Abstract
In the classic picture of morphogen-mediated patterning, cells acquire the correct spatial arrangement of specified fates by reading a precisely distributed gradient of morphogen. Xiong et al. now provide evidence for an alternate strategy—cells of the zebrafish neural tube actively sort to their correct positions following disordered specification by Sonic hedgehog.
A central paradigm of modern developmental biology is the use in many organisms of graded distributions of extracellular signaling ligands, or morphogens, to confer differential gene expression programs upon fields of initially uniform and unspecified embryonic cells (Rogers and Schier, 2011). This is captured in the classic “French flag model” of positional information—morphogens determine the arrangement and numbers of specified cells by inducing differential gene expression programs as a function of gradient activity (Wolpert, 1969). In the strictest version of the model, the formation and interpretation of gradient activity occurs within a field of static cells whose positions and numbers remain unaltered. However, in the majority of contexts, cell divisions and tissue-level rearrangements accompany morphogen-mediated fate specification, which could prohibit embryos from utilizing extracellularly diffusing ligand gradients to reliably introduce patterning (Lander, 2011). It has been challenging to assess the relationship between the two jointly occurring processes of gradient-mediated patterning and tissue morphogenesis.
New work by Megason and colleagues in this issue of Cell (Xiong et al., 2013) demonstrates an innovative approach and unique insight into the patterning of the zebrafish neural tube. In the fish embryo, tube formation begins as cells comprising the sheet of neural ectoderm converge toward the midline and undergo invagination, division, and interdigitation, forming the neural keel (Clarke, 2009). During morphogenesis, the underlying midline mesoderm secretes the signaling protein Sonic hedgehog (Shh). Shh activ ity is necessary for the appearance of ventrally positioned neural precursor fates within the neural tube. In current models of Shh-mediated patterning, the concentration and duration of Shh exposure dictate the precise position, number, and arrangement of cells expressing particular differential gene programs (Balaskas et al., 2012), but it is unclear whether such a model is compatible with the observed dramatic cellular rearrangements.
Xiong et al. (2013) utilize time-lapse confocal microscopy of intact, living zebrafish embryos to simultaneously record position, lineage relationships, and Shh-dependent green fluorescent protein (GFP) reporter activity during the full time course of neural tube development. Astonishingly, they find that specification of precursors does not occur in a highly ordered manner as expected from the gene expression patterns of cells in the neural tube. Instead, the progenitors of different lineages are intermingled and specified in a salt-and-pepper pattern (Figure 1). The expression of Shh-dependent GFP reporters appears quite early during midline migration. Once cells begin accumulating a Shh-dependent reporter to detectable levels, GFP expression only increases, even for cells positioned far from their eventual neighbors. Thus, imprecisely induced fates are maintained, even as cells continue to divide, leaving no opportunity for early imprecision to be corrected later by overriding signals.
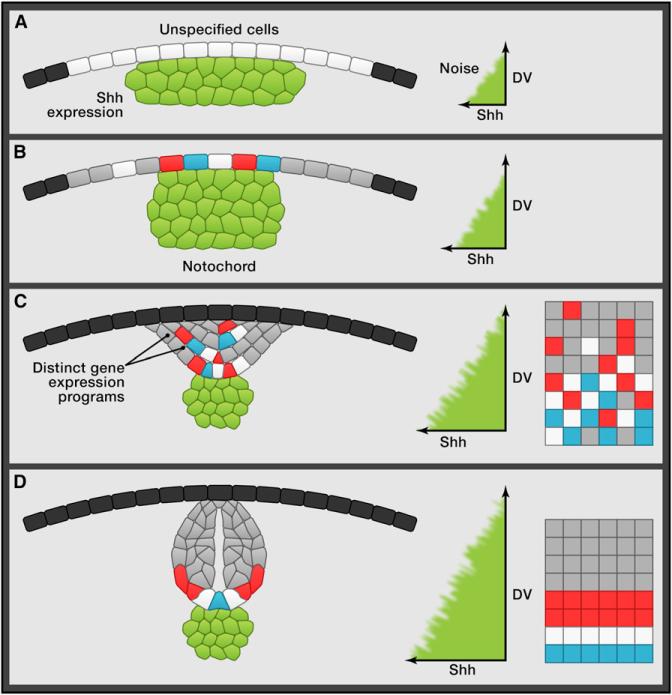
Figure 1. Cell Sorting in Morphogen-Mediated Patterning.
Cell sorting leads to precise patterns from salt-and-pepper specification of neural progenitors. (A) In a cross-section view, the unspecified cells of the neural plate (white) overlay the presumptive notochord expressing Shh (green), creating a shallow gradient (right). (B) As the notochord forms and Shh expression increases, disordered Shh-induced specification, as observed by distinct gene expression programs in the neurectoderm (blue, red, and white cells), begins as the neural plate converges toward the midline. (C) In the neural keel, highly intermingled progenitors (far right) sort to their appropriate positions, such that in the neural rod and tube (D), the neural progenitors are correctly positioned along the dorsal ventral (DV) axis.
Instead, precision is recovered in the neural keel and rod when cells reorganize themselves along the dorsal-ventral axis by undergoing extensive neighbor exchange. The changing of relative posi tions guarantees that cells always find their proper positions in the forming neural tube, such that precise boundaries between cells of different fates can be formed. Moreover, this reorganization occurs even for ectopically induced cells and requires the expression of cadherin, suggesting that cellular adhesive properties play a role in cell sorting. These findings lead the authors to propose a revised French flag model in which precise patterning arises from early heterogeneous specification that is corrected by positional sorting. Thus, Xiong et al. (2013) clearly demonstrate a context of morphogen-mediated patterning in which the signaling gradient itself cannot provide the observed precision in ordered cell fates.
These results have multiple implications for shaping future studies of Shh-mediated patterning and understanding morphogens more generally. First, we do not yet know the distribution of Shh in the early neural keel stage. It would seem likely that the spatial heterogeneity in cell fates at this stage results from stochastic fluctuations in the flux of Shh molecules at target cells. However, the data do not rule out the possibility that the Shh gradient itself exhibits some degree of precision—that is, that the same number of molecules arrive at any cell sitting at a given distance from the signaling center—and that the heterogeneity is caused not by the gradient but by imprecision in internal cellular processes that read out Shh concentration. Stochastic gene expression could, in principle, impact the degree of variability in fate determination (Raser and O'Shea, 2005). In all likelihood, a combination of both a variable gradient and noisy readout may contribute to the observed heterogeneity.
The relationship between Shh-mediated gene expression patterns and the cells’ final positions along the dorsalventral axis remains elusive. The authors speculate that other diffusible factors may play a role in directing cells to their correct position, although it is unclear why other gradients would be less susceptible than Shh to fluctuations from tissue morphogenesis or gene expression. The potential role of adhesion in directing cell sorting, as suggested by the cadherin depletion experiment, is highly intriguing and reminiscent of the expression of adhesion molecules elicited during the segmentation of the paraxial mesoderm (Aulehla and Pourquié, 2010). Adhesion may represent a signaling cue in itself (Amack and Manning, 2012). If Shh also confers differential expression of homotypic adhesion molecules, normal patterning might arise as long as enough cells of the correct fates are found in their proper locations relative to the signaling center.
Broadly correct organization of the neural plate/keel is observed in the relatively small distance between daughter cells of the same lineage; after division, daughters tend to be separated by about one to two cell diameters. The scale of the imprecision is approximately the same as that of early signaling events in contexts of restricted cell movement, such as in syncytial early Drosophila embryos (Gregor et al., 2007), where the shared cytoplasm and repeated mitotic cycles permit sharing of zygotically produced transcription factors that confer patterning decisions. This spatial averaging serves as one means by which immobilized cells, acting as passive information recipients, can improve the precision of responses to input patterning cues despite molecular fluctuations in gene expression events. The sorting behavior of the zebrafish neural tube cells demonstrates how nature has devised another powerful means of conferring developing tissues with the ability to raise the French flag.
REFERENCES
- Amack JD, Manning ML. Science. 2012;338:212–215. doi: 10.1126/science.1223953. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Pourquié O. Cold Spring Harb. Perspect. Biol. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, Briscoe J, Ribes V. Cell. 2012;148:273–284. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. Curr. Opin. Neurobiol. 2009;19:134–138. doi: 10.1016/j.conb.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor T, Tank DW, Wieschaus EF, Bialek W. Cell. 2007;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Cell. 2011;144:955–969. doi: 10.1016/j.cell.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Science. 2005;309:2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KW, Schier AF. Annu. Rev. Cell Dev. Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- Wolpert L. J. Theor. Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Xiong F, Tentner AR, Huang P, Gelas A, Mosaliganti KR, Souhait L, Rannou N, Swinburne IA, Obholzer ND, Cowgill PD, et al. Cell. 2013;153:550–561. doi: 10.1016/j.cell.2013.03.023. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]