Abstract
While there clearly is an intimate relationship between astrocytes and microglia, few studies have examined these potentially dynamic interactions. In this study, cytokine-mediated communication between microglia and astrocytes under inflammatory conditions was investigated. We have previously shown that activated microglia produce Interleukin (IL)-10, a regulatory cytokine that plays an important role in resolving neuroinflammation. Nonetheless, the mechanism by which IL-10 attenuates pro-inflammatory cytokine expression in the brain is unclear. Here we show that IL-10 re-directed astrocytes regulate the activation of microglia in a Transforming growth factor (TGF)-β dependent manner. In support of this concept, astrocytes in the brain maintained higher IL-10 receptor (IL-10R1) expression and primary astrocytes in culture were markedly more sensitive to the anti-inflammatory effects of IL-10 compared to microglia. Moreover, studies using primary cultures and an astrocyte-microglia co-culture system revealed that astrocytes mediated the anti-inflammatory effects of IL-10 on microglia through the production of TGFβ. For instance, only when astrocytes were present did IL-10 stimulation reduce the expression of IL-1β and increase expression of anti-inflammatory mediators fractalkine receptor (CX3CR1) and interleukin 4 receptor-α (IL-4Rα) in microglia. Importantly, these IL-10-astrocyte dependent effects on microglia were blocked by a TGFβ inhibitor. Furthermore, inhibition of TGFβ signaling in the brain resulted in prolonged sickness behavior and amplified pro-inflammatory cytokine expression in mice challenged with lipopolysaccharide (LPS). Taken together, IL-10 stimulated the production of TGFβ by astrocytes, which in turn, attenuated microglial activation. Overall, these findings provide novel insight into the mechanisms by which astrocytes modulate microglia under inflammatory conditions.
Keywords: interleukin-10, TGF-beta, cytokines, microglial regulation, neuroinflammation, sickness behavior
Introduction
Innate immunity within the central nervous system (CNS) is primarily provided by resident microglia. Microglia are pivotal in immune surveillance and also facilitate the coordinated responses between the immune system and the brain (Davalos et al., 2005; Nimmerjahn et al., 2005). For example, microglia interpret and propagate inflammatory signals that are initiated in the periphery. This transient microglial activation is essential for the induction and maintenance of the behavioral symptoms of sickness. This term describes neurobehavioral symptoms associated with infection, including lethargy, listlessness, decreased activity and reduced social interaction (Dantzer et al., 2008). This sickness response is mediated by pro-inflammatory cytokines, including IL-1β, and represents a reorganization of the host priorities to resolve infections (Kelley et al., 1997). Nonetheless, prolonged exposure to inflammatory cytokines in the brain has deleterious effects on neuronal plasticity, behavior, and cognition (Dantzer et al., 2008). For example, microglia in the aged brain develop a more “primed” or reactive profile (Cunningham, 2013; Sierra et al., 2007) and as a consequence of this there is amplified production of IL-1β following an innate immune challenge (Frank et al., 2010; Henry et al., 2009). Amplified neuroinflammation induced by primed microglia is associated with cognitive impairment, prolonged sickness behavior, and depressive-like behavior (Barrientos et al., 2006; Godbout et al., 2008). Exaggerated microglial responses may be related to impairments in key regulatory systems that make it more difficult to resolve microglial activation (Norden and Godbout, 2013). Thus, understanding how microglial activation is regulated in the brain is critical.
Recent findings indicate that astrocytes in the brain are active participants in both propagating and regulating neuroinflammation (Farina et al., 2007; Liu et al., 2011; Pekny and Nilsson, 2005). Astrocytes become activated by inflammatory mediators, engagement of TLRs (Bsibsi et al., 2006; Carpentier et al., 2005; Gurley et al., 2008) and cytokines including IL-1β (John et al., 2004). Once activated, astrocytes produce many regulatory factors that may influence CNS immunity and provide negative feedback to activated microglia. For instance, addition of conditioned media from astrocytes to microglia cultures increased antioxidant and anti-inflammatory gene expression (Min et al., 2006). In addition, several studies indicate that activated astrocytes release factors that are neuroprotective (Farina et al., 2007). Although these results were not directly linked to regulating microglial activation, it is plausible that this is a critical component to astrocyte-mediated neuroprotection.
Two key anti-inflammatory cytokines that modulate astrocyte and microglial activation are IL-10 and TGFβ. IL-10 is an anti-inflammatory cytokine that has immunoregulatory effects in the brain (Kremlev and Palmer, 2005). Direct administration of IL-10 into the brain suppressed LPS-induced IL-1β expression in the hippocampus and ameliorated sickness behavior (Bluthe et al., 1999; Lynch et al., 2004). In IL-10 deficient mice, peripheral LPS challenge caused prolonged neuroinflammation and sickness behavior (Richwine et al., 2009). IL-10 also has beneficial effects in neuroinflammatory disease models including experimental autoimmune encephalomyelitis (Cua et al., 2001), spinal cord injury (Ishii et al., 2013), and stroke (Frenkel et al., 2005). These findings support an anti-inflammatory and potential neuroprotective role of IL-10 within the brain. While active microglia and macrophages secrete IL-10 (Henry et al., 2009), the cellular target of IL-10 regulation in the brain is unclear.
TGFβ also has neuroprotective and anti-inflammatory effects in the CNS. These anti-inflammatory responses to TGFβ may be mediated, in part, by reducing microglial activation (Butovsky et al., 2014). For example, TGFβ inhibited pro-inflammatory cytokine and nitric oxide production in activated microglia (Orellana et al., 2013; Suzumura et al., 1993). In addition, TGFβ protected against amyloid-beta induced neurotoxicity (Caraci et al., 2008; Ramirez et al., 2005) and neuronal death in a model of Parkinson disease by inhibiting reactive oxygen species (ROS) production in microglia (Qian et al., 2008). Nonetheless, the mechanisms and cell-specific targets of IL-10 and TGFβ mediated suppression of neuroinflammation remain unknown.
Therefore, the objective of this study was to investigate microglia-astrocyte interactions under inflammatory conditions and focus on key anti-inflammatory cytokine signals between these two cell types. In this study, we provide several lines of evidence that IL-10 re-directs active astrocytes to produce TGFβ, which in turn, attenuates the activation of microglia. Inhibition of TGFβ signaling blocked these IL-10-astrocyte mediated effects on microglia. After peripheral immune challenge with LPS, we confirmed that increased IL-10 expression was specific to enriched microglia in the brain and the increased IL-10R1 and TGFβ expression was specific to enriched astrocytes. Moreover, inhibition of TGFβ signaling in the brain exaggerated microglial activation, neuroinflammation, and sickness behavior following LPS challenge. Collectively these data provide new insight into cytokine-mediated interactions between astrocytes and microglia.
Materials and Methods
Animals
Neonatal pups (postnatal day 1-3) and adult (3-4 months-old) BALB/c mice were obtained from our breeding colony kept in barrier-reared conditions in a specific-pathogen-free facility at the Ohio State University. Mice were individually housed in polypropylene cages and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Microglia and astrocyte isolation from brain
Microglia and astrocytes were isolated from brain homogenates using a Percoll density gradient as previously described (Fenn et al., 2012). In brief, tissues were homogenized and cell pellets were re-suspended in 70% isotonic Percoll. A discontinuous Percoll density gradient (70%, 50%, 35%, 0%) was layered and centrifuged for 20 min at 2000xg. Enriched microglia were collected from the interphase between 70% and 50% Percoll. Of the cells recovered from this interphase, 90% of the cells were CD11b+ microglia (Henry et al., 2009). Enriched astrocytes were collected from the interphase between 50% and 35% Percoll. Of the cells recovered from this interphase, 60% of the cells were GLAST-1+ astrocytes.
Flow cytometry
Cells were assayed for surface antigens by flow cytometry as previously described (Fenn et al., 2012; Henry et al., 2009). Percoll enriched microglia were incubated with rat anti-mouse CD11b-FITC, IL-10R1-PE, and CD45-PerCP-Cy5.5 antibodies (eBioscience). Percoll enriched astrocytes were incubated with rat anti-mouse GLAST-1-APC (Miltenyi Biotec), CD-11b-FITC, and IL-10R1-PE antibodies (eBioscience). Expression was determined using a Becton-Dickinson FACSCaliber four color Cytometer. Ten thousand events characterized as microglia or astrocytes were recorded. Microglia were identified by CD11b+/CD45low expression and astrocytes were identified by GLAST-1+/CD11b− expression. For each antibody, gating was determined based on appropriate negative isotype stained controls. Flow data were analyzed using FlowJo software (Tree Star, CA).
BV2 and Primary Cell culture
BV2 microglia, neonatal primary microglia and primary astrocyte cultures were established and maintained as previously described (Fenn et al., 2012). Mixed primary glia cultures were shaken at 160rev/min and 37 C for 3.5h to harvest microglia. Remaining cells were treated with 50 mM l-leucine methyl ester (Sigma-Aldrich) for 45 min to deplete remaining microglia. After l-leucine incubation, astrocytes recovered in growth medium supplemented with 0.1 mM l-leucine for 1-3 days (Phulwani et al., 2008). BV2 and primary microglia were plated at a density of 75,000-100,000 cells per well on poly-L-lysine coated 24-well plates and left overnight to adhere. Primary astrocytes were plated at a density of 50,000 cells/well. Before treatment, cells were washed with serum-free medium. Primary microglia were activated with 10 ng/mL LPS for 1 h (stereotype 0127:B8, Sigma-Aldrich) and treated with recombinant mouse IL-10 (10 ng/mL) or human TGFβ (1 ng/mL) (R&D Systems) for an additional 3 h. BV2 microglia and primary astrocytes were activated with 100 ng/mL LPS for 1 h and treated with TGFβ (1 ng/mL) or IL-10 (10 ng/mL), respectively, for an additional 3 h. Conditioned media was collected and stored at −80°C and RNA was isolated using Tri-Reagent.
Microglia-Astrocyte transwell co-cultures
Primary microglia were plated at 50,000-75,000 cells/well in a 24-well transwell plate (Corning Life Sciences). After incubation for 3 hours, astrocytes were added to a removable 0.4 um plycarbonate membrane at an equal number as microglia. Following incubation overnight, cells were washed with serum-free media and activated with LPS (10 ngl/mL). After 1 hour, IL-10 (10ng/mL) and SB431542 (10 μM) (Sigma-Aldrich) was added for an additional 3 h. Conditioned media was collected and RNA was isolated from the microglia using Tri-Reagent.
Immunohistochemistry and digital image analysis
Primary astrocytes or microglia were grown on glass coverslips, washed with PBS and incubated with 4% formaldehyde for 10 minutes. Cells were blocked with 5% normal goat serum and 1% bovine serum albumin (BSA) then labeled using rabbit anti-mouse GFAP (Dako), Iba-1 (Wako Chemicals) or IL-10R1 (Millipore) antibodies overnight at 4° C. Next, cells were washed and incubated with a flurochrome-conjugated secondary antibody (Alexa Fluor, anti-rabbit 488 or 594) and counterstained with a DNA stain, 4’,6-diamidino-2-phenylindole (DAPI).
Mice were deeply anesthetized and transcardially perfused with PBS followed by 4% formaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h and cryoprotected in 20% sucrose for 48 h. Preserved brains were frozen using dry-ice cooled isopentane (−165°C) and sectioned (25 μm) using a Microm HM550 cryostat. Brain sections were identified by reference markers in accordance with the stereotaxic mouse brain atlas (Paxinos and Franklin, 2004). Iba-1 staining was performed as previously described (Wohleb et al., 2012). In brief, free-floating sections were blocked and then incubated with rabbit anti-mouse Iba-1 antibody (Wako Chemicals) overnight at 4°C. Sections were washed with PBS and incubated with antibody Alexa Flour 594 secondary.
Fluorescent images were visualized using an epifluorescent Leica DM5000B microscope and captured using a Leica DFC300 FX camera and imaging software. To quantify the phenotypic changes of microglia, digital image analysis (DIA) of Iba-1 staining was performed (Donnelly et al., 2009) in the hippocampus. 6-10 representative images were taken at 20× magnification in the dentate gyrus, CA1, and CA3 regions. A threshold for positive staining was determined for each image that included all cell bodies and processes, but excluded background staining (ImageJ). Results were reported as the average percent area in the positive threshold for all representative pictures.
Determination of TGFβ protein
TGFβ1 protein concentration was determined using the DuoSet ELISA Development System according to the manufacturer's instructions (R&D Systems). In brief, 96-well enzyme immunoassay plates were incubated overnight with anti-mouse TGFβ capture antibody. Samples were activated with 1N HCl for 10 minutes and neutralized with 1.2N NaOH. Plates were incubated with samples and standards (0-320 pg/ml) for 2 h. Next, biotinylated detection antibody was added for 2 h. Bound TGFβ was detected using Streptavidin-HRP development and absorbance at 450 nm was read using a Snergy HT Plate Reader (Bio-tek instruments). Wavelength correction was subtracted at 540 nm. The assay was sensitive to 5 pg/mL TGFβ and the interassay coefficient of variation was <10%.
RNA isolation and RT-PCR
RNA was isolated from cell cultures and brain tissue using the Tri-Reagent protocol (Sigma-Aldrich). For Percoll enriched microglia and astrocytes, RNA was isolated using the PrepEase kit (USB, CA). RNA was reverse transcribed to cDNA and real-time (RT)-PCR was performed using the Applied Biosystems Taqman® Gene Expression assayAssay-on-Demand Gene Expression protocol as previously described (Wohleb et al., 2012). In brief, experimental cDNA was amplified by real-time PCR where a target cDNA (e.g., IL-1β, IL-6, CCL2) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference.
Intracerebroventricular cannulation
The i.c.v. cannulation was performed as previously described (Huang et al., 2008). In brief, mice were deeply anesthetized and positioned in a sterotaxic instrument so that the plane formed by the frontal and parietal bones was parallel to the table top. An incision was made on the cranium to reveal the bregma and a 26-guage stainless-steel guide cannula was placed in the lateral cerebral ventricle using the following stereotaxic coordinates: Lat 0.5 mm; and A-P 1.2 mm to the bregma; and Hor -2 mm from the dura mater. A dummy cannula was inserted in the guide cannula to prevent occlusion and infection. Mice were provided a minimum of 7 d to recover before any treatments were administered.
Peripheral and Central Injections
Adult mice were injected intraperitoneally (i.p.) with saline or 0.33mg/kg LPS. Mice with the indwelling cannula were injected i.c.v. with 2.5 nmoles SB431542 (Sigma-Aldrich) or vehicle (50% DMSO in PBS) one hour after i.p. injection of saline or LPS. The LPS dosage was selected because it elicits a pro-inflammatory cytokine response in the brain resulting in a transient sickness response in adult mice (Berg et al., 2004; Godbout et al., 2005). Body weight, food intake, and social exploratory behavior were determined 0, 4, 8 and 24 h after injections.
Social Exploratory Behavior
Social exploratory behavior was determined as a measure of sickness behavior as previously described (Godbout et al., 2005). A novel juvenile was introduced into the test subject's home cage for a 10-min period. Behavior was videotaped and the cumulative amount of time the experimental subject engaged in social investigation of the juvenile (e.g., anogenital sniffing, trailing) was determined. Baseline social behavior was measured immediately before experimental treatment (time 0). Results are expressed as percent of time engaged in social behavior compared to baseline.
Statistical Analysis
To ensure a normal distribution, data were subjected to the Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). To determine significant main effects and interactions between main factors, data were analyzed using one-way (i.e., Pretreatment and Treatment), two-way (i.e., Pretreatment × Treatment), and three-way (i.e., Pretreatment × Treatment × Time) ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± standard error of the mean (SEM). Values were considered significant at p-values < 0.05 and a tendency at p-values ≤ 0.1.
Results
IL-10 receptor was expressed on astrocytes and microglia isolated from the brain
Based on previous work, we are interested in how IL-10 mediates anti-inflammatory responses in the brain (Henry et al., 2009; Lynch et al., 2004; Richwine et al., 2009). Therefore, the ability of IL-10 to regulate microglia and astrocytes was investigated using adult mice and a series of primary cell culture experiments. IL-10 signals through the IL-10 receptor, which has two major components 1) the ligand binding domain (IL-10R1) and 2) the signaling domain (IL-10R2). IL-10R2 is expressed constitutively by most cells. Expression of IL-10R1, however, varies by cell type and is expressed at low levels under homeostatic conditions (Moore et al., 2001). Therefore, we focused our attention on the ligand binding domain of the IL-10 receptor (IL-10R1).
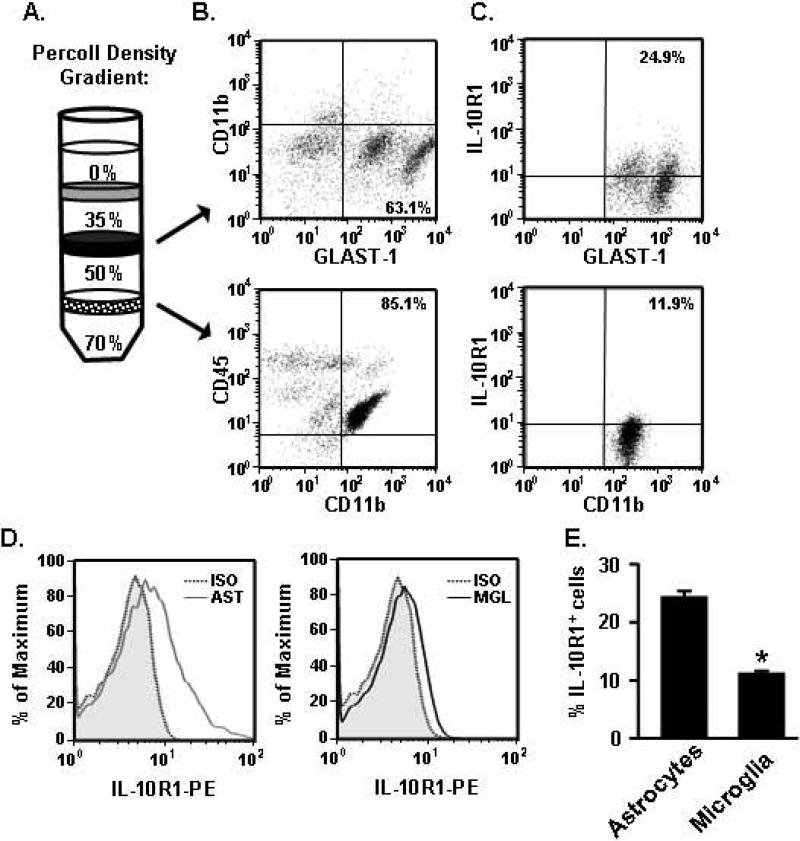
In initial studies, expression of IL-10R1 was determined on astrocytes and microglia collected from brain homogenates using a modified Percoll density gradient. The diagram in Fig.1A shows that enriched astrocytes were collected from the interphase between 35-50% Percoll and that enriched microglia were collected from the 50-70% interphase. Fig.1B shows representative dot plots of CD11b and GLAST-1 staining of astrocytes (top panel) and CD11b and CD45 staining of microglia (bottom panel). Consistent with our previous work (Henry et al., 2009), cells isolated from the 50%-70% Percoll interphase were enriched microglia (CD11b+/CD45low). In addition, the majority of cells collected from the 35%-50% Percoll interphase (over 60%) were astrocytes (CD11b−/GLAST+). Therefore, this modified Percoll gradient protocol yielded both enriched microglia and astrocytes.
Figure 1. IL-10 receptor was expressed on astrocytes and microglia isolated from the brain.
A) Microglia and astrocytes were isolated from mice brains using a Percoll density gradient. B) Representative dot plots of gated astrocytes (GLAST-1+/CD11b−) (top panel) and microglia (CD11b+/CD45low) (bottom panel). C) IL-10R1 protein expression was determined on astrocytes (top panel) and microglia (bottom panel) based on isotype controls. D) Representative histograms for MFI are shown for astrocytes (AST) and microglia (MGL) relative to isotype (ISO) control. E) Quantification of IL-10R1 positive astrocytes and microglia (n=8). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05).
Next, IL-10R1 expression was determined on astrocytes and microglia. Fig.1C shows representative dot plots of IL-10R1 staining on astrocytes and microglia. The black vertical bars (either CD11b or GLAST-1) and horizontal bars (IL-10R1) represent isotype staining. Representative histograms of mean fluorescence intensity (MFI) for IL-10R1 on astrocytes and microglia are shown in Fig.1D. Fig.1E shows the percentage of IL-10R1+ cells based on isotype labeling. These data indicate that astrocytes had increased expression of IL-10R1 compared to microglia (p<0.01). For example, 24 ± 1.0% of astrocytes were positive for IL-10R1 while only 10.8 ± 0.3% of microglia were positive for IL-10R1. Taken together, astrocytes maintained a relatively higher basal expression of IL-10R1 than microglia.
IL-10 receptor was expressed on the surface of primary astrocytes
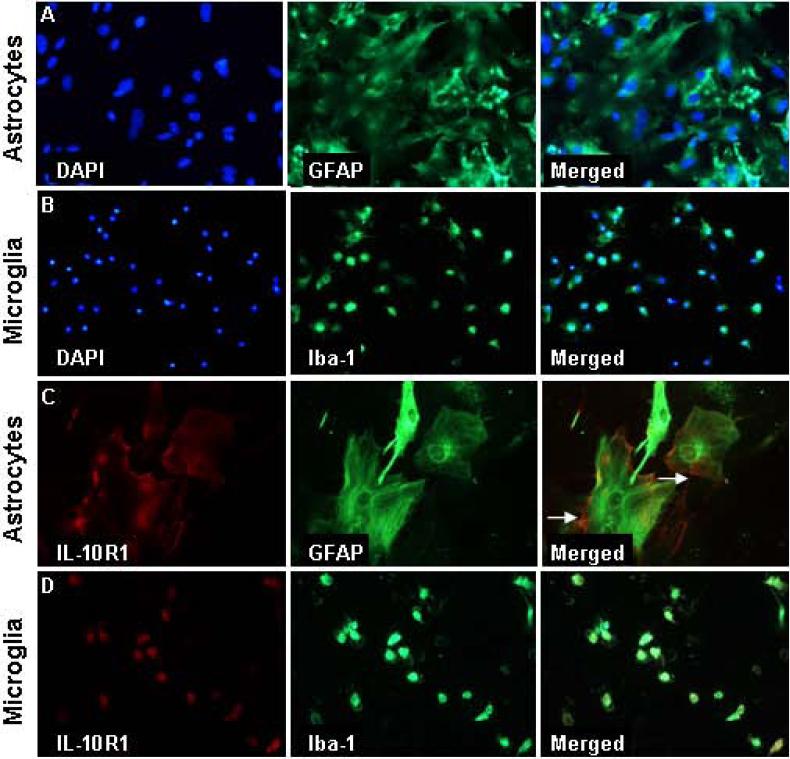
We next sought to determine IL-10R1 expression in primary cultures of microglia and astrocytes. First, the purity of the primary cultures was determined. Fig.2A shows a representative image of primary astrocytes labeled with GFAP and DAPI. Consistent with previous reports, primary astrocytes were large and formed a sheet across the bottom of the plate (Souza et al., 2013). This was a highly purified culture and every DAPI cell also co-labeled with GFAP. A similar high purity of primary microglia was detected. For example, every DAPI cell in the microglia culture also co-labeled with ionized calcium binding adapter molecule 1 (Iba-1) (Fig.2B).
Figure 2. IL-10 receptor was expressed on the surface of primary astrocytes.
Primary astrocytes and microglia were plated on cover slips. A) Representative images of DAPI, GFAP and merged DAPI/GFAP labeling of primary astrocytes. B) Representative images of DAPI, Iba-1 and merged DAPI/Iba-1 labeling of primary microglia. Representative images of IL-10R1+ staining in C) primary astrocytes or D) primary microglia. Arrows depict surface IL-10R1 labeling.
Next, the expression of IL-10R1 was determined on primary cultures of astrocytes and microglia. Fig.2C shows that IL-10R1+ labeling was prevalent on the surface of GFAP+ astrocytes. Although IL-10R1 labeling was also observed on microglia, this labeling was inconsistent with cell surface labeling (Fig.2D). Overall, both primary cell types expressed IL-10R1 when cultured, but there was an enhanced prevalence of IL-10R1 on the surface of astrocytes.
IL-10 suppressed LPS-induced IL-1β in mixed microglia-astrocyte cultures
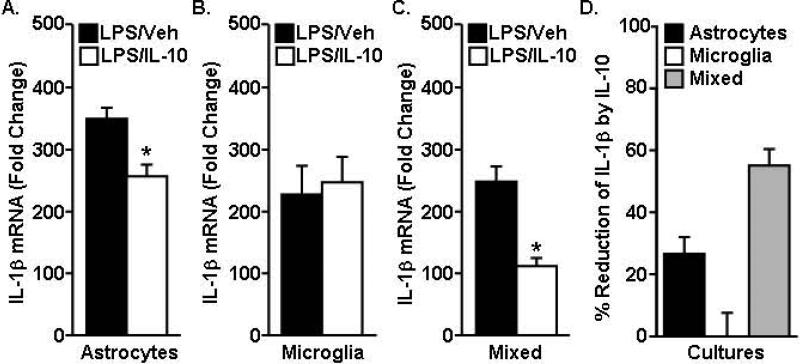
IL-10 has anti-inflammatory actions within the CNS (Lynch et al., 2004), but the cell-specific target of IL-10 regulation is unclear. Our data indicate that IL-10R1 was prevalent on the surface of astrocytes whereas surface expression of IL-10R1 on microglia was limited (Fig.1&2). Thus, astrocytes may have a higher capacity to respond to IL-10. To test this hypothesis, the ability of IL-10 to reduce IL-1β mRNA expression in primary astrocytes, microglia, or mixed microglia-astrocyte cultures was determined. In this experiment, primary cultures were activated with LPS, treated with IL-10, and IL-1β mRNA expression was determined 4 h later. IL-10 reduced the LPS-induced increase in IL-1β mRNA expression in primary astrocytes (Fig.3A, p<0.01) and in mixed microglia/astrocyte cultures (Fig.3C, p<0.01) but had no effect on the LPS-induced expression of IL-1β mRNA in primary microglia (Fig.3B). Fig.3D highlights the percent reduction of IL-1β mRNA by IL-10 treatment. For example, IL-10 reduced IL-1β mRNA expression in primary astrocytes by 26 ± 5% and mixed microglia/astrocytes by 55 ± 5%. Thus, IL-10 was most effective in reducing LPS-induced IL-1β mRNA when both microglia and astrocytes were present in the culture. Taken together, there is important interplay between microglia and astrocytes in responding to IL-10.
Figure 3. IL-10 suppressed LPS-induced IL-1β in mixed microglia-astrocyte cultures.
LPS (10ng/mL) was provided to primary cultures for 1 h followed by vehicle or IL-10 (10ng/mL) for an additional 3 h. IL-1β mRNA was determined in A) primary astrocytes, B) primary microglia or C) mixed astrocytes/microglia cultures (n=6-9). Data are expressed as fold change from saline. Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from the LPS-vehicle group. D) Percent reduction of LPS-induced IL-1β expression by IL-10.
IL-10 did not re-direct activated microglia towards a less inflammatory profile
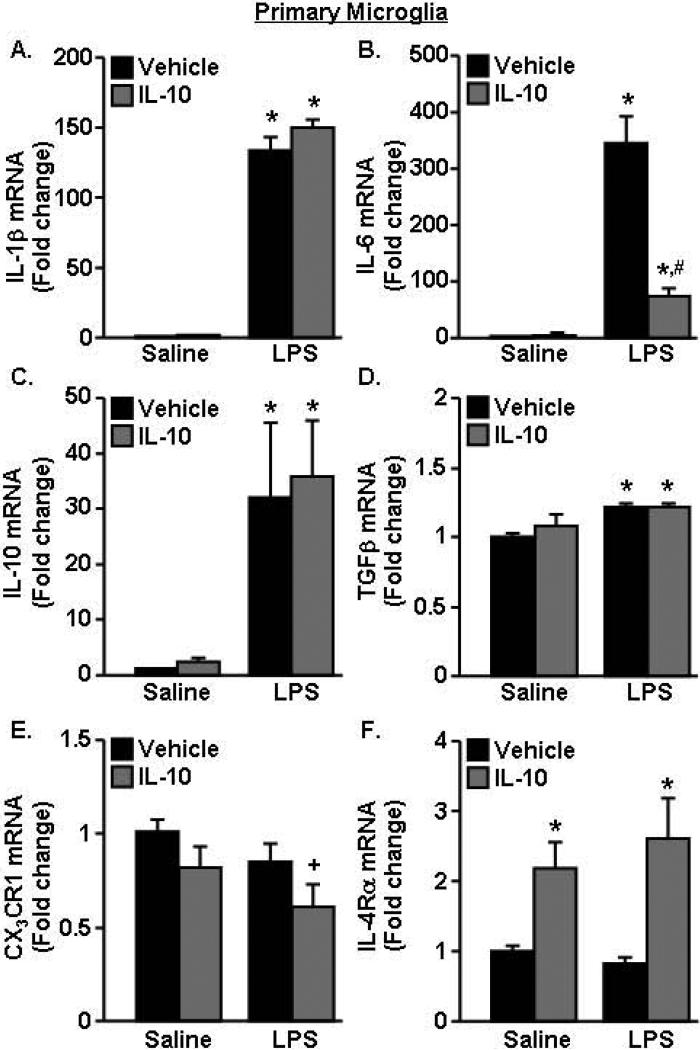
To further understand the mechanism by which IL-10 reduces the inflammatory profile of glia, separate cultures of primary microglia and astrocytes were examined. We have previously shown that IL-10 was unable to re-direct activated BV2 microglia towards a less inflammatory profile (Fenn et al., 2012). Therefore, we anticipated that IL-10 would have a limited effect on the inflammatory status of LPS activated primary microglia. In this experiment, primary microglia were activated with LPS, treated with IL-10, and expression of several inflammatory (IL-1β, IL-6), anti-inflammatory (IL-10, TGFβ) and immuno-regulatory markers (fractalkine receptor (CX3CR1) and interleukin 4 receptor-α (IL-4Rα)) were determined. As expected, LPS increased IL-1β mRNA in primary microglia, but this induction of IL-1β was unaffected by IL-10 (Fig.4A). LPS also increased mRNA expression of IL-6 mRNA (p<0.0001). In this case, the LPS-induced expression of IL-6 was reduced by IL-10 (Fig.4B, F(1,18)=23.87, p<0.002). LPS also increased expression of the anti-inflammatory markers IL-10 (Fig.4C, p<0.02) and TGFβ (Fig.4D, p<0.02), but these increases were independent of IL-10. Moreover, Fig.4E shows IL-10 reduced mRNA expression of CX3CR1 (p<0.05) and microglia treated with both LPS and IL-10 tended to have the lowest expression of CX3CR1 compared to all other groups (p=0.1). IL-10 increased IL-4Rα expression in primary microglia (p<0.002), but this increase was independent of LPS (Fig.4F). Taken together, primary microglia had some responsiveness to IL-10 with reduction of LPS-induced IL-6 and increase in IL-4Rα, but overall IL-10 was limited in suppressing LPS activated primary microglia.
Figure 4. IL-10 did not re-direct activated microglia towards a less inflammatory profile.
Primary microglia were pre-treated with saline or LPS (10ng/mL) for 1 h and vehicle or IL-10 (10ng/mL) was added for an additional 3 h. mRNA expression of A) IL-1β, B) IL-6, C) IL-10, D) TGFβ, E) CX3CR and F) IL-4Rα was determined (n=8, 2 independent experiments). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline-vehicle, means with # are significantly different (p<0.05) from LPS-vehicle, means with + tend to be different (p=0.1) from saline.
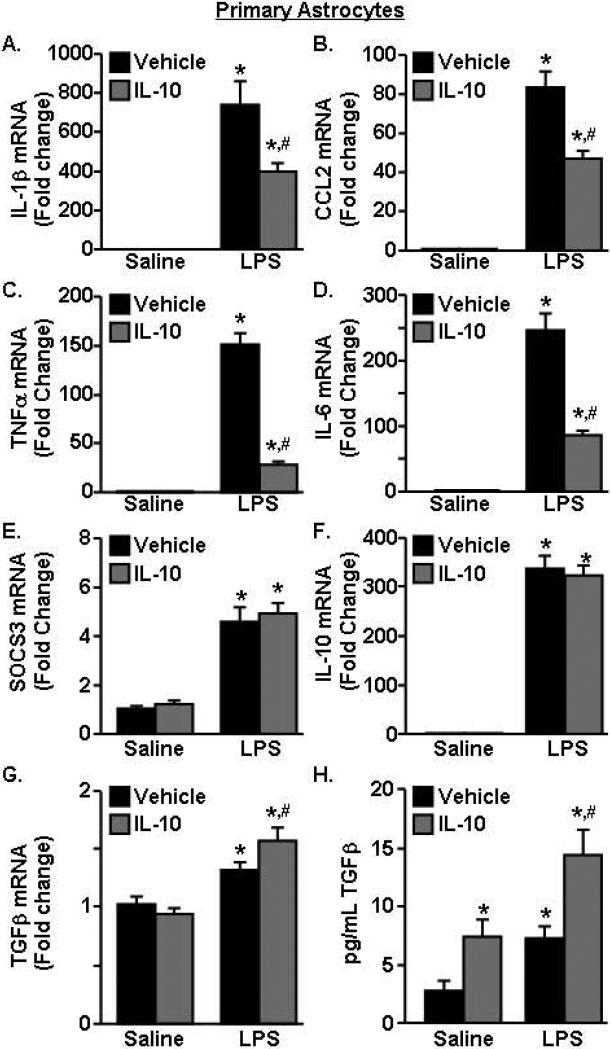
IL-10 lowered the pro-inflammatory profile of LPS-activated astrocytes
In LPS-activated microglia/astrocyte co-cultures, IL-10 significantly decreased IL-1β expression (Fig.3C&D). In microglia cultures, however, IL-10 had no effect on LPS-induced IL-1β mRNA expression (Fig.4A). Therefore, we sought to determine the extent to which IL-10 re-directed activated primary astrocytes. In this experiment, primary astrocytes were activated with LPS, treated with IL-10, and mRNA expression of several inflammatory (IL-1β, IL-6, CCL2, TNFα) and anti-inflammatory (SOCS3, IL-10, TGFβ) markers was determined. Fig.5A D shows that LPS increased mRNA expression of IL-1β, CCL2, TNFα, and IL-6 (p<0.001, for all). Moreover, IL-10 attenuated the LPS-induced mRNA expression of IL-1β, CCL2, TNFα, and IL-6 (F(1,31)=6.62, p<0.02, for each). LPS also increased expression of the anti-inflammatory genes SOCS3 and IL-10 (Fig.5E-F, p<0.0001, for each), but this was independent of IL-10 treatment. Fig.5G shows that TGFβ mRNA was increased by LPS and was significantly augmented by IL-10 (F(1,40)=5.34, p<0.03). In addition, secretion of TGFβ protein by astrocytes was increased by LPS (p<0.002) and by IL-10 (p<0.003) (Fig.5H). Furthermore, the highest level of TGFβ protein secretion was by astrocytes activated with LPS and stimulated with IL-10 (p<0.005). Overall, these data indicate that IL-10 decreased pro-inflammatory cytokine expression and increased TGFβ production in immune activated astrocytes.
Figure 5. IL-10 lowered the pro-inflammatory profile of LPS-activated astrocytes.
Primary astrocytes were pre-treated with saline or LPS (100ng/mL) for 1 h and vehicle or IL-10 (10ng/mL) was added for an additional 3 h. mRNA expression of A) IL-1β, B) CCL2, C) TNFα, D) IL-6, E) SOCS3, F), IL-10, and G) TGFβ was determined. H) TGFβ protein levels in the conditioned media were determined (n=8-10, 2 independent experiments). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline-vehicle, means with # are significantly different (p<0.05) from LPS-vehicle.
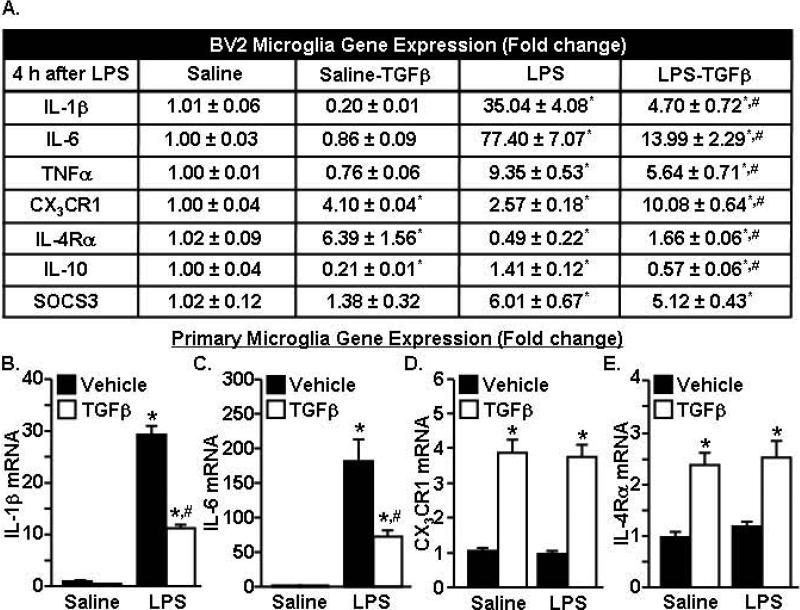
TGFβ promoted an anti-inflammatory phenotype in resting and activated microglia
TGFβ mRNA and protein was augmented by IL-10 treatment of LPS-activated astrocytes (Fig.5). To determine the degree to which TGFβ has anti-inflammatory effects on microglia, BV2 and primary microglia were activated with LPS and treated with TGFβ. A summary of the gene expression changes in BV2 microglia is shown in Fig.6A. As expected, LPS increased IL-1β, IL-6, and TNFα mRNA expression (p<0.001, for each). Moreover TGFβ attenuated IL-1β, IL-6 and TNFα mRNA expression in LPS-activated BV2 microglia (F(1,22)=12.27, p<0.003, for each). TGFβ alone increased CX3CR1 (p<0.0001) and IL-4Rα (p<0.01) expression.
Figure 6. TGFβ promoted an anti-inflammatory phenotype in resting and activated microglia.
BV2 microglia were pre-treated with saline or LPS (100ng/mL) for 1 h and vehicle or TGFβ (1ng/mL) was added for an additional 3 h. A) mRNA expression of several pro- and anti-inflammatory cytokines was determined (n=6). Primary microglia were pre-treated with saline or LPS (10ng/mL) for 1 h and vehicle or TGFβ (1ng/mL) was added for an additional 3 h. mRNA expression of B) IL-1β, C) IL-6, D) CX3CR1, and E) IL-4Rα was determined (n=8, 2 independent experiments). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline-vehicle and means with # are significantly different (p<0.05) from LPS-vehicle.
A similar experiment was completed in primary microglia. Fig.6B&C show that TGFβ attenuated the LPS-induced increase in IL-1β and IL-6 mRNA expression (F(1,32)=94.96, p<0.0001). Moreover, TGFβ alone increased CX3CR1 and IL-4Rα mRNA expression (F(1,31)=42.11, p<0.0001, for each). Taken together, TGFβ reduced pro-inflammatory cytokine expression in activated microglia and also enhanced the expression of receptors associated with microglial regulation.
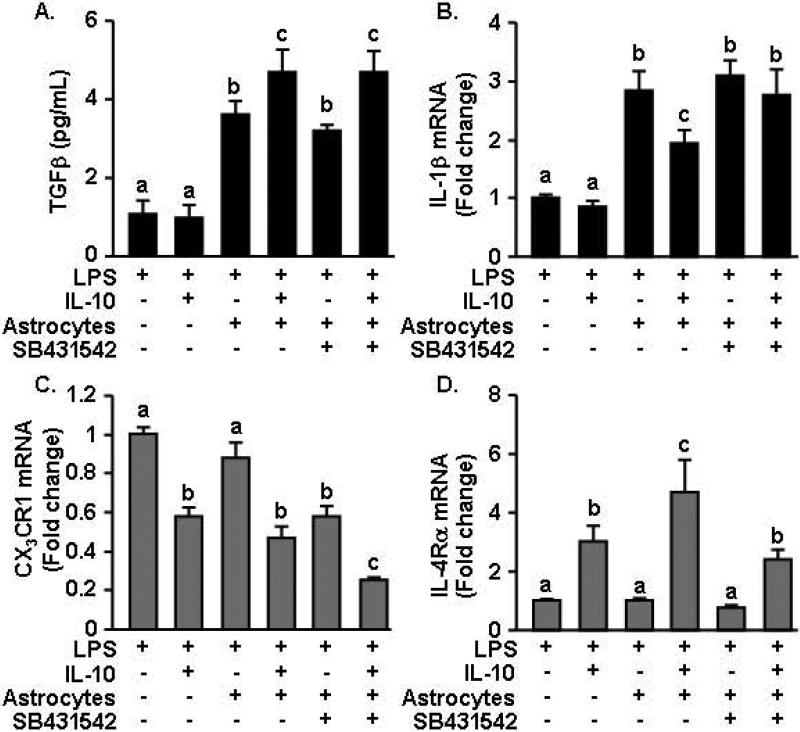
IL-10 re-directed astrocytes regulated the activation of microglia in a TGFβ dependent manner
Based on these data, we hypothesized that astrocytes are directly responsive to the anti-inflammatory effects of IL-10 and, in turn, produce TFGβ to suppress microglial activation. To address this premise, a transwell co-culture system of microglia and astrocytes was developed where microglia were plated on the bottom of the well and astrocytes were placed on a removable transwell insert. In this manner the cells shared media, but did not physically interact and the astrocytes could be readily removed. In this experiment, all wells were activated with LPS and after 1 hour, IL-10, an inhibitor of TGFβ (SB431542) (Inman et al., 2002), or respective vehicle controls were added for an additional 3 h. First, TGFβ protein levels were determined in the supernatants. Fig.7A shows that LPS-activated microglia cultured without astrocytes secreted low levels of TGFβ. When astrocytes were present on the transwell insert, TGFβ production was significantly increased (F(1,42)=33.04, p<0.0001). In addition, IL-10 tended to augment TGFβ production in LPS-activated astrocyte-microglia co-cultures (p = 0.09). This IL-10 mediated augmentation of TGFβ was absent in microglia cultures without astrocytes. The TFGβ signaling inhibitor SB431542 had no effect on the induction of TGFβ expression in the co-cultures treated with IL-10.
Figure 7. IL-10 re-directed astrocytes regulated the activation of microglia in a TGFβ dependent manner.
Primary microglia were plated at the bottom of a 24 well plate and astrocytes were added in a removable inset. Cells were pre-treated with LPS (10ng/mL) for 1 h followed by IL-10 (10ng/mL), SB431542 (10μM) or vehicle controls for an additional 3 h. A) TGFβ production in the conditioned media was determined. Microglial mRNA expression of B) IL-1β, C) CX3CR1, and D) IL-4Rα was determined (n=8-10, 2 independent experiments). Bars represent the mean ± SEM. Means with different letters (a, b, or c) are significantly different (p<0.05) from each other.
Next, microglial activation was examined by determining mRNA expression of IL-1β, CX3CR1 and IL-4Rα specifically in the cultured microglia. Because all groups were treated with LPS, the data is normalized to the levels of mRNA with LPS treatment alone. Fig.7B shows that IL-10 had no effect on LPS-induced expression of IL-1β in microglia. The addition of astrocytes in the transwells increased IL-1β expression in microglia 3-fold over LPS alone (p<0.0001). When IL-10 was added to microglia-astrocyte co-cultures, IL-1β expression was decreased (p<0.01). Importantly, the TFGβ inhibitor SB431542 restored the 3-fold induction of IL-1β expression in microglia to the same level of astrocyte-vehicle treated microglia. Thus, we interpret these data to indicate that IL-10 stimulates the release of TGFβ by astrocytes, which provides negative feedback on microglia to reduce IL-1β expression.
TGFβ secreted by IL-10 re-directed astrocytes also increased the mRNA expression of CX3CR1 and IL-4Rα in microglia. For example, CX3CR1 mRNA was decreased in microglia following stimulation with IL-10 (Fig.7C, F(1,39)=48.67, p<0.0001). Moreover, addition of the TGFβ inhibitor further reduced CX3CR1 mRNA expression (p<0.002). In a similar manner, microglia treated with IL-10 increased IL-4Rα mRNA expression (Fig.7D, F(1,40)=19.04, p<0.0001) and when co-cultured with astrocytes, further increased IL-4Rα expression (p=0.08). Addition of the TGFβ inhibitor, however, decreased IL-4Rα mRNA expression to the same level as microglia cultured without astrocytes (F(1,42)=4.71, p<0.04). Taken together, IL-10 regulates microglial activation indirectly by increasing TGFβ production by astrocytes.
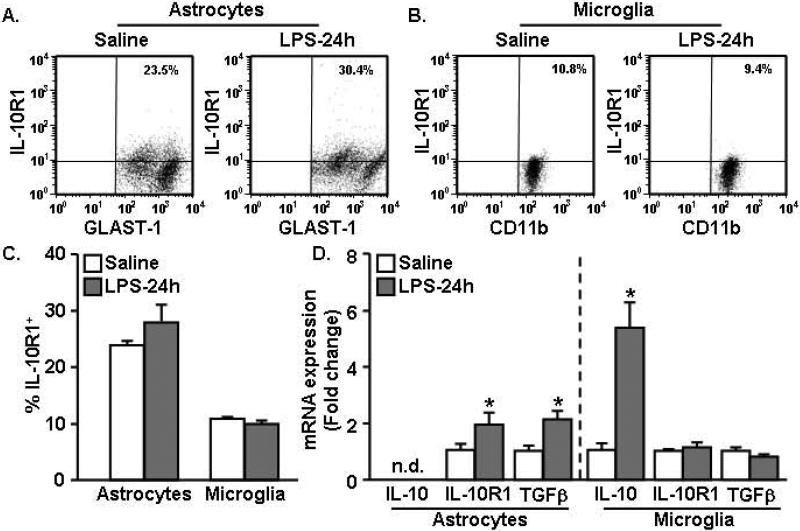
LPS injection increased IL-10R1 and TGFβ mRNA expression in enriched astrocytes
Our data indicate that TGFβ production by astrocytes following IL-10 stimulation is important for attenuating microglial activation in culture (Fig.7). Therefore, we next sought to determine if similar interactions between astrocyte and microglia existed in the brain. In these studies, the innate immune system was activated by an i.p. LPS injection and IL-10R1 surface expression was determined on astrocytes and microglia 24 h later. Fig.8A-B shows representative dot plots of IL-10R1 on astrocytes and microglia. Consistent with Fig.1, these data indicate that astrocytes had higher IL-10R1 expression compared to microglia and show that IL-10R1 expression on either cell type was not significantly altered 24 h after LPS (Fig.8C).
Figure 8. LPS injection increased IL-10R1 and TGFβ mRNA expression in enriched astrocytes.
Mice were injected i.p. with saline or LPS (0.33mg/kg) and astrocytes and microglia were isolated 24 h later. IL-10R1 expression was determined on A) astrocytes and B) microglia. C) Quantification of IL-10R1 positive (IL-10R1+) astrocytes and microglia (n=10). D) mRNA expression of IL-10, IL-10R1, and TGFβ was determined in enriched astrocytes and microglia (n=5). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline controls.
Using the same LPS injection protocol, mRNA levels of IL-10, IL-10R1 and TGFβ were determined in enriched astrocytes and microglia. While IL-10 mRNA was increased only in microglia (Fig.8D, p<0.01), IL-10R1 and TGFβ mRNA levels were increased specifically in astrocytes 24 h after LPS (Fig.8D, p<0.05, for each). mRNA for both IL-10R1 and TGFβ were detectable in microglia, but they were unaltered by LPS. These data indicate that astrocytes, but not microglia, increase mRNA expression of TGFβ following peripheral immune challenge and suggest that IL-10 signaling is important for increasing TGFβ production by astrocytes.
Inhibition of TGFβ signaling in the brain prolonged sickness behavior and exaggerated neuroinflammation after peripheral LPS challenge
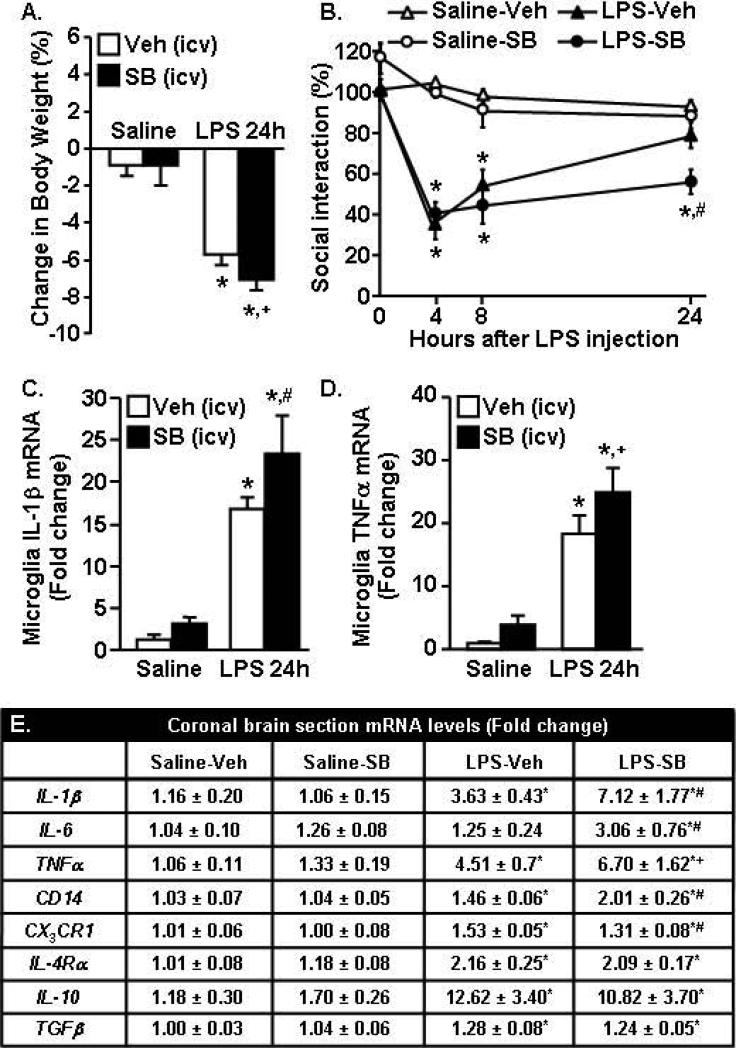
TGFβ decreased the activation profile of LPS activated primary microglia (Fig.6). In the brain, TGFβ mRNA was enhanced specifically in enriched astrocytes 24 h after LPS (Fig.8). Therefore, we next sought to determine if TGFβ regulation of microglia had a role for resolving microglial activation and reducing neuroinflammation after LPS challenge. In these studies, the innate immune system was activated by an i.p LPS injection and TGFβ signaling was blocked in the brain by a central injection (i.c.v) of the TGFβ inhibitor SB431542 (SB). Fig.9A shows that mice injected i.p. with LPS lost weight (F(1,70)=125.71, p<0.0001) over 24 h and the LPS-SB group tended to have the most weight loss compared to all other groups (p=0.10).
Figure 9. Inhibition of TGFβ signaling in the brain prolonged sickness behavior and exaggerated neuroinflammation after peripheral LPS challenge.
Mice were injected i.p. with saline or LPS (0.33mg/kg) and i.c.v. with vehicle (Veh) or 2.5 nmoles SB431542 (SB). A) Body weight was determined 24 h after LPS. B) Social exploratory behavior was determined at baseline and again at 4, 8, and 24 h after LPS. At 24 h, mice were sacrificed enriched microglia and a coronal brain section were collected. C) IL-1β mRNA and D) TNFα mRNA expression was determined in microglia (n=8). E) Expression of several inflammatory markers were determined in the coronal brain section (n=8-12). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline-vehicle, means with # are significantly different (p<0.05) from LPS-vehicle, and means with + tend to be different (p=0.1) from LPS-vehicle.
To evaluate sickness behavior, social exploratory behavior was determined at baseline and again at 4, 8, and 24 hours after LPS. As expected, LPS injection reduced social exploratory behavior (Fig.9B, F(1,130)=128, p<0.0001). Moreover, this was dependent on time (F(1,130)=28.51, p<0.0001) and TGFβ inhibition (F(1,130)=3.18, p<0.03). For example, both groups of LPS injected mice had decreased social exploratory behavior compared to controls 4 and 8 h after injection. The LPS-vehicle mice returned to baseline social exploratory behavior by 24 h. The LPS-SB mice, however, did not return to baseline behavior by 24 h and still maintained a 50% reduction in social exploratory behavior (Fig.9B, p<0.01).
After the completion of behavioral testing 24 h after LPS, microglia and a coronal brain section (through the i.c.v. injection site) were collected for RNA analysis. Fig.9C shows that LPS injection increased IL-1β mRNA expression in enriched microglia (F(1,24)=57.3, p<0.001). Moreover, TGFβ inhibition tended to increase microglial IL-1β expression (F(1,24)=3.27, p=0.08). Post hoc analysis revealed that microglia from LPS-SB mice had the highest expression of IL-1β 24 h after LPS (p<0.04). In addition, LPS injection increased TNFα expression (F(1,21)=35.22, p<0.0001) and inhibition of TGFβ tended to enhance TNFα mRNA expression (F(1,21)=2.11, p=0.10) in microglia. Post hoc analysis revealed that microglia from LPS-SB mice tended to have the highest expression of TNFα 24 h after LPS (p=0.10).
To supplement the data from enriched microglia, mRNA levels of several inflammatory markers associated with microglial activation were determined from the coronal brain section. Similar to the results from enriched microglia, LPS increased IL-1β mRNA expression in the brain (F(1,41)=15.53, p<0.0003) and TGFβ inhibition tended to further increase IL-1β expression (F(1,41)=2.73, p=0.09). For example, IL-1β mRNA expression was highest in the LPS-SB group compared to all other groups (p<0.02, Fig.9D). TGFβ inhibition also amplified IL-6 mRNA expression in the brain of LPS injected mice (F(1,41)=2.79, p=0.1) and the LPS-SB mice had the highest expression of IL-6 compared to all other groups (p<0.01). A similar pattern of expression was detected in TNFα mRNA (tendency, p=0.1) and CD14 mRNA (p<0.02). CX3CR1 mRNA was lower in the LPS-SB431542 group compared to the LPS-vehicle group (p<0.04). IL-4Rα, IL-10, and TGFβ mRNA were all increased 24 h after LPS (p<0.04 for all), but these increases were independent of TGFβ inhibition.
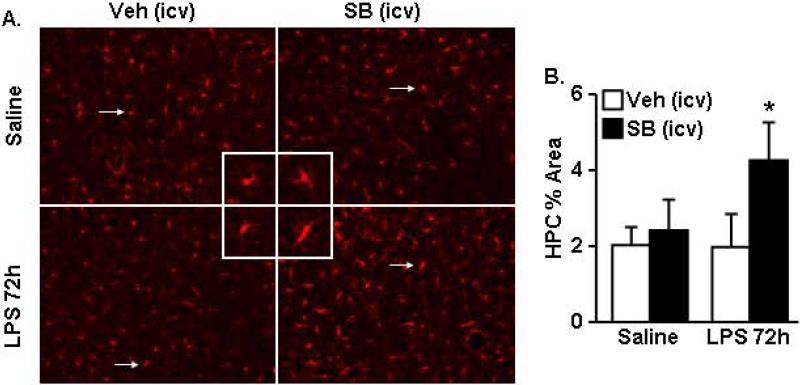
In other models of amplified microglial responses to peripheral LPS challenge (e.g., CX3CR1KO and stress) microglial Iba-1 immunoreactivity persisted 72 h after LPS injection (Corona et al., 2010; Wohleb et al., 2012). Therefore, to confirm that LPS-induced microglial activation was prolonged after TGFβ inhibition, Iba-1 immunoreactivity was determined in the hippocampus (HPC) 72 h after LPS. Microglia in the HPC of LPS-SB mice had larger cell bodies and dense processes indicating a more activated phenotype (Fig.10A). Iba-1 proportional area analysis confirmed increased Iba-1 immunoreactivity in the hippocampus of SB treated mice (F(1,17)=6.56, p<0.02). The highest Iba-1 proportional area was in the mice treated with both LPS and SB compared to all other groups (Fig.10B, p<0.04). Collectively these results indicate that interfering with TGFβ signaling in the brain caused exaggerated LPS-induced neuroinflammation and sickness behavior.
Figure 10. Inhibition of TGFβ signaling in the brain prolonged microglial activation in the hippocampus of LPS injected mice.
Mice were injected i.p. with saline or LPS (0.33mg/kg) and i.c.v. with vehicle (Veh) or 2.5 nmoles SB431542 (SB). After 72 h, brains were collected and Iba-1 immunoreactivity was determined. A) Representative Iba-1 labeling in the hippocampus. Inset includes enlarged image of Iba-1+ cell indicated by white arrow. B) Proportional area analysis of Iba-1 positive labeling (n=4). Bars represent the mean ± SEM. Means with * are significantly different (p<0.05) from saline.
Discussion
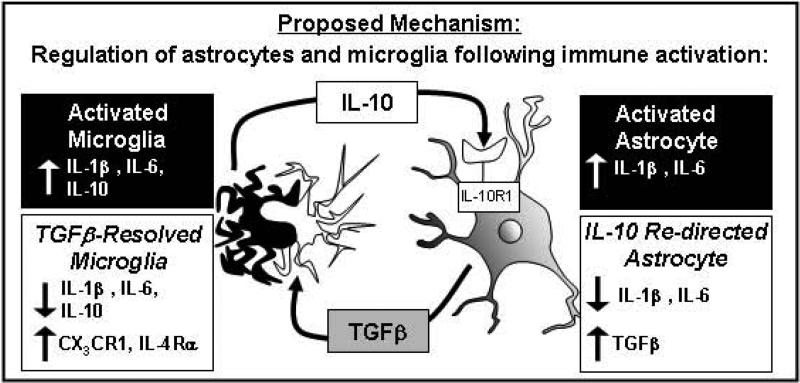
Bi-directional communication between microglia and astrocytes under inflammatory conditions is dynamic and complex. The primary objective of this study was to understand how IL-10 mediates its anti-inflammatory effects in the brain to attenuate pro-inflammatory cytokine expression. Here we report that LPS-activated astrocytes were attenuated by IL-10 to a greater extent than activated microglia. Moreover, activated astrocytes re-directed by IL-10 in culture responded with increased mRNA expression and protein production of TGFβ. Novel data are provided showing that this TGFβ production by astrocytes, in turn, modulated the activation state of microglia. For instance, only when astrocytes were present in co-cultures did IL-10 stimulation reduce expression of IL-1β and increase expression of anti-inflammatory mediators CX3CR1 and IL-4Rα in primary microglia. Moreover, these IL-10-astrocyte mediated effects on primary microglia were blocked by inhibition of TGFβ signaling. Furthermore, blockade of TGFβ signaling in the brain (i.c.v.) resulted in exaggerated LPS-induced neuroinflammation and sickness behavior. Taken together, these findings outline a mechanism by which IL-10 suppresses astrocyte activation and stimulates the production of TGFβ, which attenuates microglial activation. These new insights into cytokine mediated interactions between astrocytes and microglia are summarized in Fig.11.
Figure 11. Proposed mechanism for IL-10 mediated regulation of astrocytes and microglia after immune activation.
Immune activated microglia and astrocytes produce both pro- and anti-inflammatory cytokines. In this model of systemic LPS activation, microglia become activated and increase IL-10 expression. IL-10 has limited anti-inflammatory potential on microglia. In astrocytes, however, IL-10 decreases production of pro-inflammatory cytokines and increases TGFβ expression. TGFβ released by astrocytes signal to microglia to decrease production of IL-1β and IL-6 and increased expression of CX3CR1 and IL-4Rα.
One important aspect of this study was that IL-10R1 was more abundant on astrocytes than microglia. For example, by using a novel Percoll density gradient to separate both enriched microglia and astrocytes from the same mouse, we were able to show that astrocytes collected from the brain had higher relative surface expression of IL-10R1 compared to microglia (Fig.1). Primary astrocytes in culture also had higher levels of surface IL-10R1 expression compared to microglia (Fig.2). Examining these specific populations of microglia and astrocytes confirmed previous cell culture work showing that primary astrocytes isolated from rats had higher levels of IL-10R1 mRNA than primary microglia (Ledeboer et al., 2002). In addition, facial nerve axotomy and ischemia markedly increased IL-10R1 on astrocytes, but not microglia (Perez-de Puig et al., 2013; Xin et al., 2011). Here, LPS injection increased neuroinflammation and microglial expression of IL-10, but did not increase surface expression of IL-10R1 on either microglia or astrocytes. Nonetheless IL-10R1 mRNA was increased specifically in enriched astrocytes (Fig.8) and we surmise that IL-10R1 protein will be increased on the surface of astrocytes in a time dependent manner. It is also plausible that IL-10R1 protein was increased on astrocytes after LPS, but was internalized and degraded in the presence of IL-10 (Wei et al., 2006). Collectively these data support the premise that active microglia produce IL-10 and that astrocytes maintain a high level of IL-10R1 protein expression.
A key finding in the study was that astrocytes were identified as the cellular target of IL-10 regulation in the brain. Several reports indicate that IL-10 is an anti-inflammatory cytokine that reduces neuroinflammation (Bluthe et al., 1999; Lynch et al., 2004). The cellular target of IL-10 suppression, however, was unclear. Here, we show novel data that astrocytes respond to IL-10. For example, IL-10 attenuated LPS-induced IL-1β in mixed glia cultures and primary astrocyte cultures, but only had a limited effect on primary microglia (Figs.3&4). IL-10 reduced microglial mRNA expression of IL-6 after LPS, but had no effect on the LPS-induced increase in IL-1β or the LPS-induced reduction of CX3CR1 mRNA expression (Fig.4). These data are consistent with a previous study showing that IL-10 does not re-direct activated BV2 microglia (Fenn et al., 2012). In primary astrocyte cultures activated by LPS, however, IL-10 reduced all the pro-inflammatory cytokines determined (IL-1β, IL-6, CCL2, TNFα) and further increased expression of the regulatory cytokine TGFβ (Fig.5). In addition, analysis of enriched astrocytes/microglia after LPS challenge in vivo confirmed that TGFβ mRNA was increased specifically in enriched astrocytes and not enriched microglia (Fig.8). Thus, IL-10 robustly redirected activated astrocytes towards a less inflammatory profile and promoted expression of TGFβ.
Another relevant aspect of this study is that TGFβ produced by IL-10 re-directed astrocytes modulated microglial activation. For instance, IL-10 treatment in astrocyte-microglia co-cultures lowered IL-1β expression in microglia and this was blocked using SB4315342, a TGFβ signaling inhibitor (Inman et al., 2002). In addition, TGFβ mRNA was increased specifically in astrocytes isolated from the brain 24 h after LPS. When TGFβ was provided to LPS activated BV2 and primary microglia in culture, the LPS-induced inflammation was markedly attenuated (Fig.6). These data are consistent with a recent study showing that astrocyte conditioned media containing TGFβ inhibited nitric oxide (NO) production in microglia. When TGFβ was neutralized, however, the conditioned media from astrocytes failed to inhibit microglial NO production (Orellana et al., 2013). It is important to mention that our results were obtained from primary cultures established from neonates, which may not fully represent the functions of mature microglia and astrocytes in vivo (Butovsky et al., 2014). Therefore, these data were supplemented by examining astrocytes in vivo in the context of a peripheral LPS challenge and also the effects of TGFβ inhibition on microglial activation. Consistent with the in vitro results, inhibition of TGFβ signaling in the brain exaggerated microglial activation (IL-1β and TNFα) and corresponding neuroinflammation (i.e. higher IL-1β, IL-6, TNFα, CD14 mRNA expression) following LPS (Fig.9). Moreover, central TGFβ inhibition was associated with increased Iba-1 immunoreactivity in the hippocampus 72 h after LPS (Fig.10). These results parallel the microglia/astrocyte transwell culture data (Fig.7) and indicate that interfering with TGFβ signaling in the brain ablates the anti-inflammatory effects mediated by IL-10. Indeed, elevated IL-10 mRNA expression was maintained in the brain of mice injected with LPS independent of TGFβ inhibition. Thus, without TGFβ signaling, high IL-10 expression in the LPS-SB group was insufficient to resolve pro-inflammatory gene expression. Taken together, these data indicate that IL-10 dependent anti-inflammatory responses in the brain are mediated by astrocytes and their corresponding production of TGFβ.
Our findings indicate that an important consequence of impaired regulation of microglia by astrocytes is prolonged symptoms of sickness. For example, inhibition of TGFβ signaling in the brain contributed to more extensive weight loss, prolonged sickness behavior, and exaggerated neuroinflammation after a peripheral LPS challenge (Fig.9&10). These data are consistent with models of aging and impaired microglial regulation (i.e., CX3CR1KO) where exaggerated IL-1β expression in the brain was associated with prolonged sickness/depressive-like behaviors (Abraham and Johnson, 2009; Corona et al., 2010; Godbout et al., 2005; Wynne et al., 2010). Specifically, TGFβ increased CX3CR1 expression and reduced IL-1β expression in activated microglia. Both of these events are associated with the resolution of microglial activation and return to baseline behavior after LPS challenge (Henry et al., 2009; Wynne et al., 2010). Similar to our previous reports in aged mice, TGFβ inhibition in adult mice resulted in a more inflammatory phenotype of microglia with amplified expression of IL-1β and down-regulated expression of CX3CR1. These current data indicate that astrocytes contribute to the regulation of microglia and help restore the brain to a state of homeostasis after a transient inflammatory insult.
These new insights into the dynamic relationship between microglia and astrocytes are critically relevant to the study of neuroimmune regulation in injury, aging, and disease. For example, IL-10 signaling in astrocytes was important for neuroprotection following facial nerve axotomy and IL-10 deficient mice had decreased motor neuron survival following injury (Xin et al., 2011). Moreover, following peripheral LPS immune challenge, primed microglia from aged mice produce elevated levels of both IL-1β and IL-10 compared to adult microglia. Although IL-10 protein expression was high in the aged brain after LPS, it was insufficient to resolve microglial activation (Fenn et al., 2012; Henry et al., 2009). These results suggest that responsiveness to IL-10 may be impaired in the aged brain. A connection with these previous findings to the current study is that astrocytes in the aged brain may not be responding appropriately to IL-10 produced by microglia. For instance, mRNA expression of astrocyte inflammatory markers GFAP and vimentin (Pekny and Nilsson, 2005; Zamanian et al., 2012) were increased in the brain of aged mice compared to adults (Cotrina and Nedergaard, 2002; Godbout et al., 2005). In addition, TGFβ mRNA expression was increased in the brain of adult, but not aged mice 24 h after LPS challenge. This low TGFβ expression in the aged brain corresponded with high IL-1β expression and protracted CX3CR1 downregulation (Wynne et al., 2010). The data provided in this study support the premise that aged astrocytes are less responsive to IL-10 and fail to produce sufficient TGFβ to resolve microglia activation after a peripheral immune challenge. It is important to note that in other disease models, TGFβ may promote inflammation. In experimental autoimmune encephalomyelitis, central TGFβ signaling initiated the onset of disease (Luo et al., 2007) and aided in sustaining high levels of inflammation in the brain and spinal cord (Lanz et al., 2010). In this model however, mice are immunized against CNS which elicits an auto-immune response. Nonetheless, in the context of resolving the activation of microglia after peripheral innate immune challenge, our results indicate that TGFβ lowered microglial activation and that inhibition of TGFβ signaling in the brain amplified neuroinflammation.
In conclusion, we provide novel evidence that astrocytes interpret the anti-inflammatory signals provided by IL-10 and respond by producing TGFβ. This dynamic interaction of astrocytes provides feedback to attenuate microglial activation. These results provide a novel mechanism by which astrocytes and microglia use anti-inflammatory mediators to modulate cytokine production, and may provide new avenues to understand impaired regulation of inflammation in aging, neurological disease, and traumatic CNS injury.
Main points.
Cytokine-mediated communication between microglia and astrocytes under inflammatory conditions was investigated in this study. We provide novel data that astrocytes express IL-10 Receptor and that IL-10 re-directed astrocytes regulate the activation of microglia in a TGFβ dependent manner. We used both in vitro and in vivo studies to show that astrocytes are highly responsive to IL-10 stimulation and respond by producing TGFβ. Furthermore, TGFβ attenuates microglial activation in vitro and in vivo following systemic immune challenge with lipopolysaccharide. When TGFβ signaling was inhibited in the brain, peripheral LPS injection caused exaggerated neuroinflammation and prolonged sickness behavior.
Acknowledgements
This research was supported by NIA grant R01-AG-033028 to J.P.G. In addition, A.M.F. was supported by a Med to Grad scholarship from the Howard Hughes Medical Institute (HHMI) and an OSU Presidential Fellowship. We thank Dr. John Sheridan for the use of the FACSCaliber Cytometer and Dr. Ronald Glaser for the use of the AB PRISM 7300-sequence detection system.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, behavior, and immunity. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain, behavior, and immunity. 2004;18:149–157. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F, Sortino MA, Copani A. TGF-beta 1 protects against Abeta neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiology of disease. 2008;30:234–242. doi: 10.1016/j.nbd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. Journal of neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Hutchins B, LaFace DM, Stohlman SA, Coffman RL. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J Immunol. 2001;166:602–608. doi: 10.4049/jimmunol.166.1.602. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of neuroimmunology. 2010;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. Journal of the neurological sciences. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, Kielian T. Microglia and Astrocyte Activation by Toll-Like Receptor Ligands: Modulation by PPAR-gamma Agonists. PPAR Res. 2008;2008:453120. doi: 10.1155/2008/453120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular pharmacology. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, Fujimoto M, Takeda K, Naka T, Yamashita T. ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell death & disease. 2013;4:e710. doi: 10.1038/cddis.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. Journal of neuroscience. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Hutchison K, French R, Bluthe RM, Parnet P, Johnson RW, Dantzer R. Central interleukin-1 receptors as mediators of sickness. Ann N Y Acad Sci. 1997;823:234–246. doi: 10.1111/j.1749-6632.1997.tb48395.x. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Palmer C. Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. Journal of neuroimmunology. 2005;162:71–80. doi: 10.1016/j.jneuroim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. Journal of clinical investigation. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, Tilders FJ, Van Dam AM. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. European journal of neuroscience. 2002;16:1175–1185. doi: 10.1046/j.1460-9568.2002.02200.x. [DOI] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life sciences. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Luo J, Ho PP, Buckwalter MS, Hsu T, Lee LY, Zhang H, Kim DK, Kim SJ, Gambhir SS, Steinman L, Wyss-Coray T. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. Journal of clinical investigation. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10--a role for IL-1 beta? Journal of neurochemistry. 2004;88:635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. Journal of neuroscience. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Montero TD, von Bernhardi R. Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: Involvement of ATP released via pannexin 1 channels. Glia. 2013;61:2023–2037. doi: 10.1002/glia.22573. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd edition 2004.
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Perez-de Puig I, Miro F, Salas-Perdomo A, Bonfill-Teixidor E, Ferrer-Ferrer M, Marquez-Kisinousky L, Planas AM. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. Journal of cerebral blood flow and metabolism. 2013;33:1955–1966. doi: 10.1038/jcbfm.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181:3841–3849. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181:660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez G, Toro R, Dobeli H, von Bernhardi R. Protection of rat primary hippocampal cultures from A beta cytotoxicity by pro-inflammatory molecules is mediated by astrocytes. Neurobiology of disease. 2005;19:243–254. doi: 10.1016/j.nbd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain, behavior, and immunity. 2009;23:794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Souza DG, Bellaver B, Souza DO, Quincozes-Santos A. Characterization of adult rat astrocyte cultures. PloS one. 2013;8:e60282. doi: 10.1371/journal.pone.0060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–2158. [PubMed] [Google Scholar]
- Wei SH, Ming-Lum A, Liu Y, Wallach D, Ong CJ, Chung SW, Moore KW, Mui AL. Proteasome-mediated proteolysis of the interleukin-10 receptor is important for signal downregulation. Journal of interferon & cytokine research. 2006;26:281–290. doi: 10.1089/jir.2006.26.281. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain, behavior, and immunity. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Mesnard NA, Serpe CJ, Sanders VM, Jones KJ. IL-10 within the CNS is necessary for CD4+ T cells to mediate neuroprotection. Brain, behavior, and immunity. 2011;25:820–829. doi: 10.1016/j.bbi.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. Journal of neuroscience. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]