged in the last 10–15 years. It causes more severe disease and is resistant to fluoroquinolones.
Epidemiology
Older adults are disproportionately affected by C. difficile infection.
Nearly 1% of all hospitalizations involve C. difficile infection.
C. difficile infections place a significant financial burden on healthcare systems.
General risk factors
Antibiotic exposure and advanced age are the two greatest risk factors for C. difficile infection.
Gastric acid suppression appears to be strongly correlated with C. difficile infection, although the mechanism is unclear.
Most other risk factors reflect diminished health status (albumin ≤3.5 g/dl, underlying disease severity and mechanical ventilation).
Age-related risk factors
Older adults’ frequent interactions with healthcare systems increase their opportunity for exposure to C. difficile spores.
Older adults frequently receive antibiotics, placing them at risk for C. difficile infection.
With aging comes immune senescence and, perhaps, a less robust gut microbiome.
Disease manifestations & treatment
C. difficile infection may manifest with a range of symptoms including asymptomatic colonization, watery diarrhea and fulminant colitis requiring colectomy.
Older adults experience more severe disease and are at greater risk for recurrent disease.
Metronidazole and oral vancomycin are the mainstays of treatment.
While expensive, fidaxomicin reduces the likelihood of a first disease recurrence.
Fecal transplant is an excellent therapy that is garnering greater attention.
Prevention
C. difficile spores are difficult to remove using routine cleansing agents and may remain viable on environmental surfaces for months.
Reducing the burden of C. difficile in the environment is critical to disease prevention.
Healthcare workers may serve as vectors; hand hygiene using soap and water is the best means to remove spores from their hands.
Preventing unnecessary antimicrobial use through stewardship also reduces C. difficile infections.
Conclusion & future perspective
Morbidity and mortality due to C. difficile is mostly iatrogenic.
Fecal transplant centers may offer highly effective treatment for C. difficile infection, particularly for recurrent episodes.
Current research efforts may lead to an effective, evidence-based probiotic therapy to treat C. difficile infection.
There are ongoing efforts, including clinical trials, to develop a vaccine that will treat, and possibly prevent, C. difficile infection.
Acknowledgments
R Jump wrote this article as a recipient of the IDSA Education and Research Foundation/National Foundation for Infectious Diseases Association of Specialty Professors Young Investigator Award in Geriatrics. Special thanks to CJ Donskey for his valuable comments.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by the NIH (R03-AG040722) and the Veterans Integrated Service Network 10 Geriatric Research Education and Clinical Center (VISN 10 GRECC). R Jump also gratefully acknowledges the T Franklin Williams Scholarship with funding provided by: Atlantic Philanthropies, Inc., the John A Hartford Foundation, the Association of Specialty Professors, the Infectious Diseases Society of America and the National Foundation for Infectious Diseases. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehne SA, Cartman ST, Minton NP. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes. 2011;2:252–255. doi: 10.4161/gmic.2.4.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Barrak A, Embil J, Dyck B, et al. An outbreak of toxin A negative, toxin B positive Clostridium difficile-associated diarrhea in a Canadian tertiary-care hospital. Can Commun Dis Rep. 1999;25:65–69. [PubMed] [Google Scholar]
- 4.Lyras D, O’Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 6.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene–variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 8.Goorhuis A, Bakker D, Corver J, et al. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162–1170. doi: 10.1086/592257. [DOI] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Konstantelias AA, Loizidis G, Rafailidis PI, Falagas ME. Risk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysis. Int J Infect Dis. 2012;16:e768–e773. doi: 10.1016/j.ijid.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Bacci S, Mølbak K, Kjeldsen MK, Olsen KEP. Binary toxin and death after Clostridium difficile infection. Emerging Infect Dis. 2011;17:976–982. doi: 10.3201/eid1706.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 12.Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl Environ Microbiol. 2012;78:4683–4690. doi: 10.1128/AEM.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould CV, McDonald LC. Bench-to-bedside review: Clostridium difficile colitis. Crit Care. 2008;12:203. doi: 10.1186/cc6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams DA, Riggs MM, Donskey CJ. Effect of fluoroquinolone treatment on growth of and toxin production by epidemic and nonepidemic Clostridium difficile strains in the cecal contents of mice. Antimicrob Agents Chemother. 2007;51:2674–2678. doi: 10.1128/AAC.01582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labbé A-C, Poirier L, Maccannell D, et al. Clostridium difficile infections in a Canadian tertiary care hospital before and during a regional epidemic associated with the BI/NAP1/027 strain. Antimicrob Agents Chemother. 2008;52:3180–3187. doi: 10.1128/AAC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nature Genetics. 2013;45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirard S, Valiquette L, Fortier L-C. Lack of association between clinical outcome of Clostridium difficile infections, strain type, and virulence-associated phenotypes. J Clin Microbiol. 2011;49:4040–4046. doi: 10.1128/JCM.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55:1056–1063. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 19.Lucado J, Gould C, Elixhauser A. NCBI bookshelf. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2006. Clostridium difficile Infections (CDI) In Hospital Stays, 2009 – Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. [PubMed] [Google Scholar]
- 20.McDonald LC, Lessa F, Sievert D, et al. Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61(9):157–162. [PubMed] [Google Scholar]
- 21•.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. Current clinical guidelines for treating Clostridium difficile infection from the Society for Healthcare Epidemiology of America and Infectious Diseases Society of America. An update is in progress, with publication projected for the summer of 2014. [DOI] [PubMed] [Google Scholar]
- 22.Dumyati G, Stevens V, Hannett GE, et al. Community-associated Clostridium difficile infections, Monroe County, New York, USA. Emerg Infect Dis. 2012;18:392–400. doi: 10.3201/eid1803.102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones AM, Kuijper EJ, Wilcox MH. Clostridium difficile: a European perspective. J Infect. 2013;66:115–128. doi: 10.1016/j.jinf.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30:526–533. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 27.Health Protection Agency. Infection Data (up to July–September 2012) Health Protection Agency; London, UK: 2012. Quarterly Epidemiological Commentary: Mandatory MRSA, MSSA and E. coli Bacteraemia, and C. Difficile. [Google Scholar]
- 28.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55:1056–1063. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 29.Loo VG, Bourgault A-M, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 30.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 31.Tleyjeh IM, Abdulhak AA, Riaz M, et al. The association between histamine 2 receptor antagonist use and Clostridium difficile infection: a systematic review and meta-analysis. PLoS ONE. 2013;8:e56498. doi: 10.1371/journal.pone.0056498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case–control studies. CMAJ. 2004;171:33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna S, Aronson SL, Kammer PP, Baddour LM, Pardi DS. Gastric acid suppression and outcomes in Clostridium difficile infection: a population-based study. Mayo Clin Proc. 2012;87:636–642. doi: 10.1016/j.mayocp.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jump RLP, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother. 2007;51:2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nerandzic MM, Pultz MJ, Donskey CJ. Examination of potential mechanisms to explain the association between proton pump inhibitors and Clostridium difficile infection. Antimicrob Agents Chemother. 2009;53:4133–4137. doi: 10.1128/AAC.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers MAM, Greene MT, Saint S, et al. Higher rates of Clostridium difficile infection among smokers. PLoS ONE. 2012;7:e42091. doi: 10.1371/journal.pone.0042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naggie S, Miller BA, Zuzak KB, et al. A case–control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med. 2011;124(3):276. e1–7. doi: 10.1016/j.amjmed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Motzkus-Feagans CA, Pakyz A, Polk R, Gambassi G, Lapane KL. Statin use and the risk of Clostridium difficile in academic medical centres. Gut. 2012;61:1538–1542. doi: 10.1136/gutjnl-2011-301378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Songer JG, Trinh HT, Killgore GE, Thompson AD, McDonald LC, Limbago BM. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis. 2009;15:819–821. doi: 10.3201/eid1505.081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curry SR, Marsh JW, Schlackman JL, Harrison LH. Prevalence of Clostridium difficile in uncooked ground meat products from Pittsburgh, Pennsylvania. Appl Environ Microbiol. 2012;78:4183–4186. doi: 10.1128/AEM.00842-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limbago B, Thompson AD, Greene SA, et al. Development of a consensus method for culture of Clostridium difficile from meat and its use in a survey of US retail meats. Food Microbiol. 2012;32:448–451. doi: 10.1016/j.fm.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharmarajan T, Sipalay M, Shyamsundar R, Norkus E, Pitchumoni C. Co-morbidity, not age predicts adverse outcome in Clostridium difficile colitis. World J Gastroenterol. 2000;6:198–201. doi: 10.3748/wjg.v6.i2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. Hallmark publication describing the acquisition and transmission of C. difficile infection among hospitalized patients, including patient-to-patient transmission, immunoblot typing and environmental contamination. [DOI] [PubMed] [Google Scholar]
- 44.Sitzlar B, Vajravelu RK, Jury L, Donskey CJ, Jump RLP. Environmental decontamination with ultraviolet radiation to prevent recurrent Clostridium difficile infection in 2 roommates in a long-term care facility. Infect Control Hosp Epidemiol. 2012;33:534–536. doi: 10.1086/665310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero DM, Nerandzic MM, Jury LA, Jinno S, Chang S, Donskey CJ. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am J Infect Control. 2012;40:556–558. doi: 10.1016/j.ajic.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Jones AL, Dwyer LL, Bercovitz AR, Strahan GW. The National Nursing Home Survey: 2004 overview. Vital Health Stat. 2009;(167):1–155. [PubMed] [Google Scholar]
- 47.Guerrero DM, Nerandzic MM, Jury LA, Chang S, Jump RL, Donskey CJ. Clostridium difficile infection in a department of veterans affairs long-term care facility. Infect Control Hosp Epidemiol. 2011;32:513–515. doi: 10.1086/659765. [DOI] [PubMed] [Google Scholar]
- 48.Loeb MB, Craven S, McGeer AJ, et al. Risk factors for resistance to antimicrobial agents among nursing home residents. Am J Epidemiol. 2003;157:40–47. doi: 10.1093/aje/kwf173. [DOI] [PubMed] [Google Scholar]
- 49.Trick WE, Weinstein RA, DeMarais PL, et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 2004;52:2003–2009. doi: 10.1111/j.1532-5415.2004.52555.x. [DOI] [PubMed] [Google Scholar]
- 50.Loeb M, Simor AE, Landry L, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med. 2001;16:376–383. doi: 10.1046/j.1525-1497.2001.016006376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicolle LE, Bentley DW, Garibaldi R, Neuhaus EG, Smith PW. Antimicrobial use in long-term–care facilities. Infect Control Hosp Epidemiol. 2000;21:537–545. doi: 10.1086/501798. [DOI] [PubMed] [Google Scholar]
- 52.Peron EP, Hirsch AA, Jury LA, Jump RLP, Donskey CJ. Another setting for stewardship: high rate of unnecessary antimicrobial use in a veterans affairs long-term care facility. J Am Geriatr Soc. 2013;61:289–290. doi: 10.1111/jgs.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 54.Borriello SP, Barclay FE. An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol. 1986;21:299–309. doi: 10.1099/00222615-21-4-299. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- 56.Rea MC, O’Sullivan O, Shanahan F, et al. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol. 2012;50:867–875. doi: 10.1128/JCM.05176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kyne L, Merry C, O’Connell B, Kelly A, Keane C, O’Neill D. Factors associated with prolonged symptoms and severe disease due to Clostridium difficile. Age Ageing. 1999;28:107–113. doi: 10.1093/ageing/28.2.107. [DOI] [PubMed] [Google Scholar]
- 59.Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol. 2002;23:696–703. doi: 10.1086/501997. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention (CDC) Severe Clostridium difficile-associated disease in populations previously at low risk – four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–1205. [PubMed] [Google Scholar]
- 61.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elixhauser A, Steiner C, Gould C. HCUP Statistical brief #145. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2012. Readmissions following hospitalizations with Clostridium difficile infections, 2009. [PubMed] [Google Scholar]
- 63.Louie TJ, Miller MA, Crook DW, et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61:222–230. doi: 10.1111/jgs.12090. [DOI] [PubMed] [Google Scholar]
- 64.Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Bauer MP, Kuijper EJ, Van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clin Microbiol Infect. 2009;15:1067–1079. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 67.Cober ED, Malani PN. Clostridium difficile infection in the ‘oldest’ old: clinical outcomes in patients aged 80 and older. J Am Geriatr Soc. 2009;57:659–662. doi: 10.1111/j.1532-5415.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 68.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354–3359. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 69.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 70.Petrella LA, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis. 2012;55:351–357. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 72.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 73•.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. Randomized controlled trial showing the benefit of fecal transplant to treat recurrent C. difficile infection. Their work is also notable for the use of feces from a pool of prescreened donors. [DOI] [PubMed] [Google Scholar]
- 74.Rubin TA, Gessert CE, Aas J. Stool transplantation for older patients with Clostridium difficile infection. J Am Geriatr Soc. 2009;57:2386. doi: 10.1111/j.1532-5415.2009.02600.x. [DOI] [PubMed] [Google Scholar]
- 75.Donskey CJ. Preventing transmission of Clostridium difficile: is the answer blowing in the wind? Clin Infect Dis. 2010;50:1458–1461. doi: 10.1086/652649. [DOI] [PubMed] [Google Scholar]
- 76.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–206. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 77.Orenstein R, Aronhalt KC, McManus JE, Jr, Fedraw LA. A targeted strategy to wipe out Clostridium difficile. Infect Control Hosp Epidemiol. 2011;32:1137–1139. doi: 10.1086/662586. [DOI] [PubMed] [Google Scholar]
- 78.Manian FA, Griesnauer S, Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41:537–541. doi: 10.1016/j.ajic.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 79.Stone SP, Fuller C, Savage J, et al. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ. 2012;344:e3005. doi: 10.1136/bmj.e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 81.Fishman NO. Antimicrobial Stewardship. Am J Med. 2006;119:S53–S61. [Google Scholar]
- 82.Ludlam H, Brown N, Sule O, Redpath C, Coni N, Owen G. An antibiotic policy associated with reduced risk of Clostridium difficile-associated diarrhoea. Age Ageing. 1999;28:578–580. doi: 10.1093/ageing/28.6.578. [DOI] [PubMed] [Google Scholar]
- 83.Valiquette L, Cossette B, Garant M-P, Diab H, Pépin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis. 2007;45(Suppl 2):S112–S121. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 84.Aldeyab MA, Devine MJ, Flanagan P, et al. Multihospital outbreak of Clostridium difficile ribotype 027 infection: epidemiology and analysis of control measures. Infect Control Hosp Epidemiol. 2011;32:210–219. doi: 10.1086/658333. [DOI] [PubMed] [Google Scholar]
- 85.Jump RLP, Olds DM, Seifi N, et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a lid on antibiotic use. Infect Control Hosp Epidemiol. 2012;33:1185–1192. doi: 10.1086/668429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang Z-D, Hoang LN, Lasco TM, Garey KW, DuPont HL. Physician attitudes toward the use of fecal transplantation for recurrent Clostridium difficile infection in a metropolitan area. Clin Infect Dis. 2013;56:1059–1060. doi: 10.1093/cid/cis1025. [DOI] [PubMed] [Google Scholar]
- 87.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 88•.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. Exciting publication that highlights the potential for an evidence-based probiotic regimen to treat C. difficile infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sougioultzis S, Kyne L, Drudy D, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology. 2005;128:764–770. doi: 10.1053/j.gastro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 90•.Gerding DN. Clostridium difficile infection prevention: biotherapeutics, immunologics, and vaccines. Discov Med. 2012;13:75–83. Succinct and current review of potential vaccines to treat and prevent C. difficile infection. [PubMed] [Google Scholar]
- 91.Hebert C, Du H, Peterson LR, Robicsek A. Electronic health record-based detection of risk factors for Clostridium difficile infection relapse. Infect Control Hosp Epidemiol. 2013;34:407–414. doi: 10.1086/669864. [DOI] [PubMed] [Google Scholar]
- 92.Eyre DW, Walker AS, Wyllie D, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis. 2012;55(Suppl 2):S77–S87. doi: 10.1093/cid/cis356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile – associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 94.Howell NV., MD Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 95.Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson ES. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case–control study. Eur J Clin Microb Infect Dis. 2012;31:2601–2610. doi: 10.1007/s10096-012-1603-0. [DOI] [PubMed] [Google Scholar]
- 96.Suissa D, Delaney JAC, Dial S, Brassard P. Non-steroidal anti-inflammatory drugs and the risk of Clostridium difficile-associated disease. Br J Clin Pharmacol. 2012;74:370–375. doi: 10.1111/j.1365-2125.2012.04191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zilberberg MD, Nathanson BH, Sadigov S, Higgins TL, Kollef MH, Shorr AF. Epidemiology and outcomes of Clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136:752–758. doi: 10.1378/chest.09-0596. [DOI] [PubMed] [Google Scholar]
Websites
- 101.US Department of Health and Human Services. [Accessed 4 March 2013];National targets and metrics. www.hhs.gov/ash/initiatives/hai/nationaltargets/index.html.
- 102.Murphy SL, Xu J, Kochanek KD. National vital statistic reports. [Accessed 1 April 2013];Deaths: preliminary data for 2010. 2012 www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. [PubMed]