Abstract
Objective
Harm avoidance, a trait indicative of behavioral inhibition, is associated with disability and dementia in old age, but the basis of these associations is uncertain. We test the hypothesis that higher level of harm avoidance is associated with increased likelihood of cerebral infarction.
Methods
Older persons without dementia completed a standard measure of harm avoidance. During a mean of 3.5 years of follow-up, 257 (of 1,082) individuals died of whom 206 (80%) underwent brain autopsy. Number of chronic cerebral infarcts (microscopic plus gross; expressed as 0,1, or >1) was assessed on neuropathologic examination, completed in 192 individuals at the time of analyses.
Results
On postmortem examination, chronic cerebral infarcts were found in 89 (42 with 1, 47 with >1). Higher harm avoidance was associated with higher likelihood of infarcts (odds ratio = 1.083, 95% confidence interval 1.040–1.128). A moderately high level of the trait (score=17, 75th percentile) was associated with a 2.4-fold increase in the likelihood of infarction compared to a moderately low level of the trait (score = 6, 25th percentile). These associations persisted in models that controlled for other cardiovascular risk factors.
Conclusion
Higher level of the harm avoidance trait may be a risk factor for cerebral infarction.
Keywords: harm avoidance, clinical-pathologic study, microinfarcts, cerebral lacunes
INTRODUCTION
Harm avoidance is an anxiety related personality trait (Cloninger, 1987) that denotes behavioral inhibition (Carver & White, 1994). In old age, the trait has been associated with incident mild cognitive impairment and dementia (Wilson et al., 2011), but it does not appear to be related to postmortem levels of plaques or tangles (Wilson et al., 2011), common neurodegenerative lesions associated with late life cognitive impairment. Little is known about the relation of harm avoidance to cerebrovascular disease, a common cause of cognitive impairment in old age. However, a related construct, anxiety, has been associated with risk of cardiovascular disease (Roest, Martens, de Jonge, & Denollet, 2010).
In the present study, we test the hypothesis that higher level of the harm avoidance trait is associated with higher likelihood of cerebral infarction. Analyses are based on 192 older participants in a longitudinal study who completed a standard harm avoidance scale, died during a mean of 3.5 years of observation (SD = 1.6), and underwent brain autopsy. As part of a uniform neuropathologic examination, chronic cerebral infarcts were identified. We estimated the association of harm avoidance with likelihood of infarction.
METHODS
Participants
Participants are from the Rush Memory and Aging Project, an ongoing longitudinal clinical-pathologic study of common chronic conditions of old age that began in 1997 (Bennett et al., 2005; Bennett et al., 2012). The study involves annual clinical evaluations and brain autopsy at death. Older persons in the greater Chicago metropolitan area were recruited from retirement communities, subsidized housing facilities, and senior centers. After a presentation on the project, interested individuals discussed participation with study staff and written informed consent was obtained. The study was approved by the institutional review board of Rush University Medical Center.
Eligibility for analyses required a valid harm avoidance score and death with brain autopsy and completed neuropathologic examination. The harm avoidance scale was completed by 1,081 individuals who underwent a uniform clinical evaluation at baseline and did not meet NINCDS-ADRDA criteria for dementia (see Diagnostic Classification section). During a mean of 3.5 years of observation, 257 people died. A brain autopsy was performed on 206 (80.2%). At the time of these analyses, the neuropathologic examination had been completed in the first consecutive 192 participants. Analyses are based on this group. Autopsies were not performed either because study personnel were not contacted at the time of death or, less commonly, because the executor of the estate refused to honor the request.
Assessment of Harm Avoidance
Harm avoidance was measured at baseline with the 35-item Harm Avoidance scale from Cloninger’s Temperament and Character Inventory (Cloninger, Przybeck, Svrakic, & Wetzel, 1994). Each item consists of a statement which the participant rates as true or false about him or her. There are 4 subscales or facets: anticipatory worry (11 items), fear of uncertainty (7 items), shyness (8 items), and fatigability (9 items). The score for the full scale and each subscale is the number of item responses indicative of the trait. Previous research in this cohort has shown that the scale has adequate internal consistency (Wilson, Buchman, et al., 2006) and that it is associated with disability (Wilson, Buchman, et al., 2006) and dementia (Wilson et al., 2011).
Assessment of Covariates
Covariates were measured each year unless otherwise noted. Cognitive function was assessed with a battery of 19 tests of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Raw test scores were converted to z scores, using the baseline mean and SD of all participants, and averaged to yield a composite measure of global cognition (Wilson, Barnes, & Bennett, 2003; Wilson et al., 2005; Wilson et al., 2013). Motor function was assessed with 11 tests of manual dexterity, manual strength, gait, and balance (Wilson, Segawa, Buchman, et al., 2012). Raw motor scores were divided by the baseline SD of all participants and these standard scores were averaged to yield a composite measure of motor function. Blood pressure was assessed with a mercury sphygmomanometer using the Hypertension Detection and Follow-up Program protocol (Hypertension Detection and Follow-up Program Cooperative Group, 1977). Two readings were taken with the participant sitting and the right arm resting at heart level. The readings were averaged to yield measures of systolic and diastolic blood pressure. Diabetes was based on use of oral hypoglycemic agents or insulin, reported diagnosis, or both (Arvanitakis, Wilson, Li, Aggarwal, & Bennett, 2006). Smoking was assessed at baseline by self report. Body mass was calculated as weight in kilograms divided by height in meters squared. Physical activity was assessed with questions adapted (McPhillips, Pellettera, Barrett-Conner, Wingard, & Criqui, 1989) from the 1985 Health Interview Survey (1985 Health Interview Survey, 1985) about the frequency and duration of participation in 5 activities in the past 2 weeks. The score was minutes per week spent in the 5 activities (walking, gardening or yard work, calisthenics, bicycling, swimming or water exercise) (Wilson et al., 2007). Neuroticism was assessed at baseline with a standard 48-item scale from the NEO Personality Inventory-Revised (Costa & McCrae, 1992). Participants rated agreement with each item statement on a 5-point scale. Item responses were scored from 1 to 5, with higher scores more indicative of the trait, and added to obtain the total score. Childhood emotional neglect was assessed at baseline with a previously established retrospective report scale (Wilson, Krueger, et al., 2006; Wilson, Boyle, et al., 2012). Ratings of agreement with each item (6 items; e.g., “When you were growing up, did you feel loved?”) were added to yield the total score. In previous analyses, the scale showed adequate internal consistency (Cronbach’s coefficient alpha = 0.89) and temporal stability (6-month test-retest correlation = 0.93) (Wilson, Krueger, et al., 2006).
Diagnostic Classification
Participants had annual clinical evaluations that included a structured medical history, neurological examination, and performance tests and questionnaires (Bennett et al, 2005; Bennett et al., 2012) and that supported annual clinical classification of common chronic conditions of old age. The diagnosis of clinical stroke was based on review of the structured medical history and neurological examination (Schneider et al., 2003). Major depression (American Psychiatric Association, 1987) was based on examination and responses to questions adapted from the Diagnostic Interview Schedule (Robins, Helzer, & Croughan, 1981). Classification of Parkinson’s disease was based on medical history. Dementia was classified using the criteria of joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984) which require a history of cognitive decline plus impairment in at least 2 cognitive domains, as described in more detail elsewhere (Bennett, Schneider, Aggarwal, et al., 2006; Wilson et al., 2011). Impairment in at least one cognitive domain in the absence of dementia was classified as mild cognitive impairment (Bennett et al., 2002). Upon death, all clinical information was reviewed by a board-certified neurologist who rendered final clinical diagnoses of mild cognitive impairment and dementia.
The pathologic diagnosis of Alzheimer’s disease was made by a board certified neuropathologist blinded to all clinical data. A Bielschowsky silver stain was used to quantify neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, parietal, entorhinal, and hippocampal cortices. Pathologic Alzheimer’s disease was classified as present (high or intermediate likelihood) or absent (low likelihood or not present) based on estimated neuritic plaque burden (Mirra et al., 1991) and tangle density (Braak & Braak, 1991) as previously described (Schneider, Arvanitakis, Bang, & Bennett, 2007).
Neuropathologic Examination
Brain removal, tissue sectioning and preservation, and quantification of pathology followed a standard protocol (Schneider et al., 2003; Bennett, Schneider, Arvanitakis, et al., 2006) and was performed blinded to all clinical data, including harm avoidance scores. The cerebral hemispheres were cut into 1cm coronal slabs, the cerebellar hemispheres were cut into 1cm sagittal slabs, and the brainstem was bisected at the level of the mid pons. All brain slices were visually examined for gross cerebral infarcts. Slabs from one cerebral hemisphere, one cerebellar hemisphere, and all pathologic lesions including infarcts were fixed in 4% paraformaldehyde. The age (acute, subacute, chronic), size (height, length, width), and location of gross infarcts were recorded. Gross subcortical (including basis pontine and cerebellar subcortical) infarcts that did not extend beyond 10mm in any dimension were classified as lacunar. All grossly visualized suspected infarcts underwent further dissection for histological confirmation (Schneider et al., 2003; Arvanitakis, Leurgans, Barnes, Bennett, & Schneider, 2011).
Microinfarcts were defined as infarcts seen by microscopy but not visualized on gross inspection as previously described (Arvanitakis et al., 2011). They were examined in 1 hemisphere (102 right, 90 left) in 6-μm paraffin-embedded sections stained with hematoxylin and eosin in 9 regions: 6 cortical regions (mid frontal, middle temporal, enthorhinal, hippocampal, inferior parietal, and anterior cingulate cortices), 2 subcortical regions (anterior basal ganglia, anterior thalamus), and midbrain. The choice of hemisphere was arbitrary unless a gross lesion was evident in which case the hemisphere with the lesion was selected. The age and location of each microscopic infarct were recorded. Chronic microinfarcts included cavitated lesions with few remaining macrophages and fibrillary gliosis or incomplete infarcts.
Cerebral atherosclerosis describes the accumulation of fatty calcification plaque deposition in the walls of large vessels. It was assessed on gross examination of the anterior, middle, and posterior cerebral arteries and their proximal branches at the circle of Willis using a semiquantitative 6-point scale of 0 (none) to 5 (severe) indicating near total or total involvement of all visualized arteries. Cerebral arteriolosclerosis describes the concentric eosinophilic hyaline thickening and luminal narrowing of small vessels. It was assessed microscopically on 6- μm hematoxylin and eosin stained sections of anterior basal ganglia (including caudate, putamen, globus pallidus, and internal capsule) by a board certified neuropathologist using a semiquantitative scale of 0 (none) to 6 (severe) indicating more than twice the normal wall thickness. To ensure an adequate number of observations at each level, each rating was collapsed to a 4-point ordinal scale in analyses.
Because the study focuses on the association between infarcts and behavior, analyses are based on chronic infarcts and we excluded infarcts occurring proximate to death (i.e., acute and subacute) as in prior research (Schneider et al., 2003; Wilson, Segawa, Boyle, et al., 2012; Wilson, Segawa, Buchman, et al., 2012). The number of chronic cerebral infarcts was treated as a 3 group ordinal variable in analyses with no infarcts, 1 infarct, and >1 infarct groups. In secondary analyses, infarcts were divided into gross and microscopic subtypes, cortical and subcortical subtypes, and gross subcortical infarcts were divided into lacunar and nonlacunar subtypes.
Statistical Analysis
We estimated the hypothesized association of harm avoidance, treated as a continuous measure, with likelihood of chronic cerebral infarction, treated as 0, 1, or >1, in a series of ordinal logistic regression models. All analysis included terms for age at death, sex, and education. The initial model included a term for harm avoidance. Subsequent models added terms for cognitive and motor function, clinical and pathologic diagnoses, vascular risk factors, neuroticism with and without harm avoidance, and childhood emotional neglect. We also conducted separate analyses of each harm avoidance subscore.
We used the bivariate Dale model (Dale, 1986; Molenberghs & Lesaffre, 1994; Williamson & Kim, 1996), which is designed for bivariate ordered responses, to assess the relation of harm avoidance to pairs of correlated ordinal vascular outcomes. These included atherosclerosis and arteriolosclerosis; microscopic and gross infarction; subcortical and cortical infarction; and lacunar and nonlacunar gross subcortical infarction. The model assesses the association of covariates with each outcome, the association between outcomes, and whether the association between outcomes is conditional on covariates.
RESULTS
Participants died at a mean of age of 88.5 (SD=6.0). They had completed a mean of 14.3 years of education (SD=2.5) and 67.7% were women. During the study, clinical stroke was diagnosed in 40, major depression in 19, and Parkinson’s disease in 3. At baseline, 111 had no cognitive impairment and 81 had mild cognitive impairment. Proximate to death, 80 had no cognitive impairment, 59 had mild cognitive impairment, and 53 had dementia. On postmortem examination, 102 met National Institute on Aging-Reagan criteria for Alzheimer’s disease (19 high likelihood, 83 intermediate likelihood) and 90 did not (87 low likelihood, 3 not present).
Scores on the harm avoidance scale ranged from a low of 0 to a high of 32 (mean = 11.6, SD = 7.0) and were approximately normally distributed (skewness = 0.66). Harm avoidance was unrelated to age (r = −0.01, p = 0.853), sex (t [190] = 0.9, p = 0.399), or education (r = −0.08, p = 0.270). Higher harm avoidance was associated with lower cognitive (r = −0.14, p = 0.054) and motor (r = −0.16, p = 0.033) function but not with a pathologic diagnosis of Alzheimer’s disease (t [140] = 0.3, p = 0.796); with some vascular risk factors (physical activity r = −0.15, p = 0.042; body mass index r = −0.21, p = 0.005) but not others (diabetes t[189] = 0.1, p = 0.896; smoking t[190] = 0.5, p = 0.621; systolic blood pressure r = −0.06, p = 0.377; diastolic blood pressure = −0.12, p=0.105); and with higher levels of neuroticism (r = 0.62, p = <0.001) and childhood emotional neglect (r = 0.21, p = 0.003).
On postmortem examination of the brain, 42 individuals (21.9%) had one chronic cerebral infarct, 47 (24.5%) had more than one, and 103 (53.7%) had none. Chronic cerebral infarction was not related to age at death (r = −0.004, p = 0.959), sex (χ2 [2] = 1.9, p = 0.384), or education (r = −0.05, p = 0.451).
Harm Avoidance and Cerebral Infarcts
We assessed the relation of harm avoidance to the likelihood of cerebral infarction (treated as 0,1, or >1) in a series of ordinal logistic regression models. Terms were included in each model to control for age, sex, and education. In the initial analysis, the likelihood of cerebral infarction increased for each additional point on the harm avoidance scale (odds ratio [OR] = 1.083, 95% confidence interval [CI]: 1.040–1.128). Thus, compared to those with a relatively low level of the trait (score = 6, 25th percentile), the likelihood of infarction was 1.38-fold higher for a trait score at the 50th percentile (score = 10), 2.41-fold higher for a trait score at the 75th percentile (score = 17), and 3.31-fold higher for a trait score at the 90th percentile (score = 21).
A high harm avoidance score could be a consequence of cerebral infarction. To investigate this possibility, we repeated the analysis with terms added to control for common consequences of cerebral infarction: levels of global cognitive function and global motor function, each assessed with multiple performance tests administered at the time of personality assessment. Harm avoidance continued to be associated with cerebral infarction in this analysis (OR = 1.073, 95% CI: 1.027- 1.122) and in subsequent analyses that controlled for cognitive and motor function averaged across all evaluations (OR=1.076, 95% CI: 1.029–1.125) or from the last evaluation before death (OR=1.081, 95% CI: 1.036 – 1.127). The association of harm avoidance with cerebral infarction persisted in additional analyses that controlled for mild cognitive impairment at baseline (OR = 1.084, 95% CI: 1.040, 1.129), mild cognitive impairment and dementia proximate to death (OR = 1.074, 95% CI: 1.030, 1.120), or a pathologic diagnosis of Alzheimer’s disease (OR = 1.083, 95% CI: 1.039, 1.129).
Because of the association of harm avoidance with two vascular risk factors, physical activity and body mass, in this and other (Volkers et al., 2002; Fassino et al., 2002) cohorts, we repeated the original analysis with terms for weekly hours of physical activity and body mass index. Harm avoidance was still associated with likelihood of cerebral infarcts in this analysis (OR = 1.088, 95% CI: 1.040–1.137) and in a subsequent analysis that also controlled for diabetes, smoking, and systolic and diastolic blood pressure (OR = 1.089, 95% CI: 1.041–1.139). Results were similar in analyses adjusted for the full set of vascular risk factors averaged across all evaluations (OR=1.083, 95% CI: 1.038–1.131) or from the last evaluation before death (OR=1.103, 95% CI: 1.050–1.160). In a separate analysis, harm avoidance was not related to postmortem ratings of atherosclerosis (OR = 1.018, 95% CI: 0.982–1.055) or arteriolosclerosis (OR = 0.998, 95% CI: 0.961–1.036).
Because harm avoidance is associated with chronic anxiety (Nyman et al., 2011) and anxiety related traits have been associated with risk of cardiovascular disease (Roest et al., 2010), we examined the association of another anxiety related trait, neuroticism, to cerebral infarction. Higher neuroticism score (mean = 69.7, SD = 20.6) was associated with increased likelihood of infarction (OR = 1.021, 95% CI: 1.007–1.036). However, when harm avoidance was added to the model, the effect of neuroticism was reduced by 67% and no longer significant (OR = 1.007, 95% CI: 0.989–1.025) and the relationship of harm avoidance to infarction was comparable to the original analysis (OR = 1.071, 95% CI: 1.018–1.126).
We previously showed that retrospective ratings of emotional neglect in childhood were associated with cerebral infarction in this cohort (Wilson et al., 2012). Therefore, we repeated the original model with a term for childhood emotional neglect (mean = 4.1, SD = 4.2, skewness = 1.1). In this analysis, higher levels of harm avoidance (OR = 1.074, 95% CI: 1.028, 1.121) and childhood emotional neglect (OR = 1.216, 95% CI: 1.127, 1.311) were each associated with increased likelihood of infarction.
Harm avoidance is a multidimensional trait. Therefore, we separately analyzed each of its 4 subscores. Higher level of each component of harm avoidance was associated with higher likelihood of infarction: anticipatory worry OR = 1.196, 95% CI: 1.062–1.347; fear of uncertainty OR=1.296, 95% CI: 1.097–1.530; shyness OR = 1.144, 95% CI: 1.028–1.273; fatigability OR = 1.190, 95% CI: 1.066, 1.328.
Harm Avoidance and Infarct Type
We constructed a series of bivariate Dale models to determine whether harm avoidance was related to some types of infarction but not others. This approach allowed us to simultaneously model two types of infarction while accounting for the association between them. As shown in Table 1, higher level of harm avoidance was associated with higher likelihood of both microscopic and gross cerebral infarction. The likelihood of microinfarction was 2.4-fold higher with a moderately high (score = 17, 75th percentile) compared to moderately low (score = 6, 25th percentile) level of the trait, and the likelihood of gross infarction was 1.8-fold higher.
Table 1.
Association of harm avoidance with microscopic and gross cerebral infarction.
| Model Term | Estimate | SE | p | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Microscopic infarction | |||||
| Age | 0.002 | 0.029 | 0.935 | 0.998 | 0.943–1.056 |
| Sex | 0.046 | 0.394 | 0.906 | 0.955 | 0.441–2.067 |
| Education | −0.005 | 0.072 | 0.949 | 1.005 | 0.873–1.157 |
| Harm avoidance | −0.079 | 0.024 | 0.002 | 1.082 | 1.032–1.134 |
| Gross infarction | |||||
| Age | −0.004 | 0.025 | 0.864 | 1.004 | 0.956–1.054 |
| Sex | −0.587 | 0.329 | 0.076 | 1.799 | 0.944–3.428 |
| Education | 0.035 | 0.063 | 0.581 | 0.966 | 0.853–1.093 |
| Harm avoidance | −0.055 | 0.021 | 0.011 | 1.057 | 1.014–1.101 |
| Microscopic-gross infarction association | |||||
| Constant | 1.120 | 0.443 | 0.012 | 3.065 | 1.286–7.303 |
| Age | −0.045 | 0.049 | 0.367 | 0.956 | 0.868–1.052 |
| Sex | −0.075 | 0.048 | 0.119 | 0.928 | 0.844–1.019 |
| Education | −0.095 | 0.743 | 0.898 | 0.909 | 0.212–3.901 |
| Harm avoidance | 0.015 | 0.134 | 0.911 | 1.015 | 0.781–1.320 |
From a bivariate Dale model, SE. Standard error; CI, confidence interval.
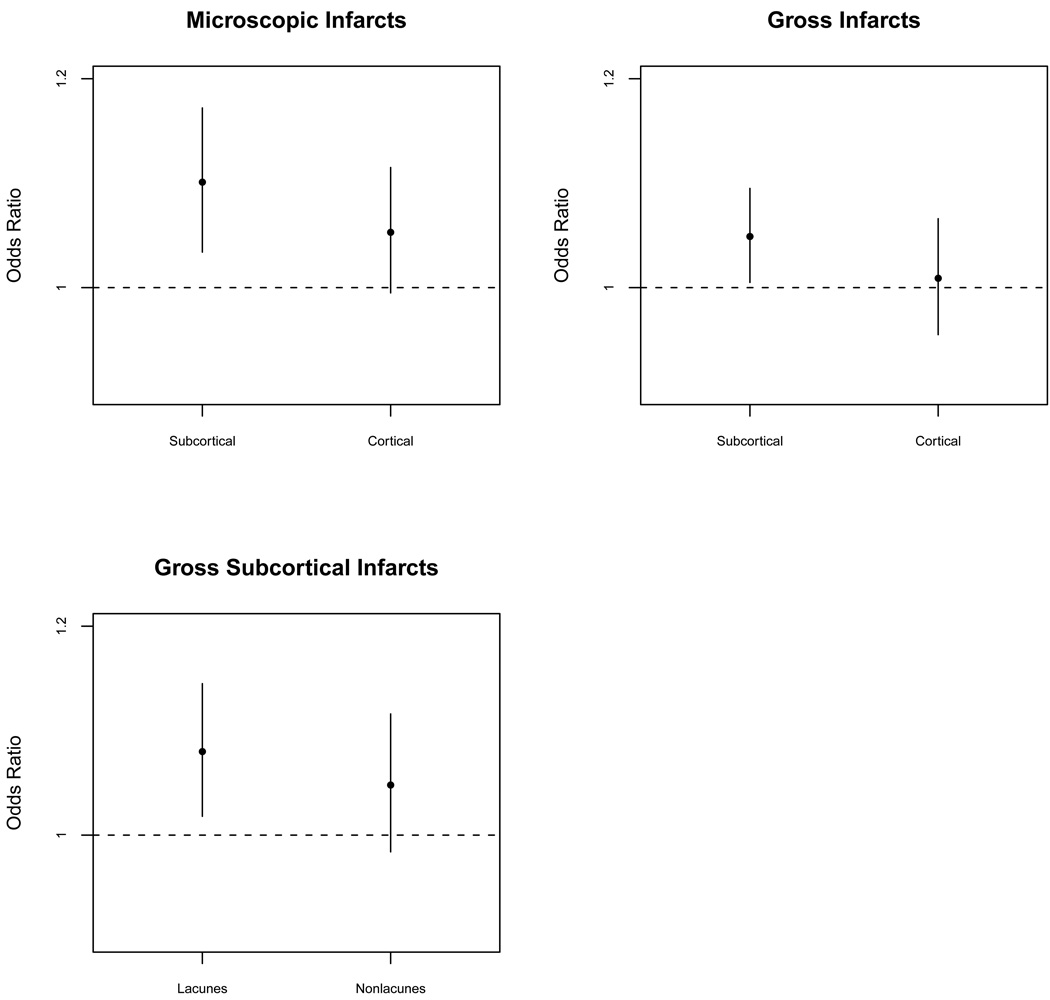
We conducted additional analyses to test whether harm avoidance was differentially related to subtypes of cortical and subcortical infarction. In the first analysis (upper left panel of Figure 1), harm avoidance was related to microscopic subcortical infarction (OR = 1.011, 95% CI: 1.034–1.172) and had a marginal association with microscopic cortical infarction (OR = 1.053, 95% CI: 0.995–1.115). In the second analysis (upper right panel of Figure 1), harm avoidance was related to gross subcortical infarction (OR = 1.049, 95% CI: 1.005–1.095) but not gross cortical infarction (OR = 1.009, 95% CI: 0.955–1.066). In a final model focused exclusively on gross subcortical infarction (lower left panel of Figure 1), harm avoidance was related to lacunar (OR = 1.080, 95% CI: 1.018–1.145) but not nonlacunar (at least 1 dimension > 10mm; OR = 1.048, 95% CI: 0.984–1.116) infarction.
Figure 1.
Harm avoidance and subcortical and cortical infarction. Relation of harm avoidance to subcortical and cortical microscopic infarction (upper left), subcortical and cortical gross infarction (upper right), and lacunar and nonlacunar gross subcortical infarction (lower left).
DISCUSSION
Harm avoidance, a trait indicative of behavioral inhibition, was assessed in older participants in a longitudinal clinical-pathologic cohort study. After a mean of 3 to 4 years of observation, nearly 200 individuals had died and undergone a brain autopsy. Higher level of harm avoidance was associated with higher likelihood of chronic cerebral infarction, and this association persisted after adjustment for potential confounders. The results suggest that high harm avoidance may be a risk factor for cerebral infarction.
There has been little prior research on the association between harm avoidance and vascular disease. The few published studies have focused mainly on cardiovascular risk factors in young adults and results have been inconsistent, with higher harm avoidance associated with both higher (Sovio et al., 2007; Puttonen et al., 2008) and lower (Kettikangas-Järvinen, Ravaja, & Viikari, 1999; Hintsanen et al., 2009) risk profiles.
Chronic anxiety has been associated with increased risk of cardiovascular disease (Roest et al., 2010). In this cohort, harm avoidance and neuroticism, another anxiety related trait, were related to cerebral infarction, but when both traits were analyzed in the same model, only the effect of harm avoidance persisted. In addition, both anxiety related (i.e., anticipatory worry, fear of uncertainty) and inhibitory (i.e., shyness, fatigability) components of harm avoidance were related to infarction. Thus, both anxiety and inhibition appear to contribute to the findings.
The present results indicate an association between harm avoidance and cerebral infarcts, but the direction of the association is uncertain. It is possible that higher harm avoidance is a consequence of cerebral infarcts. However, adjustment for common symptoms of cerebral infarction (i.e., cognitive and motor dysfunction) at the time of trait assessment,at the last evaluation before death, or during the full period of observation did not substantially alter the association, making this possibility less likely. Further research will be needed to clarify this issue.
How harm avoidance might be related to risk of cerebral infarction is not clear. Excessive anxiety and avoidance have been associated with unhealthy lifestyle behaviors (Bonnet, Irving, Terra, Nony, & Moulin, 2005). For example, in this and previous (Volkers et al., 2002) studies, higher harm avoidance was associated with lower level of physical activity. Adjustment for this association did not substantially affect the correlation of harm avoidance with infarction, but controlling for baseline physical activity may not adequately account for the cumulative impact of the trait on physical activity across the life span. In addition, chronic anxiety has been associated with biologic processes such as inflammation (Pitsavos, Panagiotakos, Papgeorgiou, et al., 2006) and autonomic nervous system dysregulation (Bleil, Gianaros, Jennings, Flory, & Manuck, 2008) that may increase vulnerability to vascular disease.
In this study, harm avoidance was more robustly associated with microscopic than macroscopic infarction and its association with macroscopic infarction was mainly with lacunes. These observations suggest that harm avoidance is particularly related to small vessel disease. The lack of an association between harm avoidance and the postmortem measure of cerebral arteriolosclerosis does not support this idea, but the measure was based on semiquantitative ratings of a single brain region. Perhaps a more extensive assessment of arteriolosclerosis or other indicators of small vessel disease such as subcortical white matter changes will be helpful in identifying the mechanism linking harm avoidance to microinfarction.
Strengths and limitation of this study should be noted. Harm avoidance was assessed with a standard psychometrically sound scale. The neuropathologic evaluation was conducted in a uniform manner by examiners blinded to all clinical data. There was a high rate of autopsy participation, minimizing the likelihood that selective attrition affected results. Findings were relatively consistent across trait subscales and infarct subtypes, suggesting that the observed association is reliable. The primary limitation is that the cohort is selected and so it will be important to replicate the findings in other groups. In addition, microscopic infarcts were only assessed in 1 hemisphere, and this may have affected results.
ACKNOWLEDGMENTS
The authors thank the Rush Memory and Aging Project participants; Traci Colvin, MPH, and Karen Skish, MS, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Woojeong Bang, MS, for statistical programming.
This research was supported by NIH grants R01AG17917, R01AG24480, R01AG24871, R01HL94966, R01AG33678, and R01AG34374, and by the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorder. 3rd ed. Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Irving K, Terra JL, Nony P, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bleil ME, Gianaros PJ, Jennings R, Flory JD, Manuck SB. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosomatic Medicine. 2008;70:328–337. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak F. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): a guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality; 1994. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Dale JR. Global cross-ratio models for bivariate, discrete, ordered responses. Biometrics. 1986;42:909–917. [PubMed] [Google Scholar]
- Fassino S, Abbate-Daga G, Amianto F, Leombruni P, Boggio S, Rovera GG. Temperament and character profile of eating disorders: a controlled study with the Temperament and Character Inventory. International Journal of Eating Disorders. 2002;32:412–425. doi: 10.1002/eat.10099. [DOI] [PubMed] [Google Scholar]
- Hintsanen M, Pulkki-Råback L, Juonala M, Viikari JSA, Raitakari OT, Keltikangas-Jarvinen L. Cloninger’s temperament traits and preclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. Journal of Psychosomatic Research. 2009;67:77–84. doi: 10.1016/j.jpsychores.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Hypertension Detection and Follow-up Program Cooperative Group. Race, education, and prevalence of hypertension. American Journal of Epidemiology. 1977;106:351–361. [PubMed] [Google Scholar]
- Keltikangas-Järvinen L, Ravaja N, Viikari JSA. Identifying Cloninger’s temperament profiles as related to the early development of the metabolic cardiovascular syndrome in young men. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1998–2006. doi: 10.1161/01.atv.19.8.1998. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McPhillips JB, Pellettera KM, Barrett-Conner E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. American Journal of Preventive Medicine. 1989;5:65–72. [PubMed] [Google Scholar]
- Mira SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessement of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Molenberghs G, Lesaffre E. Marginal modeling of correlated ordinal data using a multivariate Plackett distribution. Journal of the American Statistical Association. 1994;89:633–644. [Google Scholar]
- Nyman E, Miettunen J, Freimer N, Joukamaa M, Mäki P, Ekelund J, Peltonen L, Järvelin MR, Veijola J, Paunio T. Impact of temperament on depression and anxiety symptoms and depressive disorder in a population-based birth cohort. Journal of Affective Disorders. 2011;131:393–397. doi: 10.1016/j.jad.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Puttonen S, Elovainio M, Kivimäki M, Koskinen T, Pulkki-Råback L, Viikari JSA, Raitakari OT, Keltikangos-Järvinen L. Temperament, health-related behaviors, and autonomic cardiac regulation: the Cardiovascular Risk in Young Finns study. Biological Psychology. 2008;78:204–210. doi: 10.1016/j.biopsycho.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J. National Institute of Mental Health Diagnostic Interview Schedule: history, characteristics, validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. Journal of the American College of Cardiology. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Sovio U, King V, Miettunen J, Ek E, Laitinen J, Joukamaaa M, Veijola J, Järvelin MR. Cloninger’s temperament dimensions, socio-economic and lifestyle factors and metabolic syndrome markers at age 31 years in the Northern Finland Birth Cohort 1966. Journal of Health Psychology. 2007;12:371–382. doi: 10.1177/1359105307074301. [DOI] [PubMed] [Google Scholar]
- Volkers AC, Tulen JH, Duivenvoorden HJ, Gieteling MJ, Wegewijs-De Jong M, Van Den Broek WW. Effect of personality dimensions on the diurnal pattern of motor activity. Journal of Personality. 2002;70:233–247. doi: 10.1111/1467-6494.05004. [DOI] [PubMed] [Google Scholar]
- Williamson J, Kim K. A global odds ratio regression model for bivariate ordered categorical data from ophthalmologic studies. Statistics in Medicine. 1996;15:1507–1518. doi: 10.1002/(SICI)1097-0258(19960730)15:14<1507::AID-SIM316>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Buchman AS, Yu L, Arnold SE, Bennett DA. Harm avoidance and risk of Alzheimer’s disease. Psychosomatic Medicine. 2011;73:690–696. doi: 10.1097/PSY.0b013e3182302ale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle BA, Levine SR, Yu L, Anagnos SE, Buchman AS, Schneider JA, Bennett DA. Emotional neglect in childhood and cerebral infarction in old age. Neurology. 2012;79:1534–1539. doi: 10.1212/WNL.0b013e31826e25bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Buchman AS, Arnold SE, Shah RC, Tang Y, Bennett DA. Harm avoidance and disability in old age. Experimental Aging Research. 2006;32:243–261. doi: 10.1080/03610730600699142. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Barnes LL, Mendes de Leon CF, Bienias JL, Bennett DA. Childhood adversity and psychosocial adjustment in old age. American Journal of Geriatric Psychiatry. 2006;14:307–315. doi: 10.1097/01.JGP.0000196637.95869.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer’s disease. Archives of General Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal decline in motor function. Psychology and Aging. 2012;27:998–1007. doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychology and Aging. 2012;27:1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1985 Health Interview Survey. Hyattsville, MD: US Public Health Service; 1985 National Center for Health Statistics, Series 10; Publication No. 160 PHHS (PHS) 86-1568. [Google Scholar]