Abstract
Polyamine-depletion inhibited apoptosis by activating ERK1/2, while, preventing JNK1/2 activation. MKP-1 knockdown by SiRNA increased ERK1/2, JNK1/2, and p38 phosphorylation and apoptosis. Therefore, we predicted that polyamines might regulate MKP1 via MEK/ERK and thereby apoptosis. We examined the role of MEK/ERK in the regulation of MKP1 and JNK, and p38 activities and apoptosis. Inhibition of MKP-1 activity with a pharmacological inhibitor, sanguinarine (SA), increased JNK1/2, p38, and ERK1/2 activities without causing apoptosis. However, pre-activation of these kinases by SA significantly increased camptothecin (CPT)-induced apoptosis suggesting different roles for MAPKs during survival and apoptosis. Inhibition of MEK1 activity prevented the expression of MKP-1 protein and augmented CPT-induced apoptosis, which correlated with increased activities of JNK1/2, caspases, and DNA fragmentation. Polyamine depleted cells had higher levels of MKP-1 protein and decreased JNK1/2 activity and apoptosis. Inhibition of MEK1 prevented MKP-1 expression and increased JNK1/2 and apoptosis. Phospho-JNK1/2, phospho-ERK2, MKP-1, and the catalytic subunit of protein phosphatase 2A (PP2Ac) formed a complex in response to TNF/CPT. Inactivation of PP2Ac had no effect on the association of MKP-1 and JNK1. However, inhibition of MKP-1 activity decreased the formation of the MKP-1, PP2Ac and JNK complex. Following inhibition by SA, MKP-1 localized in the cytoplasm, while basal and CPT-induced MKP-1 remained in the nuclear fraction. These results suggest that nuclear MKP-1 translocates to the cytoplasm, binds phosphorylated JNK and p38 resulting in dephosphorylation and decreased activity. Thus, MEK/ERK activity controls the levels of MKP-1 and, thereby, regulates JNK activity in polyamine-depleted cells.
Introduction
Polyamines control cell growth and differentiation by regulating proliferation, migration, and apoptosis in normal as well as in cancer cells [1–8]. Ornithine decarboxylase (ODC) catalyzes the first rate-limiting step in polyamine biosynthesis, converting ornithine to putrescine. S-adenosylmethionine decarboxylase (SAMDC) serves as a propylamine donor, which converts putrescine and spermidine into spermidine and spermine respectively [9, 10]. DFMO (α-difluoromethylornithine) inhibits ODC activity and depletes the levels of intracellular putrescine by 6 hours, spermidine by 24 hours, and decreases spermine up to 70% by 96 hrs. Polyamine depletion prevents receptor- and genotoxic drug-induced apoptosis by preventing JNK1/2 activation. Earlier studies from our laboratory showed that increasing MEK1/ERK1/2 activity by inhibiting catalytic sub unit of protein phosphatase 2A (PP2Ac) decreased JNK1/2 activity, and protected cells from apoptosis [11, 12]. Inhibition of MEK1 by a specific inhibitor, U0126, increased JNK1/2 activity and apoptosis in response to TNF/CHX in polyamine depleted cells. These results indicated that the activity of MEK1/ERK1/2 determines the levels of JNK1/2 activity and, thereby, apoptosis. However, the mechanism by which MEK1/ERK1/2 regulates JNK activity in response to polyamine is not known.
We have shown that SiRNA-mediated knockdown of MKP-1 increased JNK1/2, and p38 activities and apoptosis in response to CPT/TNF [13]. TNF caused transient activation of ERK and JNK and that CPT-induced MKP-1 expression sustained the activity of ERK and JNK leading to apoptosis [13]. Recently, Guo et al. found that inhibition of ERK activity decreased the expression of MKP-1 protein and resulted in p38 activation in Rat-1 cells [14]. Therefore, we used CPT alone or in combination with TNF to delineate the role of ERK and MKP-1 in the regulation of JNK during apoptosis.
We predict that MEK1/ERK1/2 may regulate JNK1/2 activity via MKP-1 in polyamine dependent manner in IEC-6 cells to regulate apoptosis. We show that the activity of JNK1/2 increased while the levels of MKP-1 decreased during apoptosis. Inhibition of MKP-1 increased the levels of phosphorylated forms of JNK and p38. However, increased activity of MAPKs had minimal effect on basal apoptosis, while it augmented apoptosis induced by DNA damage and eliminated the protection conferred by polyamine depletion. Our data indicate that the expression of MKP-1 protein is regulated by the activity of MEK/ERK. Furthermore MKP-1 appears to control nuclear events associated with apoptosis, while its cytoplasmic localization and association with phospho-JNK controls apoptotic signaling in IEC-6 cells. The most important finding in this study demonstrates the formation of multi-protein signaling complex in response to apoptotic inducers.
Material and Methods
Reagents
Cell culture medium and fetal bovine serum (FBS) were obtained from Mediatech Inc. (Herndon, VA). Dialyzed FBS (dFBS) was purchased from Sigma (St. Louis, MO). Trypsin-EDTA, antibiotics, and insulin were purchased from GIBCO-BRL (Grand Island, NY). Protease inhibitors, phosphatase inhibitors, phosphate buffer saline (PBS), Dulbecco’s phosphate buffer saline (DPBS), formaldehyde were obtained from Thermo Fisher Scientific Inc. (Rockford, IL). α-difluoromethyl ornithine (DFMO) was a gift from ILEX Oncology (San Antonio, TX). TNF-α was obtained from Pharmingen International (San Diego, CA). Camptothecin (CPT) and cycloheximide (CHX) were obtained from Sigma (St. Louis, MO). Rabbit anti-JNK1/2, rabbit anti-p38, rabbit anti-phospho-ERK1/2, rabbit anti-ERK1/2, rabbit anti-cleaved-casapse-3, and mouse anti-caspase-9 antibodies were purchased from Cell Signaling (Beverly, MA). Mouse anti-actin antibody was purchased from Millipore (Billerica, MA). The rabbit anti-MKP-1 and mouse anti-phospho-JNK1/2 antibodies were purchased from Santa Cruz biotechnology (Santa Cruz, CA). Alexafluor-conjugated secondary antibodies were purchased from Molecular probes (Eugene, OR). SP-600125, SB203580, and Okadaic acid (OA) were purchased from Calbiochem (La Jolla, CA). MEK inhibitor U0126 and rabbit anti-phospho-p38 was purchased from Promega (Madison, WI). Sanguinarine (SA, MKP-1 inhibitor) was obtained from Tocris Bioscience (Ellisville, MO). Microcystin sepharose (MC-sepharose) was purchased from Millipore (Temecula, CA). Fluorometric substrates IETD-AFC (Caspase-8), LEHD-AFC (Caspase-9), and DEVD-AFC (Caspase-3) were purchased from Biomol Research laboratories (Plymouth Meeting, PA). The Cell Death Detection ELISA Plus kit was purchased from Roche Diagnostics Corp (Indianapolis, IN). Mammalian protein extraction reagent (MPER) and the Bicinchoninic acid (BCA) protein assay reagent kit were purchased from Pierce (Rockford, IL). The RC-DC protein assay reagent kit was purchased from Bio-Rad (Hercules, CA). The ECL Western blot detection system was purchased from DuPont-New England Nuclear (Boston, MA). The IEC-6 cell line (ATCC CRL 1592) was obtained from the American Type Culture Collection (Manassas, VA) at passage 13. This cell line is derived from normal rat intestine and was developed and characterized by Quaroni et al. [15]. These cells are nontumorigenic, originate from intestinal crypt cells as judged by morphological and immunologic criteria, and retain the undifferentiated character of epithelial stem cells. Tests for mycoplasma were always negative. All chemicals were of the highest purity commercially available.
Cell culture
Stock cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate in T-150 flasks and incubated at 37°C in a humidified atmosphere of 90% air-10% CO2. Stock cells were passaged once weekly and fed three times per week, and passages 15–22 were used. During experiments setup, stock cells were trypsinized with 0.05% trypsin and 0.53 mM EDTA and counted using a Beckman Coulter Counter (model Z1). Cells were grown in DMEM/5% dFBS in the presence and absence of DFMO or DFMO + putrescine (DP) to confluence for 3 days and on the 4th day were washed once with HBSS and placed in respective serum-free medium for 24 h prior to experiments.
Quantitative DNA fragmentation ELISA
The protocol for quantitative DNA fragmentation ELISA was similar to that described previously [8, 12, 13]. Briefly, cells were grown in 24 well plates for DNA fragmentation ELISA assay and protein determination. Following treatment, cells were washed twice with ice-cold DPBS, followed by the addition of lysis buffer and gentle shaking for 30 minutes at room temperature, and centrifugation at 200 rpm to remove the nuclei. An aliquot of the nuclei-free supernatant was placed in streptavidin-coated wells and incubated with anti-histone-biotin antibody and anti-DNA peroxidase-conjugated antibody. After incubation for 2 hrs at room temperature, the wells were washed three times with incubation buffer. 100μl of substrate buffer containing 2,2′-azino-di(3-ethylbenzthiazolin-sulfonate) were added to each well, and samples were incubated for an additional 5–10 min. Absorbance was read at 405-nm in a plate reader. Protein concentration was determined by the BCA method. Results are expressed as absorbance at 405 nm/mg protein/min.
Caspase activity
After treatment, cells were harvested for determination of caspase activity as described earlier [8, 12, 13]. Briefly, after washing twice, 750 μl of ice-cold DPBS was added to the 60 mm plate, and the monolayer was scraped and collected into a microfuge tube. The cells collected by centrifugation were lysed in 250 μl of ice-cold cell lysis buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 1 mM dithiothreitol (DTT), 0.1 mM EDTA). The resulting cell lysate was centrifuged at 13,000 x g at 4°C for 13 mins. The supernatant fraction was used to measure caspase activity. The fluorometric assay was carried out in a 96-well plate, and 20 μl of cell lysate, 80 μl of caspase assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA and 10% glycerol) containing caspases-3, -8, or -9 fluorometric substrates at a final concentration of 18 μM were placed in each well. The plate was incubated at 37°C for 2 h. AFC release was monitored by excitation at 400 nm at an emission of 520 nm. RC-DC protein assay reagent was used to determine protein concentration. The caspase activity was calculated as relative fluorescence units (RFU) per milligram protein per hour. In order to confirm substrate cleavage specificity, caspase-3, 8 and 9 inhibitors DEVD-FMK, IETD-FMK and LEHD-FMK were added to cell lysates incubated with caspase-3, 8 and 9 substrates.
Cell lysate preparation
Cell monolayers were washed three times with ice-cold DPBS (pH 7.4) and 350 μl of MPER buffer containing protease inhibitor and phosphatase inhibitors was added to the plate. The cells were incubated on ice for 15 min, harvested using a rubber scraper, transferred to 1.5 ml microfuge tubes, centrifuged at 13,000 X g at 4°C for 13 min, and supernatants were collected.
Nuclear and Cytosolic extract preparation
After treatment, cells were washed twice with 10 ml ice-cold DPBS containing phosphatase inhibitors. Next, cells were scraped gently with a rubber scrapper and collected into a pre-chilled 15 ml tube and spun down at 300 X g for 5 min at 4°C. After discarding the supernatant, the pellet was resuspended in 1ml ice-cold hypotonic buffer (20 mM HEPES pH 7.5, 5 mM NaF, 10 μM Na2MoO4 and 0.1 mM EDTA) by gentle mixing and transferred into a pre-chilled 1.5 ml tube. Cells were allowed to swell on ice for 15 min followed by the addition of 50 μl 10% Nonidet P-40 (0.5% final concentration) and homogenized. The homogenate was centrifuged for 30 second at 4°C and the supernatant was stored as the cytoplasmic fraction at −80°C. The nuclear pellet was resuspended in 50 μl complete lysis buffer, the tube was rocked gently for 30 min at 4°C, centrifuged for 10 min at 14,000 x g at 4°C, and the supernatant saved as nuclear extract at −80°C. The protein concentration of the extract was determined using the BCA protein assay.
Western blot analysis
25-μg protein was dissolved in 1x SDS sample buffer. The protein samples were subjected to 10–15% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore, Bedford, MA). The membranes were blocked with blocking buffer (3–5% nonfat dry milk in tris-buffered-saline containing 0.1% Tween 20) for 2 h and incubated with the indicated primary antibodies prepared in blocking buffer overnight at 4°C. All antibodies were diluted 1:1,000 with the exception of anti-actin, 1:40,000, for western blotting. Membranes were subsequently incubated with appropriate secondary antibody conjugated to horseradish peroxidase at room temperature for 1h, and immunocomplexes were visualized by the enhanced chemiluminescence (ECL) detection system.
Immunocytochemistry
Cells were seeded onto coverslips coated with poly-L-lysine and grown as described earlier (4). After being treated, cells were fixed with 3.7% formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 10 min, and washed with PBS. Coverslips were blocked with 2% BSA in PBS for 20 min and then incubated with primary antibody (MKP-1) for 2 hrs. Coverslips were then washed with 0.1% BSA in PBS for 20 min, followed by a 2 hrs incubation with an appropriate fluorescent dye-conjugated secondary antibody. Coverslips were mounted on glass slides and observed using a Nikon Eclips 80i UV epifluorescence microscope.
Statistics
All experiments were repeated three times (n = 3). Data were expressed as means ± SE. Experiments involving Western blots were performed three times with similar results, and a representative blot is shown. ANOVA with appropriate post hoc testing was used to determine the significance, and the Student’s t-test was performed to determine the significance of the differences between means. p < 0.05 was regarded as statistically significant.
Results
Effect of CPT on MKP-1, ERK1/2 and JNK1/2 activity
MKP-1 dephosphorylates p-JNK and p-p38 and regulates growth and apoptosis [16–25]. We have shown that the knockdown of MKP-1, using SiRNA, increased TNF/CPT-induced JNK activity and apoptosis in IEC-6 cells [13]. In the current study, we examined the time course of MKP-1 induction and its correlation with the activation of JNK1/2, ERK1/2, and p38. MKP-1 protein levels increased at 3 hours in response to CPT (20 μM) compared to untreated cells and then decreased gradually to basal levels by 9 hours (Fig.1A). The decrease in MKP-1 correlated with increased JNK1/2 activity (Fig. 1A). However, ERK activity remained higher during the entire treatment period (Fig.1A). CPT increased apoptosis as measured by DNA fragmentation in a time dependent manner (Fig.1B), which correlated with decreased MKP-1 and increased JNK activity (Fig.1A). These results indicate that decreased MKP-1 protein and, thereby, activity might control JNK1/2 activity during apoptotic signaling. Interestingly, MKP-1 protein showed two bands: the higher molecular weight form (upper band) changed in a time dependent manner, while the lower molecular weight form (lower band) did not change during apoptosis suggesting post-translational modification of MKP-1 protein. We have shown that the enzymatic activity of MKP-1 decreases in response to apoptotic stimuli [13].
Fig. 1. CPT induces MKP-1, JNK1/2 and ERK1/2 during apoptosis.
(a) Confluent serum starved cells grown as described in the methods were left untreated (UT) or exposed to CPT (20 μM) for the indicated time intervals. Whole cell extracts (25μg) were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1, and phosphorylated JNK1/2 (p-JNK1/2) and ERK1/2 (p-ERK1/2) using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations. (b) Confluent serum starved cells treated as described above were used to determine apoptosis. DNA fragmentation was measured using a colorimetric ELISA kit as described in methods. Values are means ± SE of triplicates. *, p < 0.05 compared with UT.
Sanguinarine (SA), an irreversible inhibitor of MKP-1 activity leads to the accumulation of inactive enzyme. This increased the phosphorylated forms of JNK1/2, ERK1/2, and p38 several-fold in a time dependent manner over those seen in untreated cells (Fig. 2A). MKP-1 protein levels began to increase after 2h and peaked at 3h in control and in the DFMO+PUT group in response to SA. However, in polyamine- depleted cells (DFMO group) the increase was noted earlier (1.0 h), and the levels of MKP-1 protein remained higher compared to the control group. Phosphorylated MEK1/ERK1/2 and JNK1/2 levels were higher in polyamine-depleted cells and began to increase earlier compared to those seen in control and DFMO+PUT groups. Addition of putrescine along with DFMO (DP group) prevented the effects of polyamine depletion on MKP-1 protein, and the activities of MAPKs were similar to those in the control group (Fig. 2A, right panel). Cells exposed to SA for 3h and then treated with TNF/CPT increased the activation of JNK and showed robust activation of caspase-3 in control and DFMO+PUT (DP) group (Fig. 2B). Polyamine depleted cells treated in a similar manner had an increased basal activation of caspase-3, however, TNF/CPT-induced caspase-3 activation was less compared to the control and DP groups (Fig. 2B) and JNK activity actually decreased (Fig. 2A, last lane in each group). The higher MKP-1 and an early activation of MEK1/2 and ERK1/2 in polyamine-depleted cells might be responsible for preventing the further activation of JNK1/2 in response to TNF/CPT and, thereby, caspase-3 activity. These results suggest that MKP-1 regulates the levels of activated ERK, JNK, and p38 in a polyamine dependent manner, which influences apoptotic signaling in IEC-6 cells.
Fig. 2. Inhibition of MKP-1 increases phosphorylation of MAPKs.
(a) IEC-6 cells were grown to confluence in control, 5 mM DFMO and DFMO + 10 μM putrescine (DFMO + PUT) containing media for 3 days followed by serum starvation for 24h. Confluent monolayers were left untreated (UT) or treated with SA (2 μM) for the indicated time periods. One group of SA treated cells was washed with HBSS and exposed to TNF/CPT for additional 3h. Whole cell extracts (25 μg) were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1, total and phosphorylated forms of JNK1/2, ERK1/2, p38 and MEK1/2 using specific antibodies. (b) Whole cell extracts (25 μg) from TNF/CPT treated cells were analyzed for the detection of active caspase-3 by western blot analysis. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations.
To delineate the role of MKP and MAPKs during apoptosis, we pretreated cells with SA for 3h to inhibit MKP-1 followed by exposure to CPT for 3h, and measured active caspases-3, -9 and DNA fragmentation (Fig. 3A). Increased levels of phosphorylated ERK1/2, JNK1/2, and p38 in SA treated cells had little effect on the activation of caspases and DNA fragmentation in the absence of CPT (Fig. 3B). However, higher levels of MAPKs pre-activated by SA significantly increased the activities of both caspases-9 and -3 (Fig. 3A) and increased DNA fragmentation (2-fold) compared to that seen with CPT (Fig. 3B). A p38 specific inhibitor, SB203580, decreased the activation of both caspases-9 and -3 seen in response to TNF/CPT without altering the levels of MKP-1 (data not shown). These results suggest that MKP-1 activity regulates the proapoptotic JNK and p38 activities.
Fig. 3. Effect of MKP-1 inhibition on CPT induced apoptosis.
(a) Confluent serum starved cells were left untreated (UT) or treated with SA for 3h. One SA treated group of cells was washed with HBSS and exposed to CPT (20 μM) for 3h. Whole cell extracts (25μg) were subjected to 10% and 15% SDS-PAGE followed by western blot analysis to detect MKP-1, and phosphorylated forms of JNK1/2, ERK1/2, p38 and MEK1/2 as well as active caspases-9 and -3. Actin immunoblotting was performed to show equal loading. Blots shown are representative of 3 observations. (b) Confluent serum starved cells treated as described above were used to determine DNA fragmentation as an index of apoptosis. Values are means ± SE of triplicates. p < 0.05 was considered significant. *, p < 0.05 compared with UT and SA. #, p < 0.05 compared with CPT.
MEK/ERK inhibition prevents MKP-1 induction and apoptosis
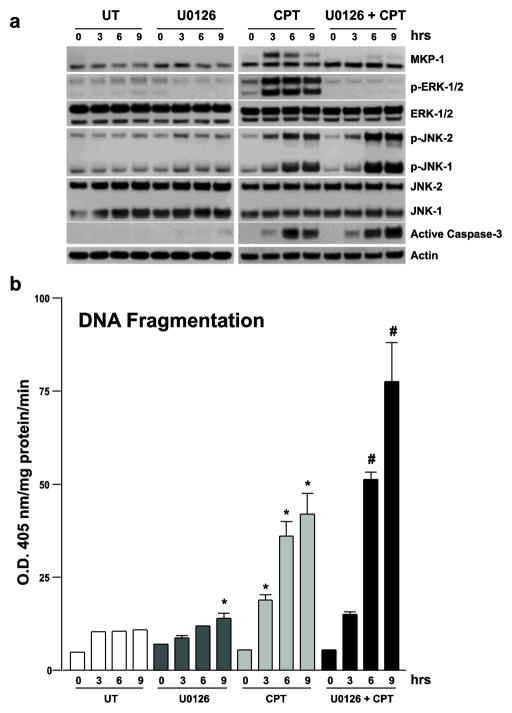
The mechanism by which MKP-1 is regulated during apoptosis is unknown. Since MEK1/ERK1/2 pathway is important in the protection of polyamine depleted cells from apoptosis and since the MEK1 is reported to regulate MKP-1, serum starved cells preincubated with U0126, MEK1 inhibitor (30 min) followed by the addition of SA for 3h were examined for MKP-1 expression. U0126 prevented the increase in ERK1/2 activity and MKP-1 expression in response to SA (Fig. 4). The increased activity of JNK due to decreased levels of MKP-1 protein in the absence of MEK/ERK augments the CPT-induced apoptosis. U0126 prevented the CPT-induced increase in the levels of MKP-1 with concomitant increases in DNA fragmentation (Fig. 5A and B) in a time dependent fashion, which was accompanied by increased activities of JNK1/2 and caspases-8, -9, and -3 (Fig. 5C).
Fig. 4. MEK1/ERK1/2 regulates MKP-1 expression and SAPKs.
Confluent serum starved cells left untreated (UT) or pretreated with U0126 (10 μM) for 30 mins. were exposed to 2 μM SA for 3h. Whole cell extracts (25 μg) were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1, total and phosphorylated forms of JNK1/2, ERK1/2, and p38 using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations.
Fig. 5. Effect of MEK1 inhibition on CPT-induced JNK1/2 and apoptosis.

(a) Confluent serum starved cells were left untreated (UT), or treated with U0126, CPT, or U0126+CPT for indicated time intervals. U0126 was added 30 minutes prior to the addition of CPT. Whole cell extracts (25 μg) were subjected to 10% and 15% SDS-PAGE followed by western blot analysis to detect MKP-1, total and phosphorylated JNK1/2, ERK1/2, and active caspase-3. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations. (b) Confluent serum starved cells treated as described above were used to determine apoptosis by measuring DNA fragmentation. Values are means ± SE of triplicates. *, p < 0.05 compared with UT. #, p < 0.05 compared with CPT. (c) Confluent serum starved cells treated as described in A were used to determine enzymatic activities of Caspases-3, -9, and -8. Values are means ± SE of triplicates. *, p < 0.05 compared with CPT.
Our previous results showing decreased activation of JNK1/2 and apoptosis in polyamine depleted cells, and that the inhibition of MEK1 augmented apoptosis by increasing JNK1/2 activity [11, 12, 26] suggest that polyamines might affect apoptosis by controlling MKP-1. Cells grown in control, DFMO and DFMO+PUT (DP) exposed to TNF/CPT were analyzed for levels of MKP-1 protein and active ERK1/2, JNK1/2 and caspase-9. Cells grown in the presence of DFMO and exposed to TNF/CPT (3h) had increased MKP-1 protein and decreased JNK1/2 and caspase-9 activities, which correlated with the decreased DNA fragmentation (Fig. 6). Furthermore, restoration of MKP-1 levels and JNK1/2 activity to control values by addition of putrescine to the DFMO containing medium also indicates that polyamines regulate the activity of MKP-1. U0126 prevented TNF/CPT-induced MKP-1 expression and augmented JNK1/2 activation and significantly increased apoptosis as judged by DNA fragmentation in polyamine depleted cells (data not shown) similar to that seen in control cells (Fig. 5b). These results clearly indicate that the MEK/ERK activity determines the levels of MKP-1 protein, and thereby, the activity of JNK and p38 kinases. Furthermore, cells treated with SA for 3h to cause accumulation of MKP-1 were washed and further incubated with DMSO, U0126 or cycloheximide (CHX) for indicated time period were analyzed to determine the stability of MKP-1 protein (Fig. 7). U0126 rapidly decreased the MKP-1 protein within 45 minutes compared to that seen in cells treated with SA for 3h (0 min.). CHX (inhibitor of protein synthesis) showed a robust reduction in the levels of MKP-1, which was greater than that seen in the presence of DMSO (Fig. 7). These results demonstrate that MKP-1 levels are predominantly regulated by the activity of MEK/ERK.
Fig. 6. TNF/CPT-induced JNK1/2 and apoptosis in Polyamine depleted cells.

(a) IEC-6 cells were grown to confluence in control, DFMO and DFMO + PUT (DP) containing media for 3 days followed by serum starvation for 24h. Confluent monolayers were left untreated (UT) or treated with TNF/CPT for 3h. Whole cell extracts (25 μg) were subjected to 10% or 15% SDS-PAGE followed by western blot analysis for detection of MKP-1, total and phosphorylated JNK1/2 and ERK1/2, procaspase-9 and PP2A. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations. (b) Confluent serum starved cells grown into control and DFMO media were treated as described above. DNA fragmentation was measured using a colorimetric ELISA kit as described in the methods. Values are means ± SE of triplicates. #, p < 0.05 compared with respective UT groups.
Fig. 7. Effect of MEK1 and ERK1/2 inhibition on the stability of MKP-1 protein.

Confluent serum starved IEC-6 cells were pretreated with SA for 3h followed by washing and further incubation in the presence of DMSO, U0126 (10 μM), or cycloheximide (CHX, 25μgh/ml) for the indicated time period. Whole cell extracts (25 μg) were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1 and phosphorylated ERK1/2. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations.
MKP-1 association with JNK1/2 and PP2Ac
Serine/threonine phosphorylation of MKP-1 has been shown to regulate its activity and stability [27]. Western blot analysis (Figs. 1 and 3) detected two bands suggesting that MKP-1 might be phosphorylated in response to apoptotic inducers. We have shown that inhibition of PP2A activity by okadaic acid augments ERK1/2 and prevents JNK1/2 activity, decreasing apoptosis [27]. Therefore, serine/threonine protein phosphatases (PP2A) might regulate the activity of MKP-1 and JNK1/2. We used sepharose-conjugated microcystin (MC-sepharose) to bind and pull down the catalytic subunit of PP2A (PP2Ac) and then detected the proteins associated with it. Input cell extracts from untreated (UT) and TNF/CPT groups and lysis buffer as a mock control (M) were used for MC-sepharose pull-down and analyzed by western blotting to detect the levels of MKP-1, phospho-JNK1/2, phospho-ERK1/2, and PP2Ac. Equal amounts of PP2Ac were bound to MC-sepharose in the UT and TNF/CPT groups, and the levels of PP2Ac were unchanged in whole cell extract (input) (Fig.8A). TNF/CPT increased both the p-ERK1/2 and p-JNK1/2 dramatically, and that p-JNK1/2 and phospho-ERK2 were bound to PP2A only in these cells. However, the pattern of MKP-1 remained constant from input extract to samples bound to PP2Ac. These results suggest that MKP-1, phospho-JNK1/2, p-ERK2 and PP2Ac formed a complex in response to the apoptotic stimulus.
Fig. 8. Association of MKP-1, p-ERK1/2, p-JNK1/2 and PP2A.
(a) IEC-6 cells were grown as described in methods. Confluent serum starved cells were left untreated (UT) or exposed to TNF/CPT for 3h. and were washed with ice-cold dPBS and lysed using MPER containing protease inhibitors. Equal amounts of cell extracts (500 μg) were incubated with 50 μl microcystine-sepharose (MC-sepharose) for 2 hrs at 4°C followed by washing twice with MPER. Proteins bound to MC-sepharose were eluted using SDS sample buffer and subjected to 10% SDS-PAGE along with input cell extracts (25 μg) followed by western blot analysis to detect the levels of MKP-1, phosphorylated JNK1/2, phosphorylated ERK1/2, and PP2A. (b) Input extracts were preincubated with Okadaic acid (OA) (100nM) or SA (10 μM) for 30 minutes at room temperature followed by MC-sepharose pull down as described above. Whole cell extracts (25μg) and MC-sepharose pull-down samples were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1, phosphorylated JNK1/2, phosphorylated ERK1/2, and PP2A. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations.
Although, we have established that MKP-1 dephosphorylates JNK1/2 and is associated with PP2Ac, we wanted to determine whether the enzymatic activity of these phosphatases is required for their interaction. Cell extracts identical to those in figure 8A (input) were preincubated with okadaic acid (OA) or SA for 30 minutes to inhibit the activities of PP2Ac and MKP-1 respectively and were used for pull down. Figure 8B shows that the amounts of MKP-1, phospho-ERK2 and phospho-JNK1/2 bound to PP2Ac were unchanged in extracts pretreated with OA. However, inhibition of MKP-1 by SA decreased the association of MKP-1 and, thereby, phospho-JNK1/2 with PP2Ac. The association of ERK2 was unaltered in OA or SA treated cell extracts. These results suggest that MKP-1 activity is necessary for the association of both the JNK and itself with PP2Ac.
Since MKP-1 is an inducible nuclear protein [16–23] and is induced in response to CPT and TNF/CPT, we determined its localization in nuclear and cytoplasmic fractions by western blotting. MKP-1 was present in the nuclear fraction of both untreated and CPT treated cells, while inactivated MKP-1 (SA treated) was exclusively found in the cytoplasmic fraction (Fig. 9). This finding suggests that inhibition of MKP-1 activity prevents its translocation to the nucleus. Immunolocalization also confirmed that both in the untreated and CPT treated cells, MKP-1 localized to the nucleus (Fig. 9B, left panel). However, MKP-1 localized in the form of bright patches within nuclei of CPT treated cells exhibiting condensation of nuclei, a typical morphological feature of apoptotic cells (Fig. 9B, right panel), suggesting its role in nuclear events associated with apoptosis. Furthermore, nuclear localization of MKP-1 in untreated cells also suggests its role in the regulation of MAPKs in the nucleus during proliferation.
Fig. 9. Nuclear and cytoplasmic localization of MKP-1.
(a) Confluent serum starved cells left untreated (UT) or exposed to CPT or SA for 3h were used to prepare nuclear, cytoplasmic and whole cell extracts as described in the methods. Cytoplasmic extracts (25 μg), nuclear extracts (15 μg), and whole cell extracts (25 μg) were subjected to 10% SDS-PAGE followed by western blot analysis to detect MKP-1. Actin immunoblotting was performed as an internal control for equal loading. Blots shown are representative of 3 observations. (b) IEC-6 cells grown on poly-L-lysine-coated coverslips in control media for 3 days followed by serum starvation for 24h were left untreated or treated with CPT for 3h. Cells were fixed, permeabilized, and stained using MKP-1 specific primary antibody followed by an appropriate secondary antibody conjugated with fluorophore. Coverslips were mounted on glass slides and images were captured using CCD camera attachment with a Nikon microscope. Representative images from three experiments carried out in triplicate are shown.
Discussion
MAPKs are sub-grouped into growth factor–activated extracellular signal regulated kinases (ERK1/2) and stress-activated MAP kinases (SAPKs) c-Jun N-terminal kinase (JNK, SAPK1) and p38 (SAPK2) [28–32]. Together they are involved in various cellular functions, such as proliferation, differentiation, inflammation, and apoptosis [33–37]. Mitogen-activated protein kinase kinases (MEK) MEK1 and MEK4/7 activate ERK1/2 and JNK1/2 respectively. Protein-tyrosine phosphatases and dual specificity (threonine/tyrosine) protein phosphatases dephosphorylate the activated MAPKs and inactivate them [17, 18]. Thus, the activity of MEKs and protein phosphatases determines the signaling output of MAPKs. The dual specificity MAPK phosphatase, MKP-1, a nuclear inducible protein, dephosphorylates and inactivates MAP kinases and SAPK in vivo and in vitro [16–24]. Over expression of MKP-1 protected cells against apoptosis by inhibiting JNK activity [20, 38]. Thus the activities of MAP kinases are tightly regulated by MKP-1.
Over the years, we have shown that polyamine depletion inhibits proliferation [5, 6], apoptosis [8, 11, 12, 26, 39, 40] and migration [4, 7] in intestinal epithelial cells. The resistance of polyamine-depleted cells to apoptotic agents is due to their inability to induce sustained JNK1/2 activity [26]. However, in response to apoptotic stimuli, ERK1/2 activation was observed earlier in polyamine-depleted cells and to a significantly greater extent than the activation of JNK1/2 and p38 [11]. Inhibition of MEK1 and, thereby, ERK1/2 sustained the activation of JNK1/2 and increased CPT-induced apoptosis in polyamine depleted cells. These results suggest that MEK1/ERK might regulate the intensity and duration of JNK1/2 activity required for the induction of apoptosis in the absence of polyamines. SiRNA-mediated knockdown of MKP-1 increased the activities of JNK1/2 and p38, and apoptosis in response to TNF/CPT [13]. Based on these findings, we hypothesized that polyamine depletion increases MKP-1 activity augmenting the dephosphorylation of JNK1/2 and p38, and, thereby, decreasing apoptosis.
Our current results demonstrate that MKP-1 protein is undetectable in normal serum starved cells, and that apoptotic stimuli induce MKP-1 early (3h) while it declines later (9h) as apoptosis progressed and JNK1/2 activity increased (Fig. 1, 9h CPT). These findings are consistent with earlier reports showing that MKP-1 was undetectable in quiescent cells, but that its expression increased in response to serum or growth factors [41]. Inhibition of MKP-1 activity by SA increased MEK1, ERK1/2, JNK1/2 and p38 in control cells suggesting that MKP-1 dephosphorylates and inactivates these MAPKs. However, in polyamine-depleted cells, SA had a stronger effect, and the activation of MAPKS occurred earlier compared to control cells (Fig. 2). Restoration of MAPK activity to control levels by putrescine indicates that polyamines play a crucial role in maintaining the balance of MAPKs by regulating the activity of MKP-1. Although, the activation of MAPKs by SA had no direct effect on survival in the absence of CPT (Fig. 2), prior activation of MAPKs significantly enhanced apoptosis (Fig. 2A and B, lane 4).
There is strong evidence that ERKs regulate the levels of MKP-1 protein. Guo et al. showed that nocodazol, a benzimidazole derivative, which rapidly depolymerizes microtubules increased ERK1/2 activity, MKP-1 expression and decreased TNF-induced p38 activity in Rat-1 cells [14]. Brondello et al. [42] demonstrated that levels of MKP-1 and MKP-2 were increased by ERK1/2 and that the p42/p44 MAPK-dependent phosphorylation of MKP1/2 decreased its degradation. Inhibition of MEK1 by U0126 prevented the induction of MKP-1 by SA (Fig. 5) and CPT-induced MKP-1 expression and augmented JNK1/2 activity and apoptosis (Fig. 6). SA induced MKP-1 protein decreased within 45 min. exposure to U0126. However, the inhibition of protein synthesis by CHX decreased MKP-1 protein in a time dependent manner at a rate similar to that seen with DMSO (Fig. 7). These results clearly demonstrate that MEK1 regulates MKP-1, controlling the phosphorylation of JNK1/2 and p38 during apoptosis.
Polyamine depleted cells exposed to TNF/CPT had increased MKP-1 and decreased JNK1/2 activity and apoptosis (Fig. 6). Decreased MKP-1 following the inhibition of ERK1/2 or MEK1 in polyamine-depleted cells significantly increased apoptosis (data not shown) indicating that MKP-1 is crucial for the survival of these cells. Furthermore, these results also indicate that the levels of activated MAPKs and MKP-1 protein are interdependent and maintain the strength and duration of apoptotic and survival signals. For example, both EGF and TNF-α transiently activate MAPKs in IEC-6 cells. Within 15 minutes, TNF-α increased ERK1/2 and JNK1/2 activities, which immediately returned to basal levels due to increased MKP-1 activity [13]. However, inhibition of MKP-1 in response to MEK1 prolonged the activation of JNK1/2 and p38 and enhanced apoptosis. In addition, the overexpression of MKP-1 has been shown to protect cells from apoptosis by inhibiting JNK1/2 [20, 31].
Since MKP-1 dephosphorylates JNK1/2, we anticipated that MKP-1 would bind phospho-JNK1/2 in response to inducers of apoptosis. Furthermore, reduced SDS-polyacrylamide gel electrophoretic mobility of MKP-1 in response to CPT, TNF/CPT, and SA suggested that MKP-1 phosphorylation might be involved in the regulation of its levels and/or activity. Brondello et al. [42] showed that p44MAPK phosphorylated MKP-1 on serine residues S-359 and S-364, and both the phosphorylated and un-phosphorylated forms hydrolyzed p-nitro-phenyl phosphate (pNPP) to a similar extent and also dephosphorylated p44MAPK suggesting the both were active [42]. Our finding that the inhibition of PP2A by OA increased ERK1/2 activity and prevented JNK activity and apoptosis [12] suggests that PP2A might play a role in the dephosphorylation of MAPKs by MKP-1. MC-sepharose pull-down showed that MKP-1 and phosphorylated forms of both the ERK2 and JNK1/2 associate with PP2Ac during TNF/CPT-induced apoptosis (Fig. 8). While the inhibition of PP2A activity by OA proved not to be essential for the association of these enzymes, inhibiting MKP-1 with SA prior to the pull down prevented its association with both PP2A and JNK1/2. These results suggest a mechanism whereby PP2A might dephosphorylate MKP-1 decreasing its stability and activity and thereby increasing the activities of MAPKs.
Although, MKP-1, a nuclear inducible protein, regulates apoptosis via JNK1/2 and p38, it is unclear whether MKP-1 regulates JNK1/2-mediated nuclear events or whether it translocates to the cytoplasm to dephosphorylate MAPKs. We have shown that the inactive form of MKP-1 (SA treated) remained in the cytoplasmic fraction, while it predominantly localized in the nuclear fraction in untreated and CPT treated cells (Fig. 9). These data indicate that MKP-1 might shuttle between the cytoplasm and the nucleus, which is consistent with the availability of its substrates in both the compartments. Nuclear ERK1/2 and JNK1/2 regulate the activities of transcription factors and, thereby, cellular function, including proliferation and apoptosis depending upon the intracellular and extracellular environments [28].
Previously we have shown that CPT significantly increases the levels of p53 and its phosphorylation during apoptosis [40]. Furthermore, TNF/CHX-induced JNK1/2 activity phosphorylates and inactivates Bcl-2, leading to cytochrome c release, caspase-9 activation, and apoptosis [26]. The current data indicate that ERK1/2 phosphorylates and stabilizes MKP-1 in control and polyamine depleted cells, leading to the dephosphorylation and inactivation of JNK and p38. This in turn inhibits the ability of these enzymes to stimulate apoptosis by modifying the activities of p53, Bax and Bcl-2.
Acknowledgments
This publication was made possible by grant DK-16505 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. This work was also supported by the Thomas Gerwin endowment.
Footnotes
Conflict of interest The authors declare that there is no conflict of interest.
References
- 1.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 2.Yang P, Baylin SB, Luk GD. Polyamines and intestinal growth: absolute requirement for ODC activity in adaptation during lactation. Am J Physiol. 1984;247:G553–G557. doi: 10.1152/ajpgi.1984.247.5.G553. [DOI] [PubMed] [Google Scholar]
- 3.Luk GD, Baylin SB. Polyamines and intestinal growth-increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983;245:G656–G660. doi: 10.1152/ajpgi.1983.245.5.G656. [DOI] [PubMed] [Google Scholar]
- 4.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990;259:G584–G592. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- 5.Ray RM, McCormack SA, Johnson LR. Polyamine depletion arrests growth of IEC-6 and Caco-2 cells by different mechanisms. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G37–G43. doi: 10.1152/ajpgi.2001.281.1.G37. [DOI] [PubMed] [Google Scholar]
- 6.Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Polyamine depletion arrests cell cycle and induces inhibitors p21 (waf1/Cip1), p27 (Kip1), and p53 in IEC-6 cells. Am J Physiol. 1999;276(3 Pt 1):C684–C691. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- 7.Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, Johnson LR. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem. 2003;278(15):13039–13046. doi: 10.1074/jbc.M208741200. [DOI] [PubMed] [Google Scholar]
- 8.Ray RM, Viar MJ, Yuan Q, Johnson LR. Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am J Physiol Cell Physiol. 2000;278(3):C480–C489. doi: 10.1152/ajpcell.2000.278.3.C480. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka K. Critical role of polyamines in Cancer: Basic mechanisms and clinical approaches. Cancer Res. 1993;53:2689–2692. [PubMed] [Google Scholar]
- 10.Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-α-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol. 2004;286:479–490. doi: 10.1152/ajpgi.00342.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ray RM, Bhattacharya S, Johnson LR. Protein phosphatase 2A regulates apoptosis in intestinal epithelial cells. J Biol Chem. 2005;280:31091–31100. doi: 10.1074/jbc.M503041200. [DOI] [PubMed] [Google Scholar]
- 13.Ray RM, Jin S, Bavaria MN, Johnson LR. Regulation of JNK activity in the apoptotic response of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G761–G770. doi: 10.1152/ajpgi.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Zhang X, Li Y, Guo Y, Wang J, Li Y, Shen B, Sun D, Zhang J. Nocodazole increases the ERK activity to enhance MKP-1 expression which inhibits p38 activation induced by TNF-α. Mol Cell Biochem. 2012;364:373–380. doi: 10.1007/s11010-012-1239-5. [DOI] [PubMed] [Google Scholar]
- 15.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epitheloid cell cultures from rat small intestine: Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase, which potently and specifically inactivates MAP kinase and suppresses its activity by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- 17.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 18.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 Cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 20.Franklin CC, Shrikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatese-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress: Inhibition of c-Jun N-terminal kinase activity and AP-1 dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 23.Raigeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinases in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 25.Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr phosphatase. J Biol Chem. 1993;268:16116–16119. [PubMed] [Google Scholar]
- 26.Bhattacharya S, Ray RM, Viar MJ, Johnson LR. Polyamines are required for activation of c-Jun NH2-terminal kinase and apoptosis in response to TNF-α in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G980–G991. doi: 10.1152/ajpgi.00206.2003. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Preheating accelerates mitogen-activated protein (MAP) kinase inactivation post-heat shock via a heat shock protein-70-mediated increase in phosphorylated MAP kinase phosphatase-1. J Biol Chem. 2005;280:13179–13186. doi: 10.1074/jbc.M410059200. [DOI] [PubMed] [Google Scholar]
- 28.Abe MK, Kuo WL, Hershenson MB, Rosner MR. Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization, and cell growth. Mol Cell Biol. 1999;19:1301–1312. doi: 10.1128/mcb.19.2.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 30.Karin M, Lin A. NF-kappa B at the crossroads of life and death. Nat immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 32.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 33.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet c and gamma radiation: Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 34.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 35.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl-2 related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 37.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Perez I, Martinez-Gomariz M, Williams D, Keyse SM, Perona R. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene. 2000;19:5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya S, Ray RM, Johnson LR. Decreased apoptosis in polyamine depleted IEC-6 cells depends on Akt-mediated NF-κB activation but not GSK3β activity. Apoptosis. 2005;10:759–776. doi: 10.1007/s10495-005-2943-3. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya S, Ray RM, Johnson LR. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal. 2009;21:509–522. doi: 10.1016/j.cellsig.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-sependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 42.Brondello J, Brunet A, Pouyssegur J, McKenzie FR. The dual Specificity Mitogen-activated protein kinase Phosphtase-1 and -2 are induced by the p42/p44 MAPK Cascade. J Biol Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]