Abstract
Purpose of review
This review will describe the clinical significance, pathogenesis and treatment of cystic fibrosis-related bone disease (CFBD).
Recent findings
CFBD continues to increase as the life expectancy of individuals with cystic fibrosis (CF) increases. According to clinical guidelines, individuals with CF should be initially screened at the age of 18 years via dual-energy x-ray absorptiometry (DXA), if not done so previously. The underlying pathogenesis of CFBD appears to be multifactorial, but increasing data imply a direct impact by the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR deficiency and/or dysfunction impair osteoblast activity and differentiation, and indirectly promotes osteoclast formation. Unfortunately, once diagnosed with CFBD, few CF-tested medical therapies exist.
Summary
CFBD is an increasingly recognized complication that has a significant impact on the overall health of the individual. Recommendations to identify CF patients who are at risk for fracture using DXA have been established. Therapeutic agents directly studied in CF patients are limited to bisphosphonates, although other potential treatment agents exist. Finally, an improved understanding of the pathologic mechanisms will aid in the study and development of therapies.
Keywords: cystic fibrosis, bone disease, fracture, CFTR
Introduction
The life expectancy of individuals with cystic fibrosis (CF) has dramatically improved in the past 75 years (1). In 1938, the life expectancy was less than one year, and only improved to 16 years by the mid-1970’s (2). CF continues to be the leading single gene life-limiting disorder in Caucasians, affecting one in 3,500 children born in the United States (1). Currently, there are approximately 30,000 individuals with CF in the United States. Manifestations of the disorder are primarily related to the defective chloride channel functioning of the cystic fibrosis transmembrane conductance regulator (CFTR), however other associated problems are believed to be the result of the cellular responses to the mutant CFTR protein or its production. The most recognized of these problems result from the mucous blockage and obstruction of the lungs, as well as the early fibrotic destruction of the pancreas. Remarkably, through improvements in therapy, the predicted life expectancy of individuals is now 36.8 years, as reported by the Cystic Fibrosis Foundation (1).
However, this increase in the life expectancy has come with costs, as new complications have emerged. Endocrine disorders, primarily CF-related diabetes (CFRD) and CF-related bone disease (CFBD) are the leading complications associated with CF (1). The CF Patient Registry reports that 19% of all individuals with CF have CFRD, occurring in 32.6% of individuals 18 years and older. Registry data also indicates that CFBD currently affects 11% of individuals with CF, although other authors report higher numbers. This finding may represent underreporting or incomplete screening. Recent advances in the impact, pathophysiology and therapy of CFBD is the focus of this review.
What is the clinical significance of CFBD?
The association of low bone density in CF was first described in 1979. Mischler et al. found that 44% of the CF subjects had a bone mineral content (BMC) more than 2 SD below controls (3). Interestingly, they reported this finding to be more prevalent in females. Furthermore, they noted that these findings were confounded by short stature, delayed bone age and low body weight. This report not only documented the first report of CFBD, but also illustrated some of the problems and systemic influences complicating bone health, with special emphasis on lung health and nutrition.
Since that time, multiple studies have documented the incidence of bone disease in this population. One such study in late-stage adult CF patients, referred for lung transplant, reported that 57% of patients had osteoporosis (4). This further translated to a 100-fold greater risk of vertebral compression fracture. In this population, compression fractures can further impair lung function and may disqualify a patient for transplantation. However, the true impact on fracture risk is debatable, especially in children. Rovner et al. evaluated the lifetime fracture history in 186 CF children and young adults with pancreatic insufficiency and mild to moderate lung disease (5). The authors found no increased fracture risk compared to healthy controls. Nevertheless, a report by Latzin et al. exemplified the worrisome nature of the problem, in an extreme case of pathologic fracture resulting in the fatality of a 16-year-old girl (6). They describe her spontaneous sternal fracture resulting in severe respiratory distress.
Briefly stated, CFBD is an example of a multifactorial disorder, contributed by vitamin D deficiency, nutrition, hypogonadism, increased inflammatory cytokines and glucocorticoid therapies. Additionally, male gender, advanced lung disease, malnutrition, and low fat-free body mass are established additional risk factors for CFBD (7-9). Furthermore, emerging data suggest a direct genetic component to the development of low bone density. The F508del-CFTR mutation is the most common mutation resulting in CF in the United States, with almost 87% of CF individuals having at least one copy and 47% homozygous for this mutation (1). F508del-CFTR in itself, compared to other mutations, is associated with a reduced bone density in adults with CF (9). These contributors and risk factors will be further discussed in this review.
Screening Recommendations
The CF Foundation currently recommends that all individuals with CF should undergo a DXA scan assessment at the age of 18 years (1, 7, 10). Patients with normal BMD should undergo repeat DXA testing every five years. If the DXA Z-score is <−1 but >−2, the study should be repeated every 2-4 years. Lastly, if the Z-score is less than or equal to −2, treatment should be considered and DXA assessment repeated yearly. Previously, the consensus on bone health in CF also recommended DXA screening of children greater than 8 years with significant risk factors for bone disease (7).
The current recommendations utilize DXA scanning for the assessment of bone density (7). However, strategies to improve screening compliance, cost and exposure to radiation are a subject of current study. De Schepper et al. recently compared the use of quantitative ultrasound bone sonometry (QUS) in 64 adolescents (>12 years) and young adults (<40 years) with CF, to the traditional DXA scan as well as peripheral quantitative computed tomography (pQCT) of the radius (11). They reported that QUS had a sensitivity of zero % and specificity of 96% for diagnosing osteopenia, based on whole body bone mineral content (BMC) Z-score <-2. Furthermore, they stated a positive and negative predictive value of zero and 90% respectively. The authors did conclude that the QUS may be utilized to identify patients with normal bone mass. Additional studies are needed to refine screening strategies, as well as to assess those individuals at greatest risk for fracture. These findings will aid in the identification of the group of patients that would most benefit from treatment.
Pathogenesis
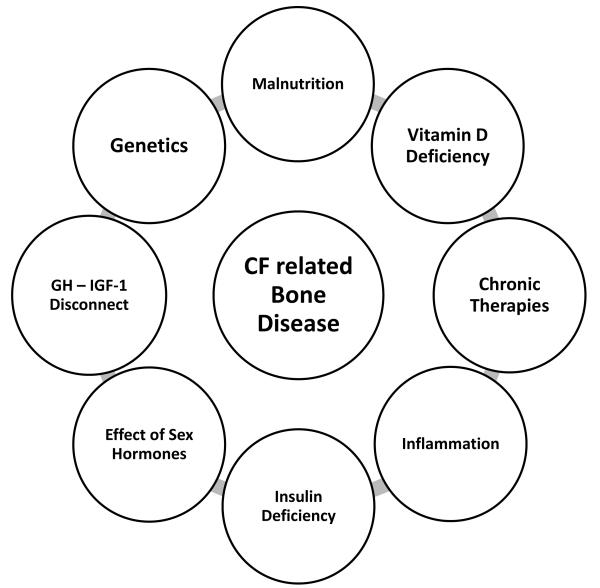
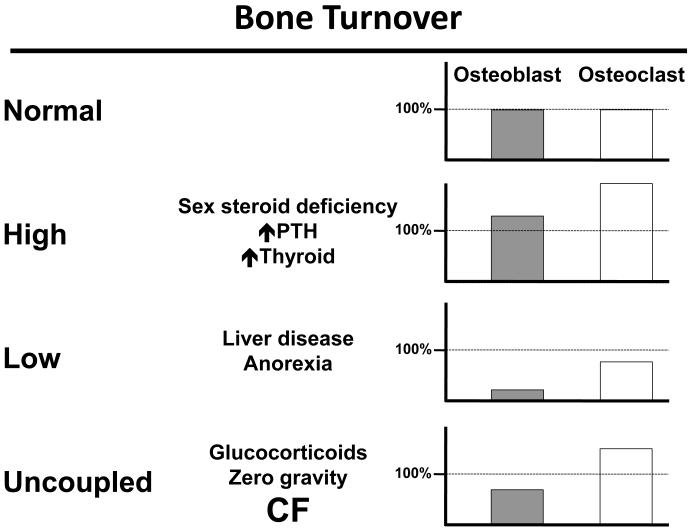
As previously mentioned, CFBD is a classic example of a multifactorial disorder. While some of these factors have established associations with CF, the remaining contributors are based on data from other disease states (Figure 1). The summation of these influences result in a bone metabolic state of uncoupled turnover. In other words, decreased osteoblast bone formation coupled with increased osteoclastic bone resorption is characteristic of CFBD (Figure 2).
Fig. 1.
Cystic Fibrosis-Related Bone Disease (CFBD) origniates from multiple systemic and direct factors associated with CF.
Fig. 2.
The dynamic status of bone turnover is uncoupled in CF. This results in a decreased bone formation, with an increased bone resorption state.
Probably the most recognized, vitamin D deficiency with resultant reduced calcium gut absorption continue to be complicating factors in CF bone health. Given the difficulties of absorption of vitamin D in CF patients, low concentrations of 25-hydroxy vitamin D is frequent. Somewhat refuting this concern, a study by Haworth et al. examined vertebral biopsy histomorphometric parameters of CF individuals (12). They found severe osteopenia, but not changes consistent with vitamin D-dependent osteomalcia. Interestingly, histomorphometric examination revealed decreased osteoblast with increased osteoclast activity, a characteristic of CFBD.
Deficiencies in vitamin D and calcium increase bone turnover through the stimulation of parathyroid hormone (PTH) production. Increased levels of PTH have been reported as a response to reduced total body calcium (13, 14). The increase in PTH stimulates osteoclastogenesis and subsequent bone reabsorption, to restore normal circulating calcium concentration.
Corticosteroid therapy is another probable culprit in CFBD, especially in the post-transplant individuals. With corticosteroids, there is a reduction in calcium absorption from the gut, and an increase in urinary calcium excretion. Additionally, corticosteroids increase osteoclastogenesis through the production of receptor activator of nuclear factor-kappa B ligand (RANKL) (15).
CFBD development may be largely related to the role of the chronic inflammatory state. In CF, there is evidence of upregulation of the nuclear factor-kappa B (NF-κB) pathway (16) that correlates with bone mineral content, and IL-6 and C-reactive protein concentrations (9, 17, 18). Furthermore, Shead et al. reported increased osteoclast precursors during CF pulmonary exacerbations (19).
New insights into CFRD suggest there is a chronic insulin deficient state (20). As an influence on bone metabolism, insulin stimulates osteoblast proliferation and function, as well as endochondral bone growth. Diabetes, whether through an insulin deficient state or hyperglycemia, is well recognized as a risk factor for the development of osteoporosis in non-CF individuals (21-23). In a recent study by Rana et al., the authors compared DXA scan results and diabetes status (based on oral glucose tolerance testing) in 81 CF subjects ≤ 18 years of age (24). They describe an association of dysglycemia and reduced bone density in the CF population.
Other pro-anabolic factors such as insulin-like growth factor I (IGF-I) and sex hormones must not be overlooked. Decreased IGF-I concentrations are described in individuals with CF, despite normal stimulated growth hormone responses (25). This finding is also noted in animal models of CF (26). Furthermore, IGF-I correlates with bone density in CF patients (27). Regarding the sex hormones, hypogonadism is well associated with decreased bone density. Although this contribution to CFBD has not been formally entrenched, both men and women with CF have a significant risk of hypogonadism (28, 29). This finding has however been associated with an increase in vertebral fractures in CF (30).
Data surrounding the genetic contribution of CF in the development of CFBD is mounting. Animal studies of CFTR deficient mice imply a direct effect on bone metabolism. Multiple reports involving the CF mouse, both theCftr knockout (Cftr−/−) and the F508del-CFTR models, demonstrate reduced bone density (31-34). The use of mouse models eliminates other clinical variables, namely lung disease and chronic malnutrition that are absent in these models (33, 35, 36). Reinforcing these findings, a validation to the idea of a genetic contribution came in a 2007 paper by Shead et al., in which the authors report detection of CFTR on human osteoblasts and osteoclasts (37).
To detail this genetic component of CFBD, we turn to data by our own laboratory. We recently reported the expression of Cftr mRNA, as well as immunohistochemistry staining of CFTR, in murine osteoblasts (38). However, we neither detected expression of Cftr mRNA nor immunohistochemical staining in the murine osteoclast. This conflicts with the above report by Shead et al., and may reflect differences in antibody specificity and/or expression differences in human versus murine osteoclasts. From these findings, we suspected an intracellular signaling component that drives the increased bone resorption that is characteristic of CFBD.
Building off the above data, we demonstrated reduced bone formation and fewer osteoblasts in Cftr−/− murine calvarial organ cultures compared to wildtype controls (38). Osteoblast primary cell cultures also exhibited less alkaline phosphatase staining in Cftr−/− cultures indicating a differentiation defect. Osteoclasts were then grown from Cftr−/− bone marrow cultures, and were found to be in greater numbers than wildtype cultures, despite the lack of osteoclast CFTR expression. A mechanistic study of this discovery revealed a decrease in osteoprotegerin (OPG) expression in Cftr−/− osteoblasts, but no differences in expression of the receptor activator of NF-κB ligand (Rankl). This results in an increase in the Rankl to OPG ratio that drives osteoclastogenesis. From these results, we concluded that CFTR deficiency impairs osteoblast differentiation and bone production, as well as indirectly increasing osteoclastic bone resorption.
Treatment
Presently, treatment for CFBD is targeted to either prevention or management of ongoing bone disease. Primarily, preventative treatment focuses on vitamin D and calcium supplementation, while maintaining nutrition and lung health (1, 7, 10). Additionally, management of CFRD and hypogonadism further enhance bone health.
Multiple clinical trials evaluating vitamin D supplementation and calcium replacement are found in the literature. Recently a Cochrane review authored by Ferguson and Chang evaluated three clinical trials consisting of vitamin D supplementation, vitamin D and calcium, and calcitriol (1,25-dihydroxy vitamin D3) (39). They found no evidence of benefit or harm in these trials. The authors concluded that current CF guidelines should be continued until new evidence surfaces. Tangpricha et al. recently published an update on guidelines for the screening and treatment of vitamin D deficiency in CF (40). They detailed the CF Foundation nutritional guidelines, suggesting the following for vitamin D supplementation: 400-500 IU/day for children 12 months and younger, 800-1000 IU/day for 1-10 years of age, and 800-2000 IU/day for 11 years and older. Additionally, the guidelines committee recommended a serum 25-hydroxyvitamin D concentration at least 30 ng/ml.
The consensus guidelines on bone health in CF suggest that adults with a Z-score of less than −2 by DXA should be offered bisphosphonate therapy (7, 10). A Cochrane Review by Conwell and Chang evaluated seven independent trials of bisphosphonate therapy in CF individuals, of at least six months duration (41). The combined number of participants was 237 adults with CF. The authors concluded that both oral and intravenous bisphosphonates are effective at increasing bone density in CF. They were unable to detect an improvement in fracture rate, and recommended larger scale trials to evaluate the efficacy of these medication. They did note that bone pain and flu-like symptoms were common with the intravenous agents.
Additional promising therapies exist, although they have yet to be studied in CF. Teriparatide stimulates osteoblast activity (42), which is diminished in CF. This could alleviate the decreased anabolic stimulation that is noted. Uncertainties persist over the use of sex steroids in CF individuals. However, other anabolic therapies may still yet play a role in improving bone health in CF. Growth hormone has previously been shown to improve bone accrual in CF (43). This could be especially effective in CF children with growth restriction. IGF-I therapy has not been tested in CF patients to improve bone health, but may this therapy may hold potential. Lastly, denosumab is a promising new drug approved for the treatment of osteoporosis but has yet to be studied in CFBD. Denosumab is a monoclonal antibody that targets RANKL and therefore acts as an antiresorptive agent for bone disease. Future clinical trials and testing in CF animal models will be needed to test the efficacy of this biologic medication in CFBD.
Future Projections
The life expectancy of CF patients is expected to increase. This is especially relevant given the new classes of drugs in development for the CFTR defect itself. One such agent is ivacaftor, which is a CFTR potentiator that targets the G551D CFTR mutation. Although it is useful in a limited number of CF individuals, it paves the way for other future drugs that target CFTR mutations directly. Since the impact of CFTR itself on bone metabolism is only beginning to be understood, these new medications may influence bone health directly.
Conclusion
CFBD is increasing as the age of the individuals with CF continues to increase. Improved screening methods for adults and children will aid in directing those in need of intervention. In CF, the dynamic state of bone formation and bone resorption is mismatched. A better understanding of the pathogenesis, and the contribution of CFTR, will further improve targets for therapy. Finally, available therapies that currently exist should be studied for this form of bone disease. We will need to not only look at strategies to correct the problem, but also develop improved methodologies to prevent occurrence.
Key Points.
Cystic fibrosis-related bone disease (CFBD) is increasing as the life expectancy of CF continues to increase.
CFBD is a multifactorial disorder, stemming from both direct and indirect contributors from the underlying CF defect.
All individuals with CF should be screened for CFBD, as well as screened for known contributors to the disease (such as vitamin D deficiency).
Acknowledgements
The authors received support by a Career Enhancement Award from the American Society for Bone and Mineral Research (GAC), NIH K08DK094784 (MSS), NIH R21AR056826 (GAC).
Footnotes
There are no conflicts of interest to disclose regarding this review.
Referecnes
- 1.Cystic Fibrosis Foundation Patient Registry, 2011 . Anual Data Report. Bethesda, Maryland: 2012. ** A detailed account of the CF Foundation Patient Registry, with data on incidence of comorbidities in CF.
- 2.Orenstein DM, Winnie GB, Altman H. Cystic fibrosis: a 2002 update. J Pediatr. 2002 Feb;140(2):156–64. doi: 10.1067/mpd.2002.120269. PubMed PMID: 11865265. [DOI] [PubMed] [Google Scholar]
- 3.Mischler EH, Chesney PJ, Chesney RW, Mazess RB. Demineralization in cystic fibrosis detected by direct photon absorptiometry. Am J Dis Child. 1979 Jun;133(6):632–5. doi: 10.1001/archpedi.1979.02130060072016. PubMed PMID: 443220. Epub 1979/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 4.Aris RM, Renner JB, Winders AD, Buell HE, Riggs DB, Lester GE, et al. Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med. 1998 Feb 1;128(3):186–93. doi: 10.7326/0003-4819-128-3-199802010-00004. PubMed PMID: 9454526. [DOI] [PubMed] [Google Scholar]
- 5.Rovner AJ, Zemel BS, Leonard MB, Schall JI, Stallings VA. Mild to moderate cystic fibrosis is not associated with increased fracture risk in children and adolescents. J Pediatr. 2005 Sep;147(3):327–31. doi: 10.1016/j.jpeds.2005.04.015. PubMed PMID: 16182670. [DOI] [PubMed] [Google Scholar]
- 6.Latzin P, Griese M, Hermanns V, Kammer B. Sternal fracture with fatal outcome in cystic fibrosis. Thorax. 2005 Jul;60(7):616. doi: 10.1136/thx.2005.041236. PubMed PMID: 15994277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005 Mar;90(3):1888–96. doi: 10.1210/jc.2004-1629. PubMed PMID: 15613415. [DOI] [PubMed] [Google Scholar]
- 8.Stalvey MS, Flotte TR. Endocrine parameters of cystic fibrosis: back to basics. J Cell Biochem. 2009 Oct 1;108(2):353–61. doi: 10.1002/jcb.22284. PubMed PMID: 19670266. [DOI] [PubMed] [Google Scholar]
- 9.King SJ, Topliss DJ, Kotsimbos T, Nyulasi IB, Bailey M, Ebeling PR, et al. Reduced bone density in cystic fibrosis: DeltaF508 mutation is an independent risk factor. Eur Respir J. 2005 Jan;25(1):54–61. doi: 10.1183/09031936.04.00050204. PubMed PMID: 15640323. Epub 2005/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 10.Boyle MP. Update on maintaining bone health in cystic fibrosis. Curr Opin Pulm Med. 2006 Nov;12(6):453–8. doi: 10.1097/01.mcp.0000245708.59138.a4. PubMed PMID: 17053497. [DOI] [PubMed] [Google Scholar]
- 11.De Schepper J, Roggen I, Van Biervliet S, Robberecht E, Gies I, De Waele K, et al. Comparative bone status assessment by dual energy X-ray absorptiometry, peripheral quantitative computed tomography and quantitative ultrasound in adolescents and young adults with cystic fibrosis. J Cyst Fibros. 2012 Mar;11(2):119–24. doi: 10.1016/j.jcf.2011.10.004. PubMed PMID: 22119452. * An evaluation of quantitative ultrasound for the screening and diagnosis of CFBD.
- 12.Haworth CS, Webb AK, Egan JJ, Selby PL, Hasleton PS, Bishop PW, et al. Bone histomorphometry in adult patients with cystic fibrosis. Chest. 2000 Aug;118(2):434–9. doi: 10.1378/chest.118.2.434. PubMed PMID: 10936137. [DOI] [PubMed] [Google Scholar]
- 13.Aris RM, Ontjes DA, Buell HE, Blackwood AD, Lark RK, Caminiti M, et al. Abnormal bone turnover in cystic fibrosis adults. Osteoporos Int. 2002;13(2):151–7. doi: 10.1007/s001980200007. PubMed PMID: 11905525. Epub 2002/03/22. eng. [DOI] [PubMed] [Google Scholar]
- 14.Aris RM, Lester GE, Dingman S, Ontjes DA. Altered calcium homeostasis in adults with cystic fibrosis. Osteoporos Int. 1999;10(2):102–8. doi: 10.1007/s001980050202. PubMed PMID: 10501788. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999 Oct;140(10):4382–9. doi: 10.1210/endo.140.10.7034. PubMed PMID: 10499489. [DOI] [PubMed] [Google Scholar]
- 16.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest. 1998 Jun 1;101(11):2598–605. doi: 10.1172/JCI2865. PubMed PMID: 9616231. Pubmed Central PMCID: 508849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ionescu AA, Nixon LS, Evans WD, Stone MD, Lewis-Jenkins V, Chatham K, et al. Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med. 2000 Sep;162(3 Pt 1):789–94. doi: 10.1164/ajrccm.162.3.9910118. PubMed PMID: 10988084. [DOI] [PubMed] [Google Scholar]
- 18.Haworth CS, Selby PL, Webb AK, Martin L, Elborn JS, Sharples LD, et al. Inflammatory related changes in bone mineral content in adults with cystic fibrosis. Thorax. 2004 Jul;59(7):613–7. doi: 10.1136/thx.2003.012047. PubMed PMID: 15223873. Pubmed Central PMCID: 1747064. Epub 2004/06/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shead EF, Haworth CS, Gunn E, Bilton D, Scott MA, Compston JE. Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006 Aug 1;174(3):306–11. doi: 10.1164/rccm.200512-1943OC. PubMed PMID: 16675777. [DOI] [PubMed] [Google Scholar]
- 20.Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care. 2010 Dec;33(12):2677–83. doi: 10.2337/dc10-1279. PubMed PMID: 21115770. Pubmed Central PMCID: 2992212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007 Jan;148(1):198–205. doi: 10.1210/en.2006-1006. PubMed PMID: 17053023. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton EJ, Rakic V, Davis WA, Chubb SA, Kamber N, Prince RL, et al. Prevalence and predictors of osteopenia and osteoporosis in adults with Type 1 diabetes. Diabet Med. 2009 Jan;26(1):45–52. doi: 10.1111/j.1464-5491.2008.02608.x. PubMed PMID: 19125760. Epub 2009/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 23.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009 Jan;84(1):45–55. doi: 10.1007/s00223-008-9195-5. PubMed PMID: 19067021. Epub 2008/12/11. eng. [DOI] [PubMed] [Google Scholar]
- 24.Rana M, Munns CF, Selvadurai H, Briody J, Craig ME. The Impact Of Dysglycaemia On Bone Mineral Accrual In Young People With Cystic Fibrosis. Clin Endocrinol (Oxf) 2012 Jul 3; doi: 10.1111/j.1365-2265.2012.04484.x. PubMed PMID: 22757766. Epub 2012/07/05. Eng. * A retrospective comparison of DXA results and dysglycemia in young CF individuals.
- 25.Laursen EM, Lanng S, Rasmussen MH, Koch C, Skakkebaek NE, Muller J. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur J Endocrinol. 1999 Apr;140(4):315–21. doi: 10.1530/eje.0.1400315. PubMed PMID: 10097250. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006 Feb;59(2):191–5. doi: 10.1203/01.pdr.0000196720.25938.be. PubMed PMID: 16439577. [DOI] [PubMed] [Google Scholar]
- 27.Gordon CM, Binello E, LeBoff MS, Wohl ME, Rosen CJ, Colin AA. Relationship between insulin-like growth factor I, dehydroepiandrosterone sulfate and proresorptive cytokines and bone density in cystic fibrosis. Osteoporos Int. 2006;17(5):783–90. doi: 10.1007/s00198-005-0058-x. PubMed PMID: 16541207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stead RJ, Hodson ME, Batten JC, Adams J, Jacobs HS. Amenorrhoea in cystic fibrosis. Clin Endocrinol (Oxf) 1987 Feb;26(2):187–95. doi: 10.1111/j.1365-2265.1987.tb00776.x. PubMed PMID: 3665116. [DOI] [PubMed] [Google Scholar]
- 29.Leifke E, Friemert M, Heilmann M, Puvogel N, Smaczny C, von zur Muhlen A, et al. Sex steroids and body composition in men with cystic fibrosis. Eur J Endocrinol. 2003 May;148(5):551–7. doi: 10.1530/eje.0.1480551. PubMed PMID: 12720539. [DOI] [PubMed] [Google Scholar]
- 30.Rossini M, Del Marco A, Dal Santo F, Gatti D, Braggion C, James G, et al. Prevalence and correlates of vertebral fractures in adults with cystic fibrosis. Bone. 2004 Sep;35(3):771–6. doi: 10.1016/j.bone.2004.05.009. PubMed PMID: 15336615. [DOI] [PubMed] [Google Scholar]
- 31.Dif F, Marty C, Baudoin C, de Vernejoul MC, Levi G. Severe osteopenia in CFTR-null mice. Bone. 2004 Sep;35(3):595–603. doi: 10.1016/j.bone.2004.05.021. PubMed PMID: 15336594. [DOI] [PubMed] [Google Scholar]
- 32.Haston CK, Li W, Li A, Lafleur M, Henderson JE. Persistent osteopenia in adult cystic fibrosis transmembrane conductance regulator-deficient mice. Am J Respir Crit Care Med. 2008 Feb 1;177(3):309–15. doi: 10.1164/rccm.200705-659OC. PubMed PMID: 18006890. [DOI] [PubMed] [Google Scholar]
- 33.Pashuck TD, Franz SE, Altman MK, Wasserfall CH, Atkinson MA, Wronski TJ, et al. Murine Model for Cystic Fibrosis Bone Disease Demonstrates Osteopenia and Sex-Related Differences in Bone Formation. Pediatr Res. 2008 Nov 26; doi: 10.1203/PDR.0b013e3181961e80. PubMed PMID: 19047917. Epub 2008/12/03. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Henaff C, Gimenez A, Hay E, Marty C, Marie P, Jacquot J. The F508del mutation in cystic fibrosis transmembrane conductance regulator gene impacts bone formation. Am J Pathol. 2012 May;180(5):2068–75. doi: 10.1016/j.ajpath.2012.01.039. PubMed PMID: 22449949. [DOI] [PubMed] [Google Scholar]
- 35.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992 Aug 21;257(5073):1083–8. doi: 10.1126/science.257.5073.1083. PubMed PMID: 1380723. Epub 1992/08/21. eng. [DOI] [PubMed] [Google Scholar]
- 36.Snouwaert JN, Brigman KK, Latour AM, Iraj E, Schwab U, Gilmour MI, et al. A murine model of cystic fibrosis. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 2):S59–64. doi: 10.1164/ajrccm/151.3_Pt_2.S59. PubMed PMID: 7533607. Epub 1995/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 37.Shead EF, Haworth CS, Condliffe AM, McKeon DJ, Scott MA, Compston JE. Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone. Thorax. 2007 Jul;62(7):650–1. doi: 10.1136/thx.2006.075887. PubMed PMID: 17600296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalvey M, Clines K, Havasi V, et al. Osteoblast CFTR Inactivation Reduces & Differentiation and Osteoprotegerin Expression in a Mouse Model of Cystic Fibrosis-Related Bone Disease. PLOS ONE. 2013 Nov 13;8(11):e80098. doi: 10.1371/journal.pone.0080098. * An in-vitro analysis of the direct and indirect actions of CFTER on osteoblasts and osteoclasts.
- 39.Ferguson JH, Chang AB. Vitamin D supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2012;4:CD007298. doi: 10.1002/14651858.CD007298.pub3. PubMed PMID: 22513949. * A comparison of multiple clinical trials of vitamin D supplimentation in CF.
- 40.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, et al. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012 Apr;97(4):1082–93. doi: 10.1210/jc.2011-3050. PubMed PMID: 22399505. * A CF guidelines committee review of the evaluation and treatment of vitamin D in individuals with CF.
- 41.Conwell LS, Chang AB. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2012;4:CD002010. doi: 10.1002/14651858.CD002010.pub3. PubMed PMID: 22513903. Epub 2012/04/20. eng. * A comparison of multiple clinical trials of bisphosphonate therapy in CF.
- 42.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003 Sep 25;349(13):1207–15. doi: 10.1056/NEJMoa031975. PubMed PMID: 14500804. [DOI] [PubMed] [Google Scholar]
- 43.Hardin DS, Ahn C, Prestidge C, Seilheimer DK, Ellis KJ. Growth hormone improves bone mineral content in children with cystic fibrosis. J Pediatr Endocrinol Metab. 2005 Jun;18(6):589–95. doi: 10.1515/jpem.2005.18.6.589. PubMed PMID: 16042327. [DOI] [PubMed] [Google Scholar]