INTRODUCTION
Xenopus laevis is an ideal model system for investigating dynamic morphogenetic processes during embryogenesis, regeneration, and homeostasis. Our understanding of these events have been greatly facilitated by lineage labeling – marking a cell or a group of cells and all their descendants using vital dyes, fluorescent molecules and transplantation techniques. Unfortunately, these techniques are limited in their spatiotemporal resolution: they do not allow long-term dynamic in vivo imaging, they are restricted to labeling cells on the surface, and are generally invasive. Genetically encoded fluorescent proteins (FPs), on the other hand, provide excellent alternative methods to traditional lineage labeling, enabling high spatio-temporal resolution labeling and tracking of cellular and subcellular structures to study patterning events. Over the past decade, FPs have evolved to allow fine control of their spectral properties – in a defined region of interest – for a greater labeling specificity. One example is EosFP; cloned from the scleractinian coral Lobophyllia hemprichii, this protein can be photoconverted from green to red fluorescence state with near UV light irradiation. Here, we describe EosFPphotoconversion of Xenopus embryos to track cells during developmental and regenerative processes, using metal halide and xenon arc-based fluorescent microscope system, which provides a simpler, less expensive alternative to photoconversion using laser microscopy.
RELATED INFORMATION
A Green to Red Photoconvertible Protein as an Analyzing Tool for Early Vertebrate Development (Wacker et al. 2007) provides an introduction to application of EosFP as a tool for the detailed analysis of early Xenopus development. Photoconvertible Fluorescent Protein EosFP: Biophysical Properties and Cell Biology Applications (Nienhaus et al. 2006) presents the spectroscopic properties of multiple EosFP variants and their applications in in vivo monitoring of cellular processes.
MATERIALS
Recipes and Caution: Please see below for recipes of reagents marked with <R> and cautions marked with <!>.
RECIPE
Marc’s modified Ringer’s (MMR) (1X)
0.1 M NaCl
<!>2.0 mM KCl
<!>1.0 mM MgSO4
<!>2.0 mM CaCl2
5.0 mM HEPES (pH 7.8)
0.1 mM EDTA
CAUTIONS
Calcium chloride
is harmful by inhalation, ingestion, or skin absorption. Wear appropriate personal protective equipment (PPE) including gloves and safety glasses. Use in a chemical fume hood.
Magnesium Sulfate
may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate PPE including gloves and safety glasses.
Potassium Chloride
may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate PPE including gloves and safety glasses.
Reagents
Ficoll solution (3% Ficoll in 1x Marc’s Modified Ringer’s (MMR))
Gentamycin
<R> MMR (1X)
mRNA delivery solution (1 0.5–1 µg/µl mRNA in H2O and 0.2 3mM Alexa Fluor 647 dextran 3000 in H2O)
-
mRNA encoding EosFP
For expression in Xenopus, capped mRNA was synthesized in vitro – from linearized pCS2+-d2EosFP – using the mMESSAGE mMACHINE kit (Ambion) following the manufacturer’s instruction.
pCS2+-d2EosFP (EosFP and its variants are available from Mobitec and its local distributors http://www.mobitec.de/de/index_distributors.html)
-
Tricaine Methanesulfonate (MS222) (0.03% prepared in 0.1x MMR and adjusted to physiological pH)
Here, we used an MS222 solution adjusted to a pH of 7.6.
-
Xenopus embryos/tadpoles, stage of interest
In this protocol, Xenopus embryo stages are given according to Nieuwkoop and Faber (Nieuwkoop and Faber 1967).
Equipment
Coverslip (e.g., No. 1.5, 24 × 50mm by Globe Scientific INC.)
Depression slide, glass (1″×3″ × 1.2mm thick with depression of 15mm in diameter)
Forceps, fine (for breaking needles)
Image processing software (e.g., Adobe Photoshop or Image J)
Injection needles (pulled glass capillaries)
Injector (e.g., Medical Systems PLI-100)
-
Metal halide, Mercury or Xenon fluorescent illumination sources
Here Lumen 200 metal halide illumination source by PRIOR Scientific and U-LH75XEAPO Xenon lamp by Olympus were used for photoconversion.
Micromanipulator, for injection (e.g., M3301R; World Precision Instruments)
Micropipettor and tips
Microscope, fluorescent dissection equipped with FITC and TRITC filtersets
-
Microscope, fluorescent, equipped with the necessary filter sets to visualize and photoconvert EosFP.
Here, an Olympus BX-61 Spinning Disc confocal microscope equipped with a Hamamatsu ORCA AG CCD camera, controlled by MetaMorph software, was used to visualize green and red fluorescent state of EosFP using the filters, EX 470/20; BS 485; EM 517/23, and EX 545/20; BS 565; EM 595/50. A DAPI filter set (EX 350/50; BS 400; EM 460/50) was used to achieve photoconversion.
Paper towels
Petri dishes (e.g., Fischerbrand 100mm × 15mm dishes)
Pipettes, disposable transfer
METHOD
Expressing EosFP in Xenopus embryos
-
1)
Synthesize capped mRNA in vitro – from linearized pCS2+-d2EosFP – using the mMESSAGE mMACHINE kit (Ambion) following the manufacturer’s instructions.
-
2)
Adjust micromanipulator (with needle) position so that it is ~ 450 with respect to the base of the dissection scope.
-
3)
Calibrate needle (e.g., using fine forceps, break needle so that it outputs 2–10 nl solution per injection).
Use needles with <1µm diameter at the tip to limit tissue damage and prevent mRNA leakage once needle is removed following injection.
-
4)
Fill needle with EosFP mRNA (e.g., 0.5 µl of 0.5–1 µg/µl concentration) solution using the “fill” button on the injector.
Keep needle tip in water to prevent clogging.
-
5)
Transfer fertilized 1cell embryos into a petri dish with Ficoll solution.
Alternatively, two or four-cell embryos can be used; however, injection volume should be limited to 2–4 nl per cell to prevent cytotoxicity.
-
6)
Inject in the 3% Ficoll solution, by holding embryo with a pair of forceps on 1 side and moving the needle on the other.
Alternatively, mesh-bottomed dish can be used to organize and inject embryos in rows and columns, enabling accurate tracking of injected embryos.
-
7)
Transfer injected embryos to a fresh 3% Ficoll solution.
-
8)
After at least an hour, transfer healthy embryos to 0.1X MMR + gentamycin (1λ/1ml) solution.
-
9)
Raise embryos at 14 or 18°C until they reach the desired developmental stage of interest.
Embryos should be kept in the dark to prevent unwanted photoconversion and photobleaching (although EosFP is extremely photostable; it exhibits long-lasting, photobleaching-resistant fluorescence).
-
10)
Monitor the expression of EosFP using the fluorescent dissecting microscope equipped with FITC filter set.
Expression of EosFP can be fluorescently detected as early as four hours post injection.
Pre-photoconversion set up
-
11)
Anesthetize EosFP-expressing tailbud/tadpole stage animals in 0.03% MS222 (~5min).
Keep anesthetized embryos in MS222during mounting, photoconversion and imaging.
Skip Step 11 for pre-tailbud embryos.
-
12)
Mount anesthetized or pre-tailbud animal by pipetting it into a depression slide; fill depression completely with MS222 solution or plain MMR, depending on the animal’s developmental stage.
If using an inverted microscope, a glass-bottomed dish may be used as an alternative for mounting and photoconversion.
-
13)
Orient animal with forceps so that region of interest for photoconversion is facing up (for an upright fluorescent microscope).
-
14)
Place a coverslip onto the depression slide with your sample.
-
15)
Draw off excess mounting solution using a paper towel, to seal the coverslip.
Photoconversion
While photoconversion of EosFP-expressing mammalian cells using laser microscopy (Wiedenmann et al. 2004) have been described, here we demonstrate a simpler, less expensive photoconversion procedure using metal halide and xenon arc-based fluorescent microscope system. Photoconversion using mercury arc lamp can be achieved in a similar fashion to metal halide photoconversion, given the near-identical emission spectra of the two illumination sources.
-
16)
After identifying the source of illumination to be used, use FITC filter set (EX 470/20; BS 485; EM 517/23) to select a region of interest.
When possible, use attenuated light to minimize photobleaching.
-
17)
Move region of interest to the center of the viewing field; close the field diaphragm to its smallest opening to make a photoconvertible spot.
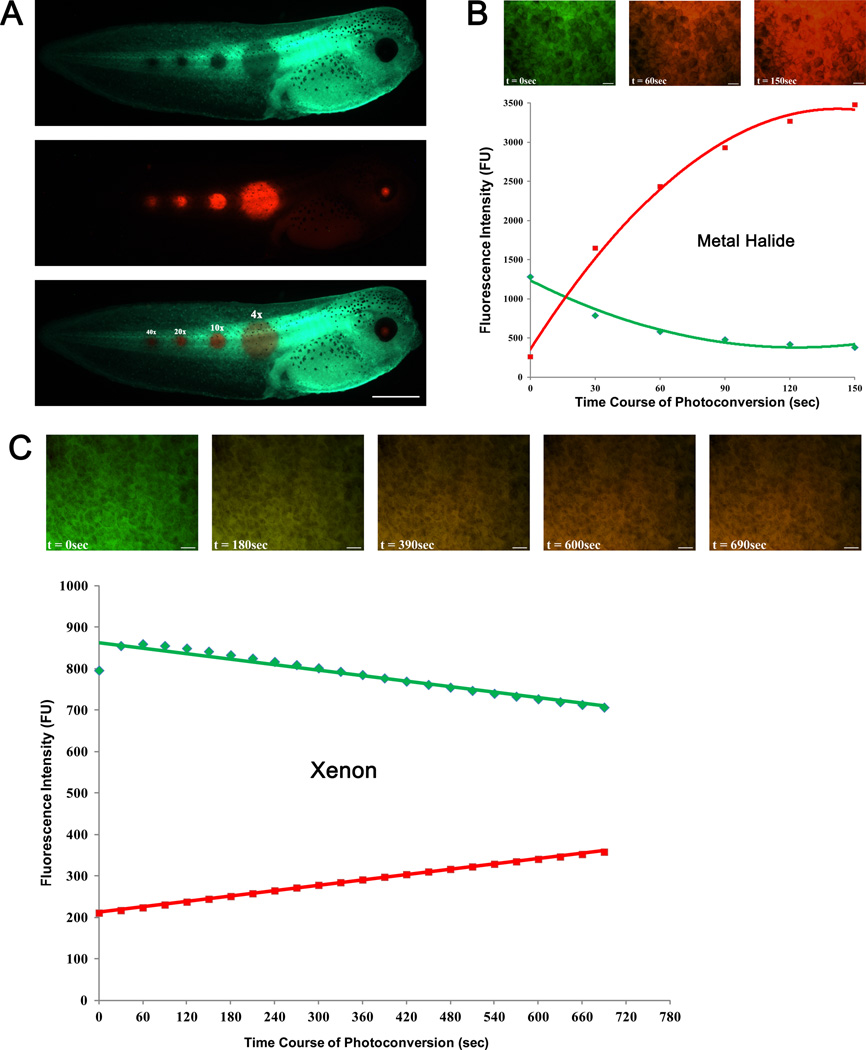
Fig. 1A shows different sizes of photoconverted spots that are generated using Olympus UPlanSApo 4x, 10x, 20x and 40x objective lenses.
-
18)
Photoconvert by irradiating the spot with UV light passed through DAPI filter set (EX 350/50; BS 400; EM 460/50). Complete photoconversion can be achieved with 60-90 seconds (for metal halide) exposure to UV light (Fig. 1B).
Incomplete photoconversion is observed with >10 minutes of UV light irradiation from a xenon lamp (Fig. 1C). See troubleshooting.
FIGURE 1.
Determining size, intensity and time course of EosFP photoconversion. (A) Photoconversion of circular area of diameters 600 µm (4x), 250 µm (10x), 130 µm (20x) and 80 µm(40x) using Olympus UPlanSApo objective is shown. FITC (I) and TRITC (II) images were acquired; the two images were overlaid (III) for simultaneous detection of both fluorescent signals. (B and C) Graphs show time course of EosFP photoconversion using metal halide and xenon lamps, respectively. Solid green and red lines represent intensities of green and red fluorescence, respectively. The inserts show overlaid FITC and TRITC images at select time points. Scale bar = 1 mm in A, 50 µm in B, C.
Imaging and tracking photoconverted cells
-
19)
Re-open the field diaphragm to its maximum aperture, and acquire green and red fluorescence images of the photoconverted sample.
-
20)
Carefully remove the coverslip by adding few drops of MMR on the edges and sliding it sideways until you can lift it. Transfer the animal to fresh 0.1xMMR and allow it to develop in a 14-22 °C incubator, depending on your requirement for developmental speed, to whatever stage desired.
-
21)
Acquire green and red fluorescence images at regular intervals to track photoconverted cells.
EosFP is very photostable, both in its green and red state, enabling tracking of cells for more than 10 days without significant reduction in fluorescence.
-
22)
Overlay green and red fluorescence images using image processing software (e.g., Adobe Photoshop, Image J, Metamorph, Axiovision or NIS-Elements).
TROUBLESHOOTING
Problem: Injection/expression of EosFP results in developmental defects.
[Steps 3-10]
Solution: 1) Consider lowering the concentration and/or volume of injected mRNA.
Problem: EosFP-expressing cells die after photoconversion
[Step 18]
Photoconversion of EosFP peaks at near-UV wavelength irradiation, which can be phototoxic to cells.
Solution: 1) Optimize step 18 for your fluorescent light source to determine the exposure time required to achieve complete photoconversion while limiting phototoxicity. Take into consideration the type and power (Watts) of the bulb you are using. For example, a 75 W xenon lamp is limited by its power and poor emission near UV wavelength, making it difficult to achieve photoconversion in fast enough that is less phototoxic to cells (Fig. 1C). On the other hand, photoconversion by a 200 W metal halide bulb can be achieved in just 60 seconds, minimizing phototoxicity while maximizing efficiency (Fig. 1B).
2) Consider slightly shifting irradiation wavelength – using different filter sets – to achieve photoconversion. For example, photoconversion can be achieved by irradiating cells, for ~120 seconds, using excitation light from a metal halide lamp passed through Chroma filter set 31036 (EX 405/20; BS 425; EM 460/50). Alternatively, photoconversion can be achieved by irradiating cells with 405-425nm light using a BP430/50 excitation filter and 425 DCLP beam splitter (Wacker et al. 2007).
DISCUSSION
Expected Results
By following the steps outlined in this protocol, highly efficient and stable photoconversion of EosFP-expressing cells can be achieved, using fluorescent microscope with a standard DAPI filter set, and metal halide or mercury arc-based illumination source. The high spatio-temporal resolution cell labeling provided by photoconversion is useful in investigating dynamic morphogenetic and patterning events, which we demonstrate here using EosFP to visualize eye and anterior spinal cord morphogenesis (Fig. 2 and (Pai et al. 2011)), and trace cellular origins of regenerating Xenopus tail following amputation (Fig. 3). In both cases, photoconversion proves to be superior to traditional labeling techniques which combine transgenesis and grafting to study developmental and regenerative processes. Importantly, expression level and fluorescence of EosFP (resistance to photobleaching) remain high enough to allow the investigation of patterning events in advanced stage tadpoles; the observed stability of EosFP and suitability for older Xenopus tadpoles expands the utility of this technique, complementing the array of EosFP applications for early stage Xenopus embryos, described by Wacker et al 2007. In addition, when compared to other photoconvertible proteins, EosFP is relatively brighter, and its monomeric structure makes it an ideal protein tag for studying spatial and temporal dynamics of cellular events. A comparison of properties of several photoconvertible proteins can be found at http://www.microscopyu.com/articles/livecellimaging/fpintro.html
FIGURE 2.
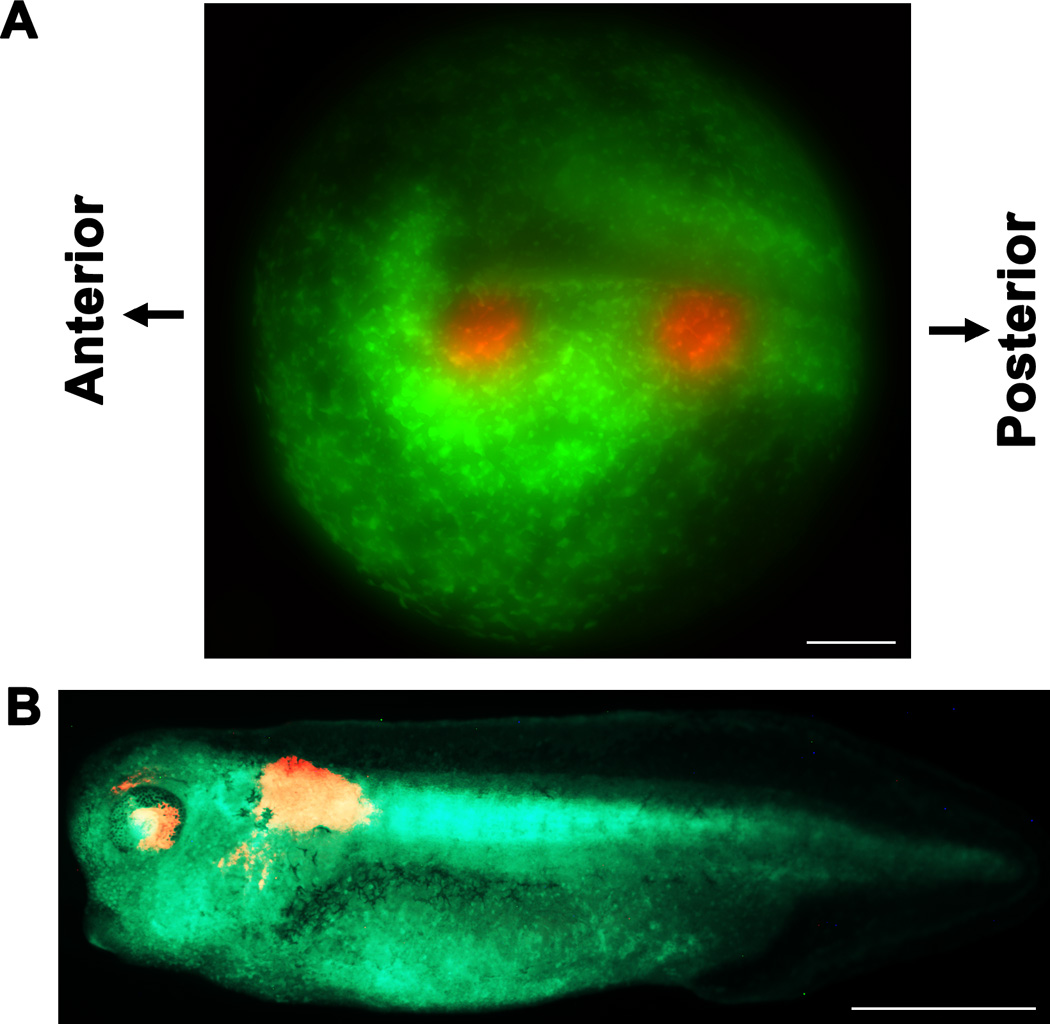
Photoconversion of target regions in stage 13 neural plate. (A) Overlaid FITC and TRITC image shows photoconversion of presumptive left eye and anterior spinal cord regions. (B) Photoconverted cells are detected in the appropriate regions in a stage 35 embryo. Scale bar = 150 µm in A, 1mm in B.
FIGURE 3.
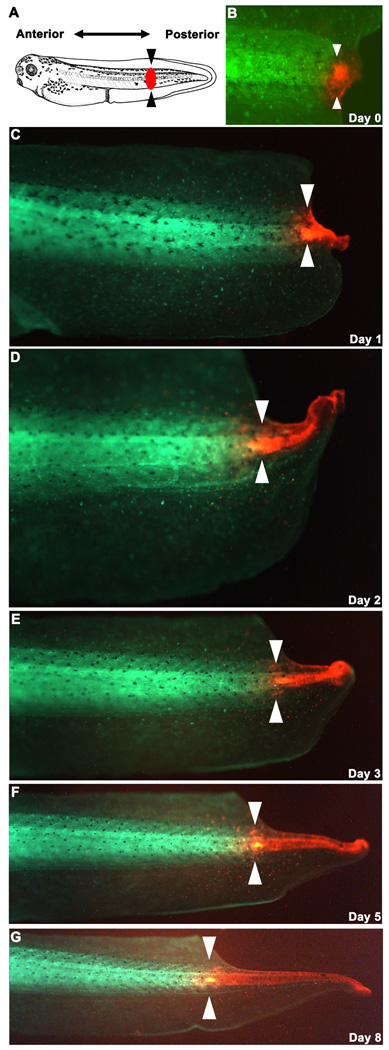
Lineage tracing during tail regeneration. (A) The tail of a stage 40 embryo (image source: Xenbase database; Bowes et al. 2009) injected with EoSFP at 1 cell was amputated through the black arrowheads. The red spot represents where blastema (mass of undifferentiated cells) starts to form following amputation. (B) The blastema was photoconverted 24 hours-post-amputation, and regeneration was followed for the following 8 days (C-G). By applying the simple technique of photoconversion, Fig. 3 panels reveal that tissues in the regenerating tails arise largely from the descendants of cells within the blastema, not as a result of significant migration of anterior tissues into the regeneration bud.
Limitations and Special Considerations
With the inherent limitation in optical resolution of standard fluorescent microscopes, it is expected that photoactivation will not be restricted entirely to the focal plane. However, this limitation does not prevent the method from being used to track a group of cells in many applications.
The smallest region that can be photoconverted is limited to a circular area of diameter of about 80 µm (achieved using UV-light passing through a maximally closed field diaphragm to a 40x objective lens). Photoconversion of a smaller region may be achieved using a slit (e.g., Olympus DSU slit-spinning disk) at the focal plane of excitation light. Alternatively, a more efficient but expensive method of laser microscopy can be employed to photoconvert smaller regions in a desired pattern. The later suggestion is especially valuable for photoconverting and tracking EosFP-fused proteins when investigating developmental and regenerative processes at the subcellular level.
ACKNOWLEDGEMENTS
We thank Franz Oswald for the kind gift of EosFP construct. Grant Sponsors: NIH (HD055850), NHTSA (DTNH22-06-G-00001), TATRC-USAMRMC (W81XWH-10-2-0058), and the G. Harold and Leila Y. Mathers Foundation. DSA gratefully acknowledges NIH K22-DE016633.
REFERENCES
- Bowes JB, Snyder KA, Segerdell E, Jarabek CJ, Azam K, Zorn AM, Vize PD. Xenbase: gene expression and improved integration. Nucleic Acids Res. 2009;38((Database issue)):D607–D612. doi: 10.1093/nar/gkp953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus GU, Nienhaus K, Holzle A, Ivanchenko S, Renzi F, Oswald F, Wolff M, Schmitt F, Rocker C, Vallone B, Weidemann W, Heilker R, Nar H, Wiedenmann J. Photoconvertible fluorescent protein EosFP: biophysical properties and cell biology applications. Photochem Photobiol. 2006;82(2):351–358. doi: 10.1562/2005-05-19-RA-533. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis (Daudin) Amesterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2011 doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker SA, Oswald F, Wiedenmann J, Knochel W. A green to red photoconvertible protein as an analyzing tool for early vertebrate development. Dev Dyn. 2007;236(2):473–480. doi: 10.1002/dvdy.20955. [DOI] [PubMed] [Google Scholar]
- Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible greento- red fluorescence conversion. Proc Natl Acad Sci U S A. 2004;101(45):15905–15910. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]