Abstract
Radionuclide renal scintigraphy provides important functional data to assist in the diagnosis and management of patients with a variety of suspected genitourinary tract problems, but the procedures are underutilized. Maximizing the utility of the available studies (as well as the perception of utility by referring physicians) requires a clear understanding of the clinical question, attention to quality control, acquisition of the essential elements necessary to produce an informed interpretation, and production of a report that presents a coherent impression that specifically addresses the clinical question and is supported by data contained in the report. To help achieve these goals, part 1 of this review covers information that should be provided to the patient before the scan, describes the advantages and limitations of the available radiopharmaceuticals, discusses quality control elements needed to optimize the study, summarizes approaches to the measurements of renal function, and focuses on recommended quantitative indices and their diagnostic applications. Although the primary focus is the adult patient, aspects of the review also apply to the pediatric population.
Keywords: 99mTc-MAG3, 99mTc-DTPA, renal scintigraphy, clearance, renal function, effective renal plasma flow, glomerular filtration rate, relative function
Radionuclide renal scintigraphy provides important functional data to assist in the diagnosis and management of patients with a variety of suspected genitourinary tract problems; however, the examination is underutilized because referring physicians may have an incomplete knowledge of the advantages and limitations of renal scintigraphy, may not know when or when not to order a study, and may fail to formulate a well-defined clinical question. Moreover, the clinical perception of the utility of renal scintigraphy can vary frominstitution to institution because of marked differences in scan quality despite the use of the same radiopharmaceutical, comparable equipment, and identical billing. Differences in scan quality are primarily related to differences in physician and technologist training and supervision. The nuclear medicine physician needs to make certain that the clinical question is clearly formulated, the scan is appropriate, and the imaging procedure incorporates the elements required to optimize the study and best answer the clinical question. To help address these goals, part 1 of this review focuses on the available radiopharmaceuticals, patient preparation, clearance measurements, and quantitative indices that aid in diagnosis. Part 2 will illustrate several pitfalls and address common clinical indications, including the evaluation of suspected obstruction and renovascular hypertension. The primary focus is the adult patient, but aspects of the review also apply to the pediatric population.
RADIOPHARMACEUTICALS
The radiopharmaceuticals generally available for assessment of renal function and anatomy can be grouped into 3 broad categories: those filtered by the glomerulus, those retained in the renal tubules via proximal tubule receptor-mediated endocytosis from the glomerular filtrate (1), and those primarily secreted by the renal tubules via the organic anion transporter (1,2).
99mTc-Diethylenetriaminepentaacetic Acid (DTPA) (Glomerular Filtration)
99mTc-DTPA is the only renal radiopharmaceutical available for routine imaging that is purely filtered by the glomerulus; consequently, it is the only imaging radiopharmaceutical that can be used to measure glomerular filtration rate (GFR) (3). In healthy subjects, the extraction fraction of 99mTc-DTPA (the percentage of the tracer extracted with each pass through the kidney) is approximately 20%; this extraction fraction is relatively low compared with the extraction fraction of tubular tracers (41%–86%) (4–6).
51Cr-Ethylenediaminetetraacetic Acid (EDTA) (Glomerular Filtration)
51Cr-EDTA is a nonimaging radiopharmaceutical used to measure GFR via plasma sampling techniques; it is not available in the United States (3).
125I-Iothalamate (Glomerular Filtration)
125I-iothalamate is used to measure GFR via plasma sampling techniques (3). 125I does not emit a photon of sufficient energy to be useful for renal imaging.
123I- and 131I-Orthoiodohippurate (OIH) (Tubular Secretion)
123I- and 131I-OIH are cleared primarily by the proximal tubules although a small component is filtered by the glomeruli. The clearance of OIH is approximately 500–600 mL/min in subjects with normal kidneys (4). 131I- and 123I-OIH are no longer commercially available in the United States because of the introduction of 99mTc-mercaptoacetyltriglycine (MAG3), the poor imaging characteristics of 131I, the potential of 131I-OIH for delivering a high radiation dose, and the unfavorable logistics resulting from the relatively short half-life of 123I (7,8).
99mTc-MAG3 (Tubular Secretion)
99mTc-MAG3 is highly protein-bound and is removed from the plasma primarily by the organic anion transporter 1 located on the basolateral membrane of the proximal renal tubules (2,4,5). 99mTc-MAG3 accumulates in the proximal tubular cell and is then transported into the tubular lumen via organic anion transporters on the apical membrane (9); if the apical membrane transporter is impaired to a greater extent than the basolateral transporter, 99mTc-MAG3 can be extracted from the plasma with greater efficiency than it is transported to the tubular lumen, resulting in retained parenchymal activity. The extraction fraction of 99mTc-MAG3 is 40%–50% (5), more than twice that of 99mTc-DTPA. Because of its more efficient extraction, 99mTc-MAG3 is preferred over 99mTc-DTPA in patients with suspected obstruction and impaired renal function and is used in approximately 70% of the renal scans performed in the United States (10–15).
The clearances of 131I-OIH and 99mTc-MAG3 are often characterized as the effective renal plasma flow (4); however, there is a common misconception that the effective renal plasma flow (OIH clearance) is equivalent to renal plasma flow or, at least, proportional to renal plasma flow. The clearance of OIH and the clearance of 99mTc tubular tracers from the plasma both require delivery to the kidney (renal plasma flow), but they also require extraction from the plasma. These parameters do not always change in a proportional fashion.
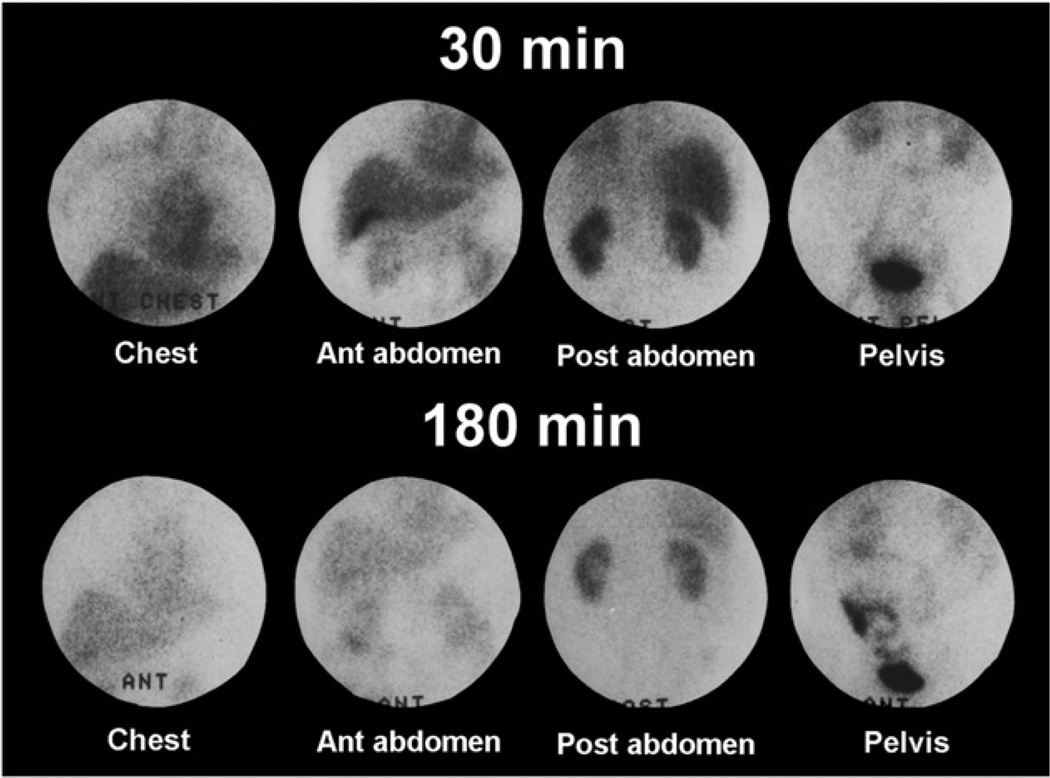
The 99mTc-MAG3 clearance, sometimes described as the tubular extraction rate, averages about 320 mL/min/1.73 m2 in adults under age 40 y and decreases by approximately 1% per year after age 40 y (5,16,17). Although the clearance of 99mTc-MAG3 is only 50%–60% that of OIH, the 99mTc-MAG3 clearance is highly correlated with the clearance of OIH, and the 99mTc-MAG3 clearance can be used as an independent measure of renal function (3,4). A small fraction of the administered dose of 99mTc-MAG3 is transported to the gut via the hepatobiliary system; this fraction increases in patients with impaired renal function (18). Occasionally, activity in the gallbladder has been mistaken for renal activity, and delayed images may show bowel accumulation (Fig. 1) (18–21).
FIGURE 1.
Images were obtained in patient with renal failure 30–45 min and 180–210 min after intravenous injection of 99mTc-MAG3. Activity can be seen in gallbladder on both early and late images, and bowel activity is clearly visualized on delayed images. Bowel activity on delayed images should not be confused with urine leak.
99mTc-l,l- and d,d-Ethylenedicysteine (EC) (Tubular Secretion)
99mTc-l,l- and d,d-EC are enantiomers; both are excellent renal radiopharmaceuticals with clearances slightly higher than 99mTc-MAG3 (18,22). Although 99mTc-d,d-EC is cleared more rapidly than 99mTc-l,l-EC (23), 99mTc-l,l-EC was first described and is available in several countries as a kit formulation.
99mTc-(CO3)Tricarbonylnitriloacetic Acid (NTA) (Tubular Secretion)
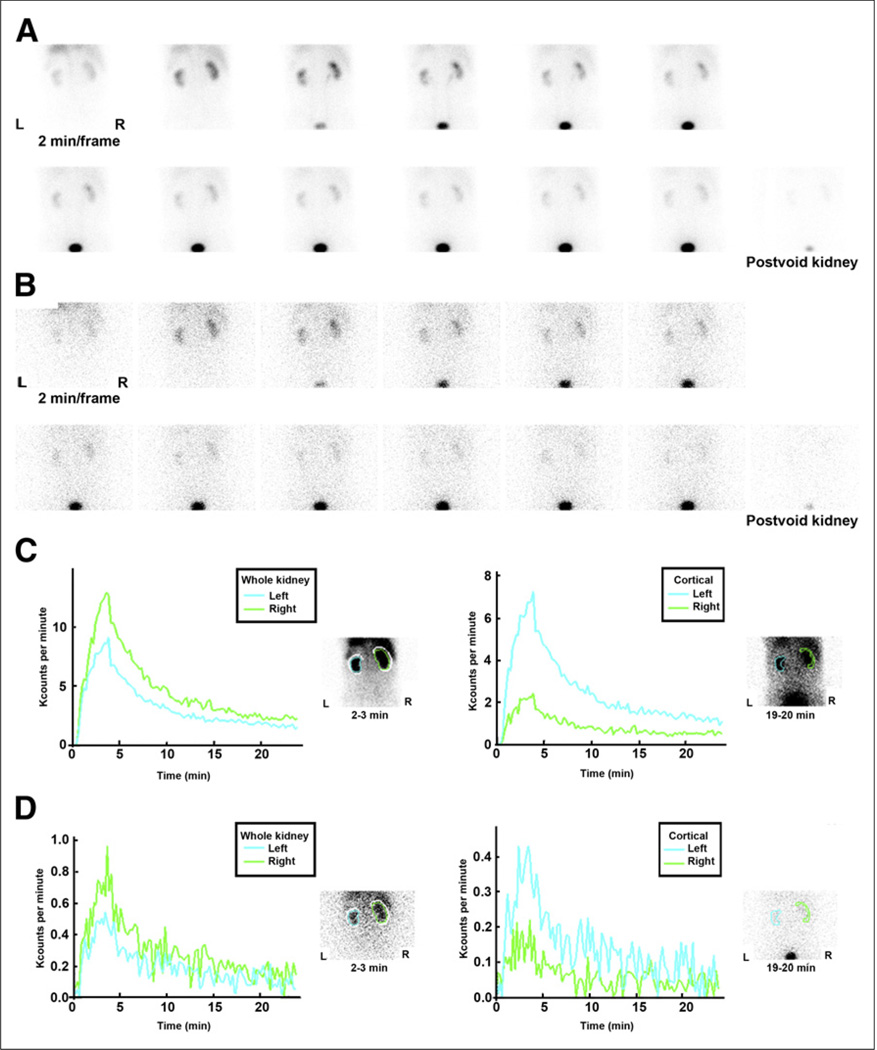
Tc-(CO3)NTA is a new 99mTc renal radiopharmaceutical with renogram curves and clearance equivalent to those of 131I-OIH (Fig. 2) (24,25). On the basis of its structure and charge distribution, 99mTc-(CO3)NTA is likely to be transported by the organic anion transporter 1. Unlike 99mTc-MAG3, however, preliminary studies in patients with chronic kidney disease show no evidence of gallbladder or gut activity (18–21,25). At the time of writing (2013), 99mTc-(CO3)NTA is still under investigation and is not commercially available.
FIGURE 2.
(A and B) Two-minute kidney images after simultaneous injection of 95.8 MBq (2.59 mCi) of 99mTc(CO)3(NTA) (A) and 11.8 MBq (0.32 mCi) of 131I-OIH (B) in 74-y-old man with stage 3 chronic kidney disease. (C and D) Whole-kidney and cortical (parenchymal) renogram curves for 99mTc(CO)3(NTA) (C) and for 131I-OIH (D). Renogram curves for 131I-OIH are quite noisy because of relatively low counting rate resulting from lower administered dose, poor capture of 364-keV photon of 131I by 9.5-mm (3/8-in) crystal, and high-energy collimator not optimized for 131I. Difference in relative height of cortical curves in C and D is due to differences in size of relative cortical ROIs. It is shape of cortical renogram curve that is important, not absolute height.
99mTc-Dimercaptosuccinic Acid (DMSA) (Cortical Retention)
99mTc-DMSA is an excellent cortical imaging agent that is used primarily in pediatrics to evaluate relative function, pyelonephritis, and renal scarring (26,27). Approximately 40% of the injected dose is retained by the renal tubules within 1 h after injection; the remainder is slowly excreted in the urine over the subsequent 24 h. Recent data suggest that 99mTc-DMSA is initially bound to α1-microglobulin; the 99mTc-DMSA α1-microglobulin complex is then filtered by the glomerulus and accumulates in the kidneys via megalin/tubulin-mediated endocytosis from the glomerular filtrate (1). Consequently, the renal uptake of 99mTc-DMSA is dependent on renal blood flow, glomerular filtration, and proximal tubule receptor-mediated endocytosis.
99mTc-Glucoheptonate (GH) (Cortical Retention and GFR)
99mTc-GH is cleared primarily by glomerular filtration, but approximately10%– 15% of the injected dose is retained in the renal tubules, allowing delayed, high-resolution static images to be obtained (28). 99mTc-GH tends to be used for static imaging if 99mTc-DMSA is unavailable; because of the parenchymal retention, it should not be used for diuretic renography.
Radiopharmaceuticals for Renal PET
PET provides high spatial resolution, sensitivity, and quantitative accuracy. Several PET radiopharmaceuticals are available for functional renal imaging; others are under development, but they are currently limited to research applications and were discussed in a previous review (29).
GENERAL PATIENT PREPARATION AND INFORMATION
Patients appreciate knowing what to expect when they are referred for an unfamiliar test. Information addressing the following topics should be provided to the patient before arrival.
Procedure and Time Required for the Study
The patient will receive an intravenous injection of the radiopharmaceutical and will lie quietly on an imaging table for 20–30 min. Depending on the protocol, there may be 2 imaging sessions.
Hydration
The patient should be told to arrive well hydrated and, in addition, to drink 2 large glasses of water just before arrival since good hydration minimizes the radiation dose to the bladder from the Auger electron of 99mTc and facilitates interpretation of the examination.
Diet and Medication
Diclofenac, a nonsteroidal antiinflammatory drug, blocks the production of prostaglandins and has been shown to inhibit spontaneous ureteric contraction, prolong the transit time, and delay the time to peak height of the renogram curve of 99mTc-MAG3 in healthy individuals (30–32). It is possible that other nonsteroidal antiinflammatory drugs may have a similar effect. Medication and dietary restriction for angiotensin-converting enzyme inhibition renography are discussed in part 2. For a basic renogram, there are no medication or dietary restrictions.
Risk and Radiation Exposure
Unlike radiographic contrast material, there is essentially no risk of an allergic or anaphylactic reaction. Depending on the radiopharmaceutical and the amount administered, the radiation exposure from a 37- to 370-MBq (1–10 mCi) injection of 99mTc-MAG3 or 99mTc-DTPA ranges from 5% to 70% of the yearly background radiation from cosmic rays and naturally occurring radioactive sources in the environment (8); this exposure is less than 5% of the yearly radiation dose considered safe for doctors and technologists who work with radiation. In patients with normal kidney function, over 95% of the 99mTc-MAG3 leaves the body by 3 h (33). Patients can go to public places and use a bathroom without risk to others.
Pregnancy
If the patient is pregnant or thinks she may be pregnant, she should discuss this possibility with the nuclear medicine physician before arrival for the test.
PROCEDURE
Voiding Before the Study
The patient should void immediately before the study. This practice will lessen the possibility that the patient needs to void during the acquisition and is essential for diuretic studies since a full bladder may delay upper tract emptying.
Administered Dose and Acquisition Parameters
Renal scans are frequently performed after the intravenous injection of approximately 370 MBq (10 mCi) of 99mTc-MAG3 or 99mTc-DTPA. Administration of activities in the range of 370 MBq may be required to obtain sufficient counts to visualize the initial bolus as it transits the aorta and kidneys or to calculate quantitative flow indices (34–36); however, except for the evaluation of renal transplants, neither 2-s flow images nor quantitative flow calculations obtained in the first few seconds after injection have been clearly demonstrated to contribute to the evaluation of relative function, suspected obstruction, or renovascular hypertension (34–36). An administered dose of 370 MBq is unnecessarily high for almost all applications, and a range from 37 to 185 MBq is much more appropriate (3,12,37–41).
Images are acquired dynamically for 20–30 min in 10- to 20-s frames and are usually displayed at 1- or 2-min intervals as the radioactive tracer is removed from the blood, transits the kidney, and enters the bladder. Postvoid views of the kidneys and bladder are recommended at the conclusion of the study (38).
Dose Injection and Extravasation
A poor bolus due to extravasation or venous obstruction can lead to inaccurate camera-based clearance measurements and result in an abnormal study with delayed uptake and washout. Injection into a limb with venous obstruction should be avoided; positioning the arm at a 90° angle to the body will minimize the likelihood of axillary retention of the tracer. A short 30- to 60-s image over the injection site at the conclusion of the study is a useful and recommended quality control procedure (37,38). The degree of extravasation or infiltration can be estimated by dividing the counts/min at the site of infiltration by the counts/min injected.
Whole Kidney Versus Cortical (Parenchymal) Regions of Interest (ROIs)
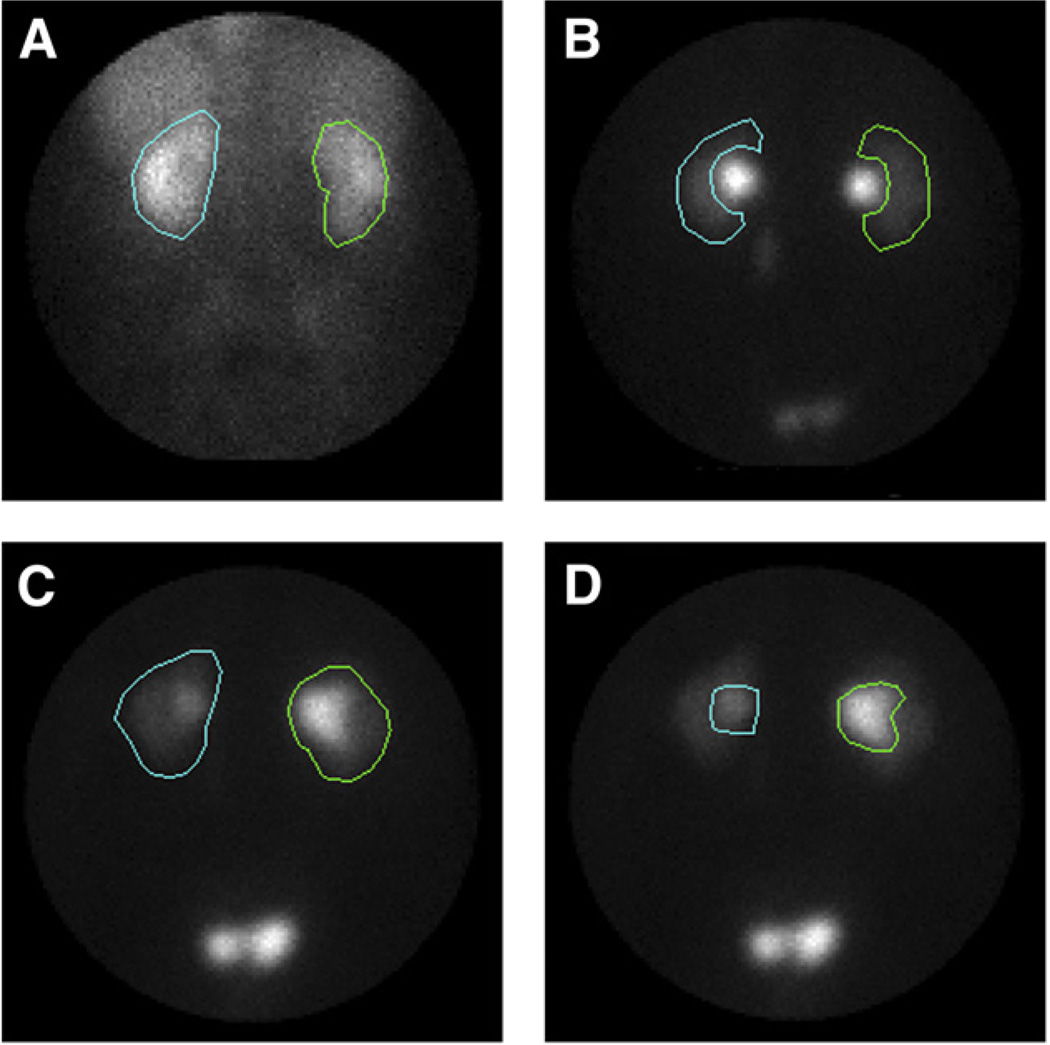
Time–activity curves are generated after placement of an ROI over each kidney, the renal cortex (parenchyma), or retained activity in the collecting system (Fig. 3). Data are recorded on the computer for subsequent analysis. The whole-kidney ROI consists of an ROI placed around the entire kidney, including the renal pelvis. Quantitative values generated using this ROI will be affected by retention of tracer in both the kidney parenchyma and the renal pelvis. Tracer retention may occur in pathologic states such as diabetic nephropathy or obstruction but may also occur in nonpathologic states such as a nonobstructed dilated collecting system or mild dehydration. To obtain a better assessment of parenchymal function, ROIs can be restricted to the renal cortex (parenchyma), excluding any retained activity in the renal pelvis or calyces.
FIGURE 3.
Whole-kidney, cortical, and collecting system ROIs are illustrated on images from baseline and postfurosemide 99mTc-MAG3 scans. (A) Whole-kidney ROI is illustrated on image obtained 2–3 min after injection. (B) Parenchymal (cortical) ROIs were assigned using 19- to 20-min image to ensure that parenchymal ROI excludes collecting system; assigning parenchymal ROI on 2- to 3-min image is problematic because collecting system is not visualized on this image. (C) Whole-kidney ROI was assigned using 1- to 2-min image after furosemide injection (2-stage diuretic acquisition protocol). (D) Collecting system ROI was assigned using 1- to 2-min image after furosemide injection. (Reprinted with permission of (50).)
Patient Position
Imaging is usually performed with the patient supine. The supine position allows a more accurate estimate of relative renal function since the kidneys are more likely to lie at the same depth (42). Nephroptosis has been observed in 22% of kidneys when patients are imaged in a seated position (43). A change in kidney position due to nephroptosis can lead to a difference in the measurement of relative uptake due to differences in attenuation rather than differences in relative function.
Background Correction
Background consists of radiotracer present in blood and the interstitial space of the kidney as well as in the tissues anterior and posterior to the kidney. For tracers with a low extraction fraction such as 99mTc-DTPA, the background counts during the second to third minute after injection can be as high as 50%–80% of the total activity in the renal ROI (37). To correct for these nonrenal counts present in the renal ROI, background correction needs to be performed. For the calculation of relative renal uptake, a perirenal background (Fig. 2C) normalized to the kidney ROI is preferred over background regions placed inferior to the kidney (37,44); automated background assignments that track the kidney ROI reduce processing time and enhance reproducibility. If the background region includes tracer that subsequently accumulates in the renal pelvis, background may be over-subtracted in the latter part of the study, leading to errors in generating quantitative parameters such as the 20-min/maximum count ratio. Further details regarding background correction methods and quality control of quantitative measurements of renal function obtained from the renogram are available in an ISCORN consensus report (37).
Time–Activity (Renogram) Curves
Whole kidney and cortical (parenchymal) time–activity curves are illustrated in Figure 2; the difference in the relative height of the cortical curves is due to the differences in the size of the relative cortical ROIs. The function of the cortical curve is to display the transit time through the cortex without contaminating the curve from activity in the collecting system; the cortical (parenchymal) ROIs are not drawn to have equal areas but to exclude the renal pelvis (45). Unless the cortical counts are normalized for area, a cortical ROI with a larger area will have more counts than a contralateral cortical ROI with a smaller area; consequently, the renogram curve with the larger cortical ROI will have a greater maximum. It is the shape of the cortical renogram curve that is important, not the absolute height. It is important to note that parameters generated from the cortical renogram curves may be spurious because of patient motion; parameters generated from cortical curves derived from poorly functioning kidneys may also be spurious because of low counts and noise.
Patients with a Percutaneous Nephrostomy or Neobladder
When a patient presents with a percutaneous nephrostomy, the nuclear medicine physician needs to decide whether to clamp the nephrostomy. This decision depends on the clinical question to be answered and may require consultation with the referring urologist. If possible, a neobladder with cutaneous drainage should be catheterized before the study to minimize reflux. Both of these issues will be discussed in more detail in part 2.
QUANTITATIVE INDICES
Relative Perfusion
Renal perfusion is evaluated by the visual or quantitative analysis of the initial bolus as it transits the abdominal aorta and enters the renal arteries. With the exception of renal transplant evaluation, quantitative measurements of renal perfusion have not been demonstrated to contribute to renal scan interpretation and can be omitted (See “Administered Dose and Acquisition Parameters” [above] and “Areas for Future Research” [part 2]) (34–38).
Relative Function
The relative uptake of the radiopharmaceutical provides a measure of differential renal function (the specific function depends on the radiopharmaceutical) and should be reported. For 99mTc-MAG3 and DTPA, the measurement is usually made by placing an ROI over each kidney and measuring the integral of the counts in renal ROI during the 1- to 2-, 1- to 2.5-, or 2- to 3-min period after injection or using the Rutland–Patlak plot (37). In subjects with normal renal function, the 95% confidence interval for the relative uptake of 99mTc-MAG3 using a perirenal background correction ranges from 42% to 58% (16). With the integral approach, the measurement needs to be made before any tracer is eliminated by either kidney. In patients with bilaterally impaired function and delayed excretion, the kidney-to-background ratio will be reduced compared with normally functioning kidneys; in this setting, the differential function measurement will be more accurate if the measurement is obtained not during the 1- to 3-min postinjection period but rather in the 1-min period just before any tracer leaves the kidney ROI (46).
Renal Size
Knowledge of renal size can assist in the interpretation of 99mTc-MAG3 renal scans. Several chronic renal diseases will result in bilaterally small kidneys, whereas the kidneys may be bilaterally enlarged in early diabetic renal disease, acute interstitial nephritis, HIV nephropathy, and amyloidosis. A unilaterally small kidney is obvious, but a small kidney may not be obvious on a 99mTc-MAG3 renal scan when the contralateral kidney is similarly reduced in size; conversely, an abnormal increase in renal size may not be recognized if both kidneys are similarly enlarged but have a normal configuration. Correlative imaging studies may provide size information, but unpublished results from our institution indicate that approximately 50% of patients referred for renal scintigraphy lack prior imaging studies; moreover, even when reports are available, there is often no comment regarding renal size. Renal size (length in cm and area in cm2) can be determined from the pixel length and area of the whole-kidney ROI. Regression equations to define the upper and lower limits of renal size normalized for body surface area have recently been developed for adults (47). Routine measurement of renal size at the time of the 99mTc-MAG3 scan may assist in the detection of unsuspected bilateral increases or decreases in renal size and facilitate scan interpretation.
Time to Peak, or Tmax
The time to peak, or Tmax, simply refers to the time from radiopharmaceutical injection to the peak height of the renogram curve. 99mTc-MAG3 and 99mTc-DTPA renograms normally peak by 5 min and decline to half-peak height by 15 min after injection; however, physiologic retention of the tracer in the renal calyces or pelvis can alter the shape of the whole-kidney renogram curve in normal kidneys and lead to prolonged values for the time to peak, 20-min/maximum count ratio, and T½.
The T½
The T½ refers to the time it takes for the activity in the kidney to fall to 50% of its maximum value. The use of this value in scan interpretation will be discussed in part 2.
Postvoid Kidney Counts/Maximum or 1- to 2-Minute Kidney Counts
Drainage from the renal pelvis can be facilitated by maximizing the pressure differential between the renal pelvis and bladder and by gravity. Requiring the patient to assume an upright posture for a few minutes and empty the bladder maximizes the pressure differential between the renal pelvis and bladder and allows gravity to facilitate urine drainage. Simple ratios that incorporate gravity-facilitated drainage from the kidneys such as counts in the postvoid kidney divided by the maximum counts in the kidney or counts in the postvoid kidney divided by counts at 1–2 min (normalized residual activity) appear to provide more robust measurements of drainage than the T½ (38,48–51).
20-Min/Maximum Count Ratio
The 20-min/maximum count ratio is the ratio of the kidney counts at 20 min to the maximum (peak) counts; this measurement provides an index of the transit time and parenchymal function and is often obtained for both whole kidney and cortical (parenchymal) ROIs. For 99mTc-MAG3, the normal 20-min/maximum count ratio for cortical ROIs averages 0.19, with SDs of 0.07 and 0.04 for the right and left kidneys, respectively (16). If the patient is not dehydrated and the 20-min/maximum count ratio for the cortical ROI exceeds 0.35 (greater than 2–3 SDs above the mean), the kidney is likely to be abnormal. In addition to detecting abnormal function, the 20-min/maximum and 20-min/1- to 2-min count ratios can be useful in monitoring patients with suspected urinary tract obstruction and renovascular hypertension (39,40,48,52).
Residual Urine Volume
Residual urine volume can be measured on the basis of the counts in pre- and postvoid ROIs over the bladder and a measurement of the voided volume (53). This measurement assumes a constant postvoid volume and is relatively easy to perform; it is routine at some institutions and may detect unsuspected urinary retention. To maintain a consistent geometry, pre- and postvoid images should be obtained with the patient in the same position. The main source of error is tracer in the renal pelvis that drains into the bladder after the patient voids; when possible, patients should walk around for several minutes to facilitate and complete gravity-assisted drainage before voiding and obtaining the postvoid image.
Urine Flow Rate
The rate of urine flow can be useful in assessing the adequacy of diuresis after furosemide administration and can be calculated by dividing the voided volume at the conclusion of the study by the interval between the time of voiding before the study and time of voiding at the conclusion of the study.
Clearance Measurements
The most common measure of renal function in clinical practice is serum creatinine; however, serum creatinine is affected by several factors (muscle mass, diet, age, certain drugs, illness, tubular secretion), and there is a wide range of serum creatinine values for all levels of GFR (54). Measurement of creatinine clearance using a 24-h urine collection is cumbersome, inconvenient, and often unreliable; moreover, repeated measurements in stable patients have shown a disappointingly high 25% coefficient of variation (SD/mean) (54,55). The initial evaluation of renal function can be improved using the 4-variable, Modification of Diet in Renal Disease study equation (56). Even with this equation, however, 20%–30% of subjects have an estimated GFR outside the relatively large 30% (70%–130%) accuracy range of the measured GFR (57–59). A more accurate measurement of renal function may be needed in patients at the extremes of age and body mass; in patients with severe malnutrition, grossly abnormal muscle mass (amputation, paralysis), high or low intakes of creatinine or creatine (vegetarian diet, dietary supplements), rapidly changing renal function; and in patients before kidney donation and before dosing with high-toxicity drugs excreted by the kidney. Measurement of renal function at the time of the scan can aid in the interpretation of a radionuclide study, provide a measurement of renal function independent of the estimated GFR, and serve as a baseline for monitoring changes. The 2 most widely used radionuclide techniques for evaluating renal function are plasma sample and camera-based clearance measurements.
Plasma Sample Clearances
The gold standard for measuring renal clearances involves timed urine collections after a constant infusion technique to maintain the plasma concentration of the tracer at a constant level (3). A variant of this technique uses a subcutaneous injection; however, both these methods are cumbersome, time-consuming, expensive, and limited to research applications. An alternative approach substitutes the plasma clearance for the renal clearance and avoids the necessity for urine collections. The plasma clearance is typically obtained using the single-injection, 2-compartment model of the plasma disappearance curve (60). The plasma disappearance curve can be characterized by multiple blood samples obtained over 90 min for tubular tracers but requires a longer time for GFR tracers because of their slower clearance (3,61,62).
Simplified techniques based on 1 or 2 plasma samples have been developed to estimate the multisample clearance (3). The 99mTc-MAG3 clearance can be estimated from the dose injected and the amount of radioactivity in a single blood sample obtained approximately 45 min after injection (3,17,61), and the GFR can be estimated from the dose injected and the activity in 1 or 2 plasma samples obtained 1–4 h after injection (3,62). These techniques generally provide reliable results, can be performed at the time of a standard renal scan using 99mTc-MAG3 or 99mTc-DTPA, and allow chemotherapy to be optimized to an individual patient’s underlying renal function (62).
Plasma sampling techniques have several sources of error. They assume a normal volume of distribution; if a patient has ascites, marked edema, or a large effusion, the tracer can diffuse into these extra fluid spaces and plasma clearance will not provide an accurate measure of renal clearance. Plasma sample clearances require meticulous technique and attention to detail. If the measurement is performed by a poorly trained or inexperienced individual, technical errors are easy to make and the results will be spurious. Because of the need for specialized training, extra effort, the necessity of handling plasma samples, and lack of reimbursement, plasma sample clearances are rarely available in the United States.
Camera-Based Clearances
Camera clearance methods that do not require blood or urine samples have been developed for 13II-OIH, 99mTc-DTPA, and 99mTc-MAG3 (63–67). The principle is based on the fact that the initial tracer accumulation by the kidneys is proportional to the renal clearance. Camera-based techniques determine the tracer accumulation (counts) in the kidneys at a defined period shortly after injection and divide by the counts injected to obtain a percentage injected dose in the kidneys. The percentage injected dose is converted to a clearance measurement using a validated nomogram (67). Camera-based clearances appear to be reproducible and are superior to creatinine clearance for monitoring changes in renal function (68). Although camera-based clearances are considered to be less accurate than plasma sample clearances (3), they avoid sources of error (timing of plasma samples, correction for radioactive decay, dilution of standards, pipetting small volumes) inherent in plasma sample techniques.
Camera-based clearance measurements, however, have their own sources of error. To obtain the percentage injected dose in each kidney, kidney counts have to be corrected for background, infiltration, attenuation, and renal depth. Two common sources of error are background subtraction and the estimation of renal depth. Since 99mTc-MAG3 is extracted more than twice as efficiently as 99mTc-DTPA, the kidney-to-background ratio will be much higher for 99mTc-MAG3 than 99mTc-DTPA and any potential error introduced by background subtraction will be reduced.
Renal depth is usually estimated from a nomogram based on height and weight (42,69). To the degree that a population-derived nomogram fails to fit a particular individual, the clearance measurement will vary from the true clearance. The sources of error due to background, self-attenuation, and renal depth tend to be reflected in a wider confidence interval associated with the accuracy of camerabased clearance compared with plasma sample clearances; however, in adult patients, these parameters tend to be constant in sequential studies and have less effect on reproducibility. Commercial camerabased techniques are currently available for measuring GFR (99mTc-DTPA), effective renal plasma flow (131I-OIH), and the 99mTc-MAG3 clearance. In evaluating the reliability of commercial software, it is important to confirm that the vendor has incorporated the appropriate quality control features and provided citable validation studies to confirm that the software is performing as claimed. Preliminary results suggest that the accuracy of γ-camera clearance methods can be improved using software adapted to a dualacquisition protocol (70).
CONCLUSION
Radionuclide renography can be a complex subject since several radiopharmaceuticals are available to image the kidney and monitor renal function; multiple quantitative indices can be generated, and protocols vary depending on institutional preference and clinical setting. Part 1 of this review has focused on the advantages and limitations of available radiopharmaceuticals, the quality control elements needed to optimize the study, and measurement of common quantitative indices designed to assist in scan interpretation. Part 2 will use the information presented in part 1 to focus on specific diagnostic applications, including suspected obstruction and renovascular hypertension. Potential pitfalls are included in both part 1 and part 2 and in a series of SAM questions designed to supplement and highlight important procedural elements and aspects of scan interpretation.
Learning Objectives: On successful completion of this activity, participants should be able to describe (1) the advantages and limitations of the available radiopharmaceuticals for radionuclide renal scintigraphy; (2) quality control elements needed to optimize the study; (3) approaches to the measurements of renal function; and (4) recommended quantitative indices and their diagnostic applications.
Acknowledgments
Financial Disclosure: This review was partially supported by a grant from the National Institutes of Health (NIH/NIDDK R37 DK38842). Andrew T. Taylor is entitled to a share of the royalties for the use of QuantEM software for processing MAG3 renal scans, which was licensed by Emory University to GE Healthcare in 1993. He and his coworkers have subsequently developed in-house, noncommercial software that was used in this study and could affect their financial status.
Footnotes
The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies. The author of this article has indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNMMI Web site (http://www.snmmi.org/ce_online) through April 2017.
REFERENCES
- 1.Weyer K, Nielsen R, Petersen SV, Christensen EI, Rehling M, Birn H. Renal uptake of 99mTc-dimercaptosuccinic acid is dependent on normal proximal tubule receptor-mediated endocytosis. J Nucl Med. 2013;54:159–165. doi: 10.2967/jnumed.112.110528. [DOI] [PubMed] [Google Scholar]
- 2.Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Transport of 99mTc-MAG3 via rat renal organic anion transporter 1. J Nucl Med. 2004;45:80–85. [PubMed] [Google Scholar]
- 3.Blaufox MD, Aurell M, Bubeck B, et al. Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med. 1996;37:1883–1890. [PubMed] [Google Scholar]
- 4.Eshima D, Taylor A., Jr Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new 99mTc renal tubular function agent. Semin Nucl Med. 1992;22:61–73. doi: 10.1016/s0001-2998(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 5.Bubeck B, Brandau W, Weber E, Kälble T, Parekh N, Georgi P. Pharmacokinetics of technetium-99m-MAG3 in humans. J Nucl Med. 1990;31:1285–1293. [PubMed] [Google Scholar]
- 6.Schaap GH, Alferink TH, de Jong RB, Oe PL, Roos JC, Donker AJ. 99mTc-MAG3: dynamic studies in patients with renal disease. Eur J Nucl Med. 1988;14:28–31. doi: 10.1007/BF00252614. [DOI] [PubMed] [Google Scholar]
- 7.Marcus CS, Kuperus JH. Pediatric renal I-123 orthoiodohippurate dosimetry. J Nucl Med. 1985;26:1211–1214. [PubMed] [Google Scholar]
- 8.Stabin M, Taylor A, Eshima D, Wooten W. Radiation dosimetry for technetium-99m-MAG3, technetium-99m-DTPA, and iodine-131-OIH based on human biodistribution studies. J Nucl Med. 1992;33:33–40. [PubMed] [Google Scholar]
- 9.Uchino H, Tamai I, Yamashita K, et al. p-aminohippuric acid transport at renal apical membrane mediated by human inorganic phosphate transporter NPT1. Biochem Biophys Res Commun. 2000;270:254–259. doi: 10.1006/bbrc.2000.2407. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A, Ziffer JA, Eshima D. Comparison of Tc-99m MAG3 and Tc-99m DTPA in renal transplant patients with impaired renal function. Clin Nucl Med. 1990;15:371–378. doi: 10.1097/00003072-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A, Clark S, Ball T. Comparison of Tc-99m MAG3 and Tc-99m DTPA scintigraphy in neonates. Clin Nucl Med. 1994;19:575–580. doi: 10.1097/00003072-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly P, Aurell M, Britton K, Kletter K, Rosenthal L, Testa T. Consensus on diuresis renography for investigating the dilated upper urinary tract. Radionuclides in Nephrourology Group. Consensus Committee on Diuresis Renography. J Nucl Med. 1996;37:1872–1876. [PubMed] [Google Scholar]
- 13.Gordon I, Colarinha P, Fettich J, et al. Guidelines for standard and diuretic renography in children. Eur J Nucl Med. 2001;28:BP21–BP30. [PubMed] [Google Scholar]
- 14.Shulkin BL, Mandell GA, Cooper JA, et al. Procedure guideline for diuretic renography in children 3.0. J Nucl Med Technol. 2008;36:162–168. doi: 10.2967/jnmt.108.056622. [DOI] [PubMed] [Google Scholar]
- 15.Conway JJ, Maizels M. The “well tempered” diuretic renogram: a standard method to examine the asymptomatic neonate with hydronephrosis or hydroureteronephrosis—a report from combined meetings of the Society for Fetal Urology and members of the Pediatric Nuclear Medicine Council–The Society of Nuclear Medicine. J Nucl Med. 1992;33:2047–2051. [PubMed] [Google Scholar]
- 16.Esteves FP, Taylor A, Manatunga A, Folks RD, Krishnan M, Garcia EV. 99mTc-MAG3 renography: normal values for MAG3 clearance and curve parameters, excretory parameters, and residual urine volume. AJR. 2006;187:W610–W617. doi: 10.2214/AJR.05.1550. [DOI] [PubMed] [Google Scholar]
- 17.Russell CD, Taylor AT, Dubovsky EV. Measurement of renal function with technetium-99m-MAG3 in children and adults. J Nucl Med. 1996;37:588–593. [PubMed] [Google Scholar]
- 18.Taylor A, Jr, Eshima D, Christian PE, Wooten WW, Hansen L, McElvany K. Technetium-99m MAG3 kit formulation: preliminary results in normal volunteers and patients with renal failure. J Nucl Med. 1988;29:616–622. [PubMed] [Google Scholar]
- 19.Sanchez J, Friedman S, Kempf J, Abdel-Dayem H. Gallbladder activity appearing-6 minutes after the intravenous injection of Tc99m MAG3 simulating a picture of obstructive uropathy of the right kidney. Clin Nucl Med. 1993;18:30–34. doi: 10.1097/00003072-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Rosen JM. Gallbladder uptake simulating hydronephrosis on Tc-99m MAG3 scintigraphy. Clin Nucl Med. 1993;18:713–714. doi: 10.1097/00003072-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Shattuck LA, Eshima D, Taylor AT, Jr, et al. Evaluation of the hepatobiliary excretion of technetium-99m-MAG3 and reconstitution factors affecting radiochemical purity. J Nucl Med. 1994;35:349–355. [PubMed] [Google Scholar]
- 22.Van Nerom CG, Bormans GM, De Roo MJ, Verbruggen AM. First experience in healthy volunteers with Tc-99m-L,L-ethylenedicysteine: a new renal imaging agent. Eur J Nucl Med. 1993;20:738–746. doi: 10.1007/BF00180902. [DOI] [PubMed] [Google Scholar]
- 23.Taylor A, Hansen L, Eshima D, et al. Comparison of technetium-99m-ll–EC isomers in rats and humans. J Nucl Med. 1997;38:821–826. [PubMed] [Google Scholar]
- 24.Taylor AT, Lipowska M, Marzilli LG. 99mTc(CO)3(NTA): A 99mTc renal tracer with pharmacokinetic properties comparable to those of 131I-OIH in healthy volunteers. J Nucl Med. 2010;51:391–396. doi: 10.2967/jnumed.109.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AT, Lipowska M, Cai H. 99mTc(CO)3(NTA) and 131I-OIH: comparable plasma clearances in patients with chronic kidney disease. J Nucl Med. 2013;54:578–584. doi: 10.2967/jnumed.112.108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piepsz A, Colarinha P, Gordon I, et al. Guidelines for 99mTc-DMSA scintigraphy in children. Eur J Nucl Med. 2001;28:BP37–BP41. [PubMed] [Google Scholar]
- 27.Rossleigh MA. Renal infection and vesico-ureteric reflux. Semin Nucl Med. 2007;37:261–268. doi: 10.1053/j.semnuclmed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Arnold RW, Subramanian G, McAfee JG, Blair RJ, Thomas FD. Comparison of 99mTc complexes for renal imaging. J Nucl Med. 1975;16:357–367. [PubMed] [Google Scholar]
- 29.Szabo Z, Xia J, Mathews WB. Radiopharmaceuticals for renal positron emission tomography imaging. Semin Nucl Med. 2008;38:20–31. doi: 10.1053/j.semnuclmed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Brough RJ, Lancashire MJ, Prince JR, et al. The effect of diclofenac (Voltarol) and pethidine on ureteric peristalsis and the isotope renogram. Eur J Nucl Med. 1998;25:1520–1523. doi: 10.1007/s002590050330. [DOI] [PubMed] [Google Scholar]
- 31.Kinn AC, Larsson SA, Nelson E, Jacobsson H. Diclofenac treatment prolongs renal transit time in acute ureteral obstruction: a renographic study. Eur Urol. 2000;37:334–338. doi: 10.1159/000052366. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa S, Elgazzar AH. Effect of the NSAID diclofenac on 99mTc-MAG3 and 99mTc-DTPA renography. J Nucl Med. 2013;54:801–806. doi: 10.2967/jnumed.112.109595. [DOI] [PubMed] [Google Scholar]
- 33.Taylor A, Eshima D, Fritzberg AR, Christian PE, Kasina S. Comparison of iodine-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 1986;27:795–803. [PubMed] [Google Scholar]
- 34.Hilson AJW, Maisey MN, Brown CB, Ogg CS, Bewick MS. Dynamic renal transplant imaging with Tc-99m DTPA (Sn) supplemented by a transplant perfusion index in the management of renal transplants. J Nucl Med. 1978;19:994–1000. [PubMed] [Google Scholar]
- 35.Peters AM, Brown J, Crossman D, et al. Noninvasive measurement of renal blood flow with technetium-99m-DTPA in the evaluation of patients with suspected renovascular hypertension. J Nucl Med. 1990;31:1980–1985. [PubMed] [Google Scholar]
- 36.el Maghraby TA, van Eck-Smit BL, de Fijter JW, Pauwels EK. Quantitative scintigraphic parameters for the assessment of renal transplant patients. Eur J Radiol. 1998;28:256–269. doi: 10.1016/s0720-048x(97)00179-4. [DOI] [PubMed] [Google Scholar]
- 37.Prigent A, Cosgriff P, Gates GF, et al. Consensus report on quality control of quantitative measurements of renal function obtained from renogram. Semin Nucl Med. 1999;29:146–159. doi: 10.1016/s0001-2998(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 38.Taylor AT, Blaufox MD, De Palma D, et al. Guidance document for structured reporting of diuresis renography. Semin Nucl Med. 2012;42:41–48. doi: 10.1053/j.semnuclmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor A, Nally J, Aurell M, et al. Consensus report on ACE inhibitor renography for detecting renovascular hypertension. J Nucl Med. 1996;37:1876–1882. [PubMed] [Google Scholar]
- 40.Taylor AT, Jr, Blaufox MD, Dubovsky EV, et al. Society of Nuclear Medicine procedure guideline for diagnosis of renovascular hypertension. [Accessed January 29, 2014];Society of Nuclear Medicine and Molecular Imaging website. http://interactive.snm.org/docs/pg_ch16_0403.pdf. Published August 2003. [Google Scholar]
- 41.O’Reilly PH. Upper urinary tract: standardization of the renogram technique for investigating the dilated upper urinary tract and assessing the results of surgery. BJU Int. 2003;91:239–243. doi: 10.1046/j.1464-410x.2003.04050.x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor A, Lewis C, Giacometti A, Hall EC, Barefield KP. Improved formulas for the estimation of renal depth in adults. J Nucl Med. 1993;34:1766–1769. [PubMed] [Google Scholar]
- 43.Tartaglione G, D’Addessi A, De Waure C, et al. 99mTc-MAG diuretic renography in diagnosis of obstructive nephropathy in adults: a comparison between F-15 and a new procedure F+10(sp) in seated position. Clin Nucl Med. 2013;38:432–436. doi: 10.1097/RLU.0b013e31828da3f5. [DOI] [PubMed] [Google Scholar]
- 44.Taylor A, Jr, Thakore K, Folks R, Halkar R, Manatunga A. Background subtraction in Tc-99m-MAG3 renography. J Nucl Med. 1997;38:74–79. [PubMed] [Google Scholar]
- 45.Taylor A, Kipper MS. The qualitative I-131 hippuran renogram: a potential problem. Clin Nucl Med. 1983;8:149–154. doi: 10.1097/00003072-198304000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Sennewald K, Taylor A., Jr A pitfall in calculating differential renal function in patients with renal failure. Clin Nucl Med. 1993;18:377–381. doi: 10.1097/00003072-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Taylor AT, Shenvi N, Folks RD, Garcia EV, Savir-Baruch B, Manatunga A. Reference values for renal size obtained from MAG3 scintigraphy. Clin Nucl Med. 2013;38:13–17. doi: 10.1097/RLU.0b013e318270866f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piepsz A, Kuyvenhoven JD, Tondeur M, Ham H. Normalized residual activity: usual values and robustness of the method. J Nucl Med. 2002;43:33–38. [PubMed] [Google Scholar]
- 49.Donoso G, Kuyvenhoven JD, Ham H, Piepsz A. 99mTc-MAG3 diuretic renography in children: a comparison between F0 and F + 20. Nucl Med Commun. 2003;24:1189–1193. doi: 10.1097/00006231-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Bao J, Manatunga A, Binongo JNG, Taylor AT. Key variables for interpreting 99mTc-mercaptoacetyltriglycine diuretic scans: development and validation of a predictive model. AJR. 2011;197:325–333. doi: 10.2214/AJR.10.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piepsz A, Tondeur M, Ham H. NORA: a simple and reliable parameter for estimating renal output with or without frusemide challenge. Nucl Med Commun. 2000;21:317–323. doi: 10.1097/00006231-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Blaufox MD, Fine EJ, Heller S, et al. Prospective study of simultaneous orthoiodohippurate and diethylenetriaminepentaacetic acid captopril renography. J Nucl Med. 1998;39:522–528. [PubMed] [Google Scholar]
- 53.Strauss BS, Blaufox MD. Estimation of residual urine and urine flow rates without ureteral catheterization. J Nucl Med. 1970;11:81–84. [PubMed] [Google Scholar]
- 54.Levey AS, Madaio MP, Perrone RD. Laboratory assessment of renal disease: clearance, urinalysis and renal biopsy. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia, PA: WB Saunders Company, Harcourt Brace, Jovanovich Inc.; 1991. pp. 919–968. [Google Scholar]
- 55.Bröchner-Mortensen J, Rodbro P. Selection of routine method for determination of glomerular filtration rate in adult patients. Scand J Clin Lab Invest. 1976;36:35–43. doi: 10.1080/00365517609068016. [DOI] [PubMed] [Google Scholar]
- 56.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 57.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 58.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Codkcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 59.Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Sapirstein LA, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearances of intravenously injected creatinine in the dog. Am J Physiol. 1955;181:330–336. doi: 10.1152/ajplegacy.1955.181.2.330. [DOI] [PubMed] [Google Scholar]
- 61.Bubeck B. Renal clearance determination with one blood sample: improved accuracy and universal applicability by a new calculation principle. Semin Nucl Med. 1993;23:73–86. doi: 10.1016/s0001-2998(05)80064-9. [DOI] [PubMed] [Google Scholar]
- 62.Murray AW, Barnfield MC, Waller ML, Telford T, Peters AM. Assessment of glomerular filtration rate measurement with plasma sampling: a technical review. J Nucl Med Technol. 2013;41:67–75. doi: 10.2967/jnmt.113.121004. [DOI] [PubMed] [Google Scholar]
- 63.Schlegel JU, Hamway SA. Individual renal plasma flow determination in 2 minutes. J Urol. 1976;116:282–285. doi: 10.1016/s0022-5347(17)58783-2. [DOI] [PubMed] [Google Scholar]
- 64.Gates GF. Split renal function testing using Tc-99m DTPA: rapid technique for determining differential filtration. Clin Nucl Med. 1983;8:400–407. doi: 10.1097/00003072-198309000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Taylor A, Jr, Corrigan PL, Galt J, et al. Measuring technetium-99m-MAG3 clearance with an improved camera-based method. J Nucl Med. 1995;36:1689–1695. [PubMed] [Google Scholar]
- 66.Bocher M, Shrem Y, Tappiser A, et al. Tc-99m mercaptoacetyltriglycine clearance: comparison of camera-assisted methods. Clin Nucl Med. 2001;26:745–750. doi: 10.1097/00003072-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Taylor A, Jr, Manatunga A, Morton K, et al. Multicenter trial validation of a camera based method to measure Tc-99m mercaptoacetyltriglycine or Tc-99m MAG3, clearance. Radiology. 1997;204:47–54. doi: 10.1148/radiology.204.1.9205222. [DOI] [PubMed] [Google Scholar]
- 68.Halkar R, Taylor A, Manatunga A, et al. Monitoring renal function: a prospective study comparing camera-based Tc-99m mercaptoacetyltriglycine clearance and creatinine clearance. Urology. 2007;69:426–430. doi: 10.1016/j.urology.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tonnesen KH, Munck O, Hald T. Influence on the radiorenogram of variation in skin to kidney distance and the clinical importance hereof. In: Zum Winkel K, Blaufox MD, Funck-Bretano JL, editors. Proceedings of the International Symposium on Radionuclides in Nephrourology 1974; Thieme; Stuttgart, Germany. 1974. pp. 79–86. [Google Scholar]
- 70.Samal M, Ptacnik V, Skibova D, Jiskrova H, Kubinyi J. Simple model-based method for gamma-camera measurement of 99m-MAG3 plasma clearance [abstract] J Nucl Med. 2013;54(suppl):170P–171P. [Google Scholar]