Abstract
Previous research has shown that comprehension of complex sentences involving wh-movement (e.g., object-relative clauses) elicits activation in the left inferior frontal gyrus (IFG) and left posterior temporal cortex. However, relatively little is known about the neural correlates of processing passive sentences, which differ from other complex sentences in terms of representation (i.e., noun phrase (NP)-movement) and processing (i.e., the time course of syntactic reanalysis). In the present study, 27 adults (14 younger and 13 older) listened to passive and active sentences and performed a sentence-picture verification task using functional Magnetic Resonance Imaging (fMRI). Passive sentences, relative to active sentences, elicited greater activation in bilateral IFG and left temporo-occipital regions. Participant age did not significantly affect patterns of activation. Consistent with previous research, activation in left temporo-occipital cortex likely reflects thematic reanalysis processes, whereas, activation in the left IFG supports processing of complex syntax (i.e., NP-movement). Right IFG activation may reflect syntactic reanalysis processing demands associated with the sentence-picture verification task.
Keywords: fMRI, sentence processing, syntactic processing, thematic processing
1. Introduction
A considerable body of research has investigated the neural basis of sentence comprehension by comparing complex to simple sentences. One dimension of sentence complexity is the presence vs. absence of wh-movement, which influences the mapping of verb arguments onto the surface representation of sentences. Compare a simple active sentence (1a) to an object wh-question (1b):
-
a.
The boy is hugging the girl.
-
b.
Whoi is the boy hugging ti?
-
c.
The girli was hugged ti by the boy.
Both sentences require the listener to build a syntactic structure that guides the integration of the verb hug with its arguments (i.e., event participants that receive a thematic role from the verb): the agent (performer of the action) and the theme (undergoer of the action). However, these processes are more difficult for (1b) than (1a). Some linguistic theories (e.g., Government and Binding Theory; [1]) claim that in object wh-questions, the theme argument originates in the post-verbal position, as it does in its active counterpart, and is displaced to the beginning of the sentence, leaving behind a “trace” of the movement operation, co-indexed with the moved wh-word. As a result, object wh-questions have a noncanonical verb-argument structure in which the theme precedes the agent, in contrast with canonical active sentences in which the agent is mapped to the subject position, preceding the theme, which is mapped to the object position. Thus, object wh-questions are more complex than simple active sentences. Previous psycholinguistic studies have found that the moved wh-word (the filler) is reactivated immediately after the verb (the gap, i.e., the hypothesized trace site), lending support to the psychological reality of wh-movement [2,3,4,5,6,7]. For example, using a visual world paradigm, wh-movement structures (including object-extracted wh-questions and object cleft structures) elicit automatic eye movements to the filler at the gap site in healthy adults and listeners with aphasia [2,3]. Additional evidence comes from event-related potential (ERP) studies [8], indicating that wh-embedded questions—as compared to whether-questions that do not entail gap-filling—elicit a positivity at the gap position around 400–700 ms.
Passive sentences (1c) also are noncanonical and, hence, are more complex than canonical, active forms (1a). In passive sentences, the theme is the grammatical subject, and the agent is mapped to an adjunct prepositional phrase. According to some theorists, passive sentences also involve syntactic movement [1], i.e., NP-movement (due to the movement of a noun phrase), in contrast with wh-movement, in which a wh-word is displaced. In movement-based accounts of passive sentences, the theme originates post-verbally in the direct object position and moves to the grammatical subject position. However, in other linguistic theories (e.g., Head-Driven Phrase Structure Grammar [9] and Lexical-Functional Grammar [10]), passive sentences do not involve movement; instead, passive sentences are distinguished from actives solely on the basis of lexical/thematic structure.
These accounts make different predictions about the processing of passive sentences. Movement-based accounts predict that identification of a passive structure during language comprehension triggers syntactic reanalysis, in which a trace is constructed and co-indexed with the grammatical subject, whereas lexical/thematic accounts do not. Psycholinguistic studies have found that passive sentences elicit delayed or absent reactivation of the filler at the hypothesized gap site, in contrast with wh-movement structures. One cross-modal priming study [11] found evidence of delayed reactivation of the grammatical subject, with effects reaching significance only 1000 ms after the verb. Similarly, delayed reactivation effects have been reported for unaccusative structures (e.g., The leafi fellti) that are also hypothesized to involve NP-movement [12,13,14]. These findings are consistent with the claim that syntactic reanalysis does take place in structures with NP-movement, though on a slowed time course. Other studies, however, have failed to show gap-filling effects for passive structures with either healthy or aphasic listeners either at the gap site or downstream from it [3]. Thus, it remains an open question whether passive sentences require syntactic reanalysis.
Both accounts predict that passive sentences, like other noncanonical sentences, should trigger a process of thematic reanalysis, i.e., revision of an initial mapping of thematic roles. Psycholinguistic studies have shown that healthy listeners tend to interpret sentence-initial noun phrases as agents unless there are additional cues to thematic mapping in the linguistic representation, such as case-marking [15], or in the discourse context [16]. This “agent-first bias” has been demonstrated through visual-world eye tracking studies in which listeners tend to direct initial looks to scenes in which the first noun phrase is the agent ([15,17,18,19,20], although cf. [21]). In the case of active (canonical) sentences, the agent-first bias corresponds to the true structure of the sentence, and no reanalysis is required. However, passive (noncanonical) sentences require thematic reanalysis as soon as the structure is identified as passive (in English, upon encountering the past participial morphology characteristic of the passive voice). At this point, the thematic role assignment of the first noun phrase must be revised from agent to theme. Some studies have found longer reaction times for passive as compared to active sentences [22,23], which may be due to the processing costs of thematic reanalysis and/or syntactic reanalysis.
Studies examining the neural mechanisms of sentence processing have primarily focused on wh-movement structures by comparing neural activation patterns elicited by complex versus simple sentences, such as object and subject relative structures [24,25,26,27,28]. These studies consistently report activation in left inferior frontal gyrus (IFG) (see [29], for review), with some researchers suggesting that this activation reflects syntactic movement operations [30,31,32,33,34]. Although few neuroimaging studies examining NP-movement structures have been reported, all studies contrasting passive and active sentences have also found activation in the left IFG ([26,35,36,37,38]; cf. [39], who report activation in the left frontal operculum). Shetreet, Friedmann and Hadar [34] also found left IFG activation in an fMRI study of Hebrew unaccusative sentences, which like English passive structures, involve NP-movement (but, see [40], who did not find IFG activation for a different NP-movement structure in German). Notably, the majority of passive sentence processing studies have been conducted with Japanese-speaking participants and, as pointed out by Yokoyama et al. [37], in Japanese, passive verbs are morphologically marked (i.e., by the morpheme rare), whereas active verbs are uninflected. Thus, the IFG activation for passive sentences may be attributable to morphological complexity, rather than movement operations. Japanese also is a verb-final language and, therefore, the thematic and syntactic reanalysis processes take place after both noun phrases are presented (see [38] for discussion). In addition, Japanese has three types of passive sentences [41]. For these reason, it is an open question whether English passive sentences elicit patterns like those for Japanese passives.
Several neuroimaging studies of complex wh-movement sentences also have reported left posterior perisylvian activation; however, fewer NP-movement structure studies find this pattern (see [35,36,37,38], who reported no posterior activation). These regions include the posterior middle and superior temporal gyri (pMTG, pSTG) and the inferior parietal cortex, (i.e., angular gyrus (AG)). Notably, these regions have been found to reflect verb-argument structure processing [42,43,44], as well as the integration of verbs with their arguments ([45,46]; see [47] for review). These findings suggest that left posterior perisylvian regions may support thematic reanalysis, but this raises the question of why activation in these regions has not emerged consistently for passive sentences. One possible explanation is that task demands influence the likelihood of observing activation in this region, with activation more likely when the task places significant demands on thematic reanalysis processes.
Hirotani et al. [26] aimed to disentangle the neural correlates of thematic and syntactic reanalysis by comparing active sentences (which require neither thematic nor syntactic reanalysis), passive sentences (which are assumed by the authors to require both) and causative sentences (which are claimed to require thematic reanalysis, but not syntactic reanalysis, because they do not involve syntactic movement). Relative to active sentences, both passive and causative sentences elicited activation in regions, including the left IFG (pars triangularis) and the left posterior superior temporal gyrus (pSTG), whereas direct comparison of passive to causative sentences showed no differential activation. However, the time course of activation differed for the two sentence types in the left IFG (greater activation for passive than causative sentences approximately 8 s after the critical point of the sentence), but not in the pSTG. The authors argue that both the left IFG and pSTG support thematic reanalysis, whereas the left IFG additionally supports syntactic reanalysis.
Studies of aphasia also provide insight into the neural basis of complex sentence processing. Individuals with agrammatic (Broca’s) aphasia, resulting from stroke or head-injury, typically have impaired production and comprehension of noncanonical sentences, including both wh- and NP-movement structures [48,49,50,51]. Because agrammatic aphasia often is associated with damage to Broca’s area, this pattern suggests that Broca’s area plays a crucial role in complex sentence processing. Other research, however, shows that stroke-induced lesions in agrammatic aphasia often extend well beyond Broca’s area and include cortical (and subcortical) tissue in temporoparietal regions, as well (see [52,53], for lesions associated with stroke-induced agrammatic aphasia). In addition, studies directly investigating the relationship between complex sentence processing and lesion location have reported mixed results: damage to anterior and/or posterior perisylvian regions can result in deficits in complex sentence processing [54,55,56]. Two recent studies examining cortical atrophy in patients with the agrammatic variant of primary progressive aphasia (PPA-G) found correlations between cortical atrophy in the left IFG and impaired complex sentence processing in these patients [57,58]. However, Caplan and colleagues [55] argued for a primary role of posterior perisylvian regions in syntactic processing in stroke-induced aphasia. The authors found that stroke-induced lesions in both Wernicke’s area, and the anterior inferior temporal lobe were associated with accuracy on a sentence-to-picture matching task, whereas lesions in the inferior and superior parietal lobe predicted performance on a task tapping thematic role assignment and co-indexation for syntactically complex sentences, as compared to simple sentences. In addition, Thothathiri, Kimberg, and Schwartz [59], in a voxel-based lesion mapping (VLSM) study in stroke-induced aphasia, found correlations between noncanonical sentence comprehension deficits and lesions in the left temporo-parietal cortex, as well as other regions, but not in Broca’s area. These mixed results may be due in part to variable deficits in thematic and/or syntactic reanalysis processes across individuals with aphasia.
Collectively, the results of both neuroimaging studies with healthy participants and lesion-deficit correlation studies suggest that both anterior and posterior regions are engaged for complex sentence processing. However, because of the dearth of studies examining NP-movement structures in English, as well as the mixed findings derived from Japanese studies focused on these sentences, further research is needed. In addition, further evidence is necessary to test the hypothesis that the left IFG supports syntactic reanalysis whereas, left posterior perisylvian regions support thematic reanalysis during complex sentence processing (cf. [26]).
The present study investigated the neural correlates of processing passive and active sentences in English, using a sentence-picture verification task to probe comprehension. This task was selected in order to maximize demands on thematic mapping and reanalysis processes by requiring the participant to integrate the linguistic representation with an external representation of the event (the visual scene). Following previous studies, we expected to find activation in the left IFG for passive as compared to active sentences. Despite mixed findings with respect to left posterior perisylvian activation, we expected activation in these regions, as well, due to our use of a thematically-demanding task. If passive sentences entail syntactic reanalysis, as suggested based on linguistic descriptions of passives as NP-movement structures, we expected greater IFG activation for passive as compared to active sentences. Additionally, if passive sentence computation engages thematic reanalysis, posterior perisylvian activation would be expected.
2. Results and Discussion
2.1. Behavioral Results
Participants performed overall very well on both active (97.7%) and passive (98%) sentences. Accuracy was equally good when the sentence matched the picture displayed on the screen and when sentence and picture did not match (98.1% and 97.5%, respectively). Reaction times (RT) obtained on correct trials were faster for active sentences (3519 ± 362 ms) as compared to passive sentences (3578 ± 375 ms) and for matched as compared to mismatched sentences (3497 ± 335, 3601 ± 395). A mixed-effects regression analysis was conducted by introducing one predictor at a time: sentence type (active, passive), condition (match, mismatch) and age, to evaluate the contribution of each to the variance. ANOVAs between consecutive models were run with subjects and items introduced as random factors for all. Random slopes for significant predictors were also introduced in the model one at a time, and their contribution to the explanation of variance was evaluated in the same way as the fixed factors. Prior to the analysis, reaction times were log-transformed in order to render a normal distribution. Results indicated a significant main effect of sentence type (t = 2.2, p = 0.031), with longer RT for passive as compared to active sentences and of condition, where sentences that did not match the picture elicited longer RT than sentences matching the picture (t = 3.7, p < 0.001). The introduction of random slopes for both sentence type and condition significantly improved the model (χ2 = 6.8, p = 0.033 and χ2 = 26.4, p < 0.001 respectively). No significant interaction between sentence type and condition emerged (t = −0.4, p = ns). Age did not reach significance as a predictor (t = 1.15, p = ns).
2.2. fMRI Results
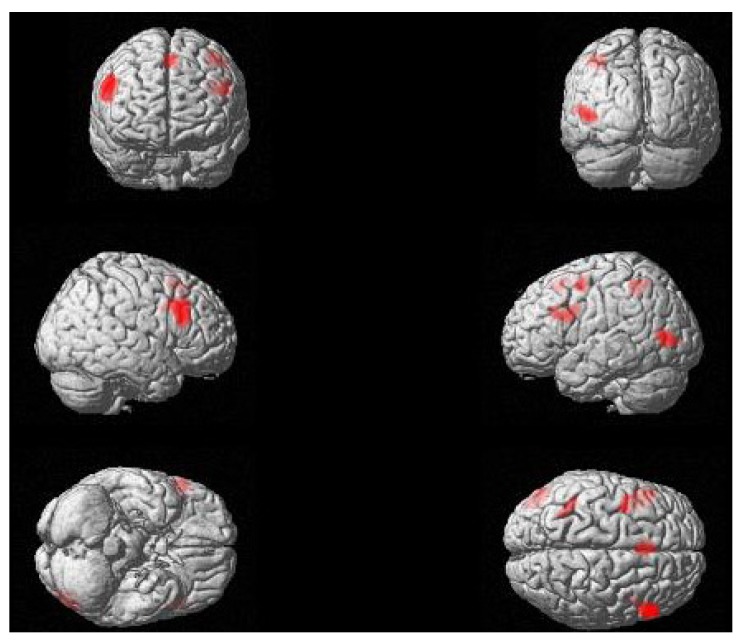
Passive sentences, as compared to active sentences, elicited significant clusters of activation in the bilateral inferior frontal gyrus (pars opercularis and pars triangularis, overlapping with BA’s 44 and 45), as well as in the left temporo-occipital junction (middle occipital gyrus and posterior middle temporal gyrus); see Table 1 and Figure 1. Additional clusters that did not survive correction for multiple comparisons were observed in the bilateral supplementary motor area, the left superior parietal lobule, and the left precentral gyrus. The reverse contrast, of active over passive sentences, did not reveal any significant areas of activation. In addition, no significant effects of age were observed for either contrast (passive > active, active > passive).
Table 1.
Areas of differential activation for passive and active sentences. Peak Montreal Neurological Institute (MNI) coordinates, cluster sizes (k), maximal t-values, and cluster-corrected (family-wise error rate) p-values are reported (voxel-wise threshold of p < 0.001, uncorrected, k ≥ 85). Notes: LH, left hemisphere; RH, right hemisphere; IFG, inferior frontal gyrus; pMTG, posterior middle temporal gyrus; SMA, supplementary motor area; SPL, superior parietal lobule.
| Contrast | Region | Peak Coordinates | k | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Passive > Active | RH IFG (pars opercularis, pars triangularis) | 56 | 24 | 28 | 497 | 6.09 | 0.005 |
| LH IFG (pars opercularis, pars triangularis) | −36 | 4 | 32 | 349 | 4.52 | 0.019 | |
| LH middle occipital gyrus, pMTG | −46 | −76 | 4 | 265 | 5.21 | 0.048 | |
| Bilateral SMA | −2 | 22 | 54 | 217 | 4.77 | 0.084 | |
| L SPL | −40 | −46 | 52 | 136 | 3.60 | 0.227 | |
| L precentral gyrus | −38 | −2 | 54 | 117 | 4.2 | 0.289 | |
| Active > Passive Age |
None | ||||||
| (Passive > Active, Active > Passive) |
None | ||||||
Figure 1.
Regions of differential activation for passive as compared to active sentences (voxel-wise threshold of p < 0.001; k ≥ 85).
2.3. Discussion
The present study investigated the neural correlates of processing passive sentences in English. Consistent with previous studies, we found activation for passive sentences in the left IFG, including tissue in both the pars opercularis and pars triangularis (cf. [26,36,37,38,39]); additionally, activation was found in homologous regions in the right hemisphere. We also found activation in the left temporo-occipital junction, including the pMTG. As pointed out earlier, some previous studies have reported posterior perisylvian activation for passive as compared to active sentences [26,39], whereas others have not found activation in this region [36,37,38]. These results contribute to our understanding of the neural basis of complex sentence processing, in particular, the functions of the left and right IFG and left posterior temporal regions in supporting noncanonical sentence comprehension.
2.3.1. Roles of the Left and Right IFG in Passive Sentence Comprehension
Several explanations have been proposed for the observation that complex sentence comprehension consistently elicits activation in the left IFG. It has been argued by some researchers that the left IFG supports the processing of complex syntactic representations [24,25,53,60,61,62] or, more specifically, syntactic movement [30,31,32,33,34], whereas other accounts suggest that the IFG supports morphological processing [37], semantic/pragmatic aspects of argument processing [63,64,65,66,67] or working memory [68]. Results of the present study are not consistent with morphological, semantic or working memory accounts of left IFG activation. First, the experimental sentences were controlled for morphological complexity, through the use of auxiliary verbs combined with present participles in active sentences and past participles in passive sentences. Second, the experimental sentences were semantically reversible, with the same arguments used in passive and active sentences, and therefore, left IFG activation is not likely due to processing the intrinsic semantic features of arguments. Third, working memory demands were low in the present study, due to the use of short sentences; furthermore, in contrast with wh-structures, passive sentences are unlikely to place enhanced demands on working memory, because the subject NP is not identifiable as a filler until the verb is processed. Thus, the IFG activation found here is most likely associated with the greater syntactic and/or verb-argument structure complexity of passive as compared to active sentences. The present results, however, do not directly address whether the source of left IFG activation is syntactic movement and/or noncanonical verb-argument structure mapping. Previous research suggests that the left IFG is involved in both aspects of syntactic complexity, supporting both thematic and syntactic reanalysis processes [26]. Studies also show that these processes may be supported by different subregions of the IFG, although research examining the neural substrates of syntactic movement report peak activation in the pars opercularis (roughly BA 44) [25,27], as well as in the pars triangularis (roughly BA 45) [26,35,36,38]; see discussion in [29]. Similarly, both the pars opercularis and pars triangularis have been linked to noncanonical argument mapping (see discussion in [64]).
Thus, the present results are consistent with the view that the left IFG supports syntactic movement [30,31,32,33,34]. However, they are incompatible with accounts claiming that the IFG supports only certain types of movement: that is, wh-movement, but not NP-movement found in passive sentences. For example, Christensen [69] argues that movement to the complementizer phrase (CP) domain (i.e., movement to the left of the grammatical subject position), which takes place in wh-movement structures, should elicit activation in the left IFG and posterior perisylvian regions, whereas movement within the inflectional phrase (IP) domain (i.e., movement to or to the right of the grammatical subject position), which occurs in NP-movement structures, such as passive sentences (under standard assumptions), should elicit activation in the left anterior temporal cortex instead. Santi and Grodzinsky [28] propose that left IFG activation is elicited only by movement that is predictable at the point of the antecedent (i.e., wh-movement structures in which the wh-word is immediately identifiable as a filler). However, consistent with previous studies on passive sentence processing in Japanese and Chinese [26,35,36,37,38,39], our findings suggest that if indeed, the left IFG supports syntactic movement; it does so not only for wh-movement structures, but also for NP-movement structures. Furthermore, despite differences between Japanese and English passives with respect to linguistic representation [41] and processing costs [39], the present results indicate that English passives, like Japanese passives, elicit left IFG activation, possibly because passive sentences in both languages require syntactic reanalysis.
In addition, passive sentences elicited greater activation in the right IFG. This finding contrasts with previous studies of passive sentence comprehension, which did not find activation in the right IFG ([26,35,36,37,38,39]), and in general, effects of syntactic complexity in the IFG tend to be strongly left-lateralized (for a review, see [29]). However, some evidence suggests that right IFG supports syntactic reanalysis of complex sentences, particularly in the context of integration of a linguistic representation with a visual scene. In an fMRI study, Meltzer, McArdle, Schafer and Braun [62] investigated the neural correlates of syntactic reanalysis demands during comprehension of complex (noncanonical object-relative) and simple (canonical subject-relative) sentences. In 50% of the trials, participants simply listened to sentences (sentence-alone condition); in the other 50% of the trials, participants performed a sentence-picture matching task. Syntactic reanalysis demands were higher in the sentence-picture matching condition, because participants were required to hold the linguistic representation of the sentence in memory, compare it to the picture probes, then reanalyze it, if necessary. The authors reported main effects of syntactic complexity (object-relatives > subject-relatives) in the left IFG across tasks; however, in sentence-picture matching trials, effects of syntactic complexity were observed in bilateral IFG. The authors propose that the right IFG specifically supports conscious and effortful syntactic reanalysis. Similarly, right IFG activation has been found in previous studies that required participants to generate linguistic judgments about syntactic structures [70,71]. The present study also placed high demands on syntactic reanalysis processes, as participants were required to build a linguistic representation of the sentence and compare it to a visual scene. Thus, high syntactic reanalysis demands are one plausible explanation for the right IFG activation observed for passive sentences. However, we note that the design of the present study (passive/active blocks) makes it difficult to distinguish the neural correlates of reanalysis processes that are due to sentence type (passive vs. active sentences) from those that are due to the correspondence between the sentence and picture (mismatch vs. match trials).
A less likely explanation for the presence of bilateral IFG activation is the inclusion of both young and older adult participants. Some previous research has suggested that healthy aging is associated with increased bilateral language activation, especially in frontal regions [72,73,74]. Therefore, one might hypothesize that bilateral IFG activation for passive sentences in the present study was driven by the older participants. However, we did not observe any significant effects of age on the processing of passive (or active) sentences. For this reason, it is more likely that bilateral IFG activation is due to the high syntactic reanalysis demands imposed by the sentence-picture verification task.
2.3.2. The Role of Left Posterior Temporal Cortex in Passive Sentence Comprehension
Previous research has shown that left posterior temporal cortex supports thematic reanalysis in passive sentences [26], and more generally, the integration of verbs with their arguments [29,43,44,45,47]. Therefore, the left temporo-occipital activation observed for passive sentences in the present study is likely due to thematic reanalysis in the context of a sentence-picture verification task. However, it is an open question why previous studies of passive sentence processing have yielded mixed results with respect to activation in this region. One possible explanation is that posterior perisylvian activation is more sensitive to the choice of task than is left IFG activation, such that tasks that place greater demands on thematic mapping and reanalysis processes are more likely to elicit posterior perisylvian activation. Consistent with this hypothesis, Caplan, Chen and Waters [61] found left IFG activation for noncanonical as compared to canonical sentence comprehension across three tasks (sentence verification, plausibility judgment and non-word detection), whereas posterior perisylvian activation was found only for the sentence verification and plausibility judgment tasks, both of which target thematic role mapping. Studies of passive sentence processing have reported nonhomogeneous findings that nonetheless provide some support for this hypothesis. For example, Yokoyama et al. [37] used a lexical decision task that did not target thematic role mapping and did not elicit posterior perisylvian activation. Studies that targeted thematic role mapping within the sentence (e.g., plausibility judgments, comprehension questions about thematic role assignment) have yielded mixed results, with some studies [26,39] eliciting temporo-parietal activations and others not [36,38]. In addition, one study [35] used a sentence-picture verification task, as in the present study. The authors did not find posterior perisylvian activation for passive as compared to active sentences, but did find posterior temporal (pMTG/pSTG) activation for another type of noncanonical structure (scrambled sentences) as compared to active sentences. Thus, there is some evidence that activation for passive sentences may emerge in left posterior perisylvian regions only with tasks that place explicit demands on verb-argument integration.
The left posterior temporo-occipital activation found in the present study is located inferiorly and posteriorly to the pSTG activation reported by Hirotani et al. [26] and in studies of verb-argument integration [29,47]. However, we note that some studies of complex sentence processing have elicited activation in the left temporo-occipital junction [62,75,76]. Meltzer and colleagues [62] also found left pMTG/pSTG activation for semantically reversible relative to non-reversible sentences in both sentence-alone and sentence-picture matching conditions. However, this activation was shifted posteriorly in the sentence-picture matching condition (peak MNI coordinates: (−49 −73 4); cf. peak coordinates in the present study: (−46 −76 4)) relative to the sentence-alone condition (peak coordinates: (−53 −46 11)). This suggests that integration of auditorily-presented linguistic stimuli with visually-presented scenes may result in a shift posteriorly to the temporo-occipital junction for thematic processing (see, also, the discussion of task effects on activation patterns in [29]).
3. Experimental Section
3.1. Participants
Fourteen healthy young adults (mean age: 24.9; range = 19–38; two males) and thirteen healthy older adults (mean age: 61.2; range = 54–70; seven males) participated in the study. All were right-handed native speakers of English with normal vision and hearing and no history of speech/language, learning or neurological disorders. The study was approved by the Institutional Review Board at Northwestern University, and all participants gave informed consent.
3.2. Materials
Twenty verbs were selected for inclusion in the experiment. All were semantically reversible, frequently-occurring (M log frequency = 4.33; Corpus of Contemporary American English (COCA); [77]) and had a regular passive form (-ed). Each verb was embedded in four sentences, all including the same noun phrase participants: two active sentences (e.g., The brother was pushing the sister; The sister was pushing the brother) and two passive sentences (e.g., The brother was pushed by the sister; The sister was pushed by the brother). All sentences contained past-tense verb forms; the passive sentences included a past-tense auxiliary combined with the past participle (was V-ed), whereas the active sentences contained a past-tense auxiliary with a progressive main verb (was V-ing), in order to control for morphological complexity across the two conditions. The two sentence types were also controlled for length in syllables (active M = 6.15; passive M = 6.3, p =0.42). The nouns referring to participants were all frequently-occurring (M log frequency = 5.00, COCA) and referred to humans or animals (e.g., brother, cat, mouse, woman).
Sentences were recorded by a female native English speaker in a sound proof booth, using Audacity. Maximum amplitude of the sound files was normalized to −3 dB. All sentences were between 2.5 and 3 s, with 100 ms of silence added at the offset of each sentence. An additional variable period of silence was added at the beginning of each sentence, so that all sound files were 3500 ms long.
Twenty black and white drawings were prepared, one for each verb. All drawings depicted two animate participants engaged in an event, such that the agent acted upon the theme (see, e.g., Figure 1). The same line drawing was used for all four sentences constructed with the same verb.
3.3. Procedures
In each trial, a picture and a sentence were presented simultaneously, with the picture remaining on the screen for 6000 ms, followed by 1000 ms fixation. Participants held a response box in their left hand and were asked to press with their index finger if the picture matched the sentence and with their middle finger if it did not. Participants could respond at any point during the trial, and reaction times were measured relative to the onset of stimulus presentation. The experiment was presented using E-Prime, and participant responses were recorded using Cedrus RB610 response boxes.
The experiment used a block design, such that each block included four trials of the same syntactic structure (active or passive), with matched and mismatched trials pseudorandomized within and across blocks. Each block ended with a 12 s fixation cross, for a total of 40 s per block. The experiment included 20 blocks, which were pseudorandomized and assigned to two runs of 6:40 min each. The order of presentation of the two blocks was counterbalanced across participants.
3.4. Data Acquisition
MRI data were acquired using a Siemens 3T Tim Trio scanner with a 32-channel head coil. At the beginning of each scan, a T1-weighted anatomical image was acquired, with the following parameters: time to repeat (TR) = 2300 ms, time to echo (TE) = 2.91 ms, flip angle = 9 degrees; matrix size = 256 × 256; field of view (FOV) = 256 mm; voxel size = 1 × 1 × 1 mm; 176 slices). During the experimental task, blood oxygen level dependent (BOLD) contrast images were acquired using the following parameters: TR= 2000 ms; TE = 30 ms, flip angle = 80 degrees; matrix size = 64 × 64; FOV = 220.16 mm; voxel size = 3.44 × 3.44 × 3 mm; 32 slices.
3.5. Data Analysis
3.5.1. Behavioral Data
Accuracy and reaction time (RT) data were analyzed by means of logistic (for accuracy) or linear (for RT) regression with mixed-effects, following the approach described by Jaeger [78] and Baayen, Davidson and Bates [79]. Subject and item were introduced as random effects in the regression analysis, and the contribution of each predictor to the explanation of the variance was evaluated by performing ANOVA comparisons between models.
3.5.2. Neuroimaging Data
Preprocessing and statistical analysis of the MRI data were performed using SPM8. Preprocessing consisted of slice-timing correction of the functional scans, realignment of the functional scans to a mean functional volume, normalization of the anatomical and functional scans to the MNI 152-subject template brain, reslicing of functional and anatomical scans to a 2 × 2 × 2 mm voxel and smoothing of the functional images using a 9 mm Gaussian kernel.
In order to eliminate scanner drift, a high-pass filter of 128 s was used in the first-level statistical analysis. In addition to the two experimental conditions (active, passive), a parameter for run and six motion parameters were entered into this analysis. In second-level analyses, contrast maps from each participant (passive > active sentences; active > passive sentences) were entered into ANCOVA analyses. Participant age was entered as a covariate to control for and evaluate the potential effects of age on passive and active sentence processing. The results were evaluated using a voxel-level threshold of p < 0.001 with a minimum cluster size (k) of 85 (680 mm3), applying family-wise error rate correction at the cluster level to identify clusters of significant activation. This is equivalent to, or more stringent than, the threshold used in some previous studies testing subtle linguistic contrasts (e.g., [42] and references therein).
4. Conclusions
The results of the present study suggest that in both younger and older adults, comprehension of passive sentences is supported by bilateral IFG and left posterior temporo-occipital regions. These findings are largely consistent with previous research on the comprehension of complex sentences, which has linked left IFG activation to syntactic complexity and left posterior temporal activation to verb-argument structure integration (see, e.g., [29,80]). Thus, despite the linguistic and psycholinguistic differences between passive sentences and other complex structures, they may be largely supported by the same brain regions. The right IFG activation found in the present study may reflect effortful reanalysis processes required by the sentence-picture verification task. Together with previous studies [62,70,71], this suggests that the right IFG may play a role in controlled syntactic reanalysis and the generation of linguistic judgments.
Acknowledgments
This research was supported by NIH grant R01DC01948-19 to C.K. Thompson. The authors would like to thank Chien-Ju Hsu and Julia Schuchard for help with stimuli construction, Ellyn Riley, Ellen Fitzmorris and Shezena Samsair for assistance with data analysis and Caitlin Radnis for assistance with manuscript preparation.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Chomsky N. Lectures on Government and Binding. Foris; Dordrecht, The Netherlands: 1981. [Google Scholar]
- 2.Dickey M.W., Choy J.J., Thompson C.K. Real-time comprehension of wh-movement in aphasia: Evidence from eyetracking while listening. Brain Lang. 2007;100:1–22. doi: 10.1016/j.bandl.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickey M.W., Thompson C.K. Automatic processing of wh- and NP-movement in agrammatic aphasia: Evidence from eyetracking. J. Neurolinguist. 2009;22:563–583. doi: 10.1016/j.jneuroling.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love T., Swinney D., Walenski M., Zurif E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain Lang. 2008;107:203–219. doi: 10.1016/j.bandl.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicol J., Fodor J.D., Swinney D. Using Cross-Modal lexical decision tasks to investigate sentence processing. J. Exp. Psychol. Learn. 1994;20:1229–1238. doi: 10.1037/0278-7393.20.5.1229. [DOI] [PubMed] [Google Scholar]
- 6.Sussman R.S., Sedivy J.C. The time-course of processing syntactic dependencies: Evidence from eye movements. Lang. Cogn. Process. 2003;18:143–161. doi: 10.1080/01690960143000498. [DOI] [Google Scholar]
- 7.Zurif E., Swinney D., Prather P., Solomon J., Bushell C. An on-line analysis of syntactic processing in Broca’s and Wernicke’s aphasia. Brain Lang. 1993;45:448–464. doi: 10.1006/brln.1993.1054. [DOI] [PubMed] [Google Scholar]
- 8.Fiebach C.J., Schlesewsky M., Friederici A.D. Separating syntactic memory costs and syntactic integration during parsing: The processing of German WH-questions. J. Mem. Lang. 2002;47:250–272. doi: 10.1016/S0749-596X(02)00004-9. [DOI] [Google Scholar]
- 9.Pollard C., Sag I.A. Head-Driven Phrase Structure Grammar. The University of Chicago Press; Chicago, IL, USA: 1994. [Google Scholar]
- 10.Bresnan J. Lexical-Functional Syntax. Blackwell; Oxford, UK: 2000. [Google Scholar]
- 11.Osterhout L., Swinney D.A. On the temporal course of gap-filling during comprehension of verbal passives. J. Psycholinguist. Res. 1993;22:273–286. doi: 10.1007/BF01067834. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt P., Piñango M.M., Wong K. The role of the anterior left hemisphere in real-time sentence comprehension: Evidence from split intransitivity. Brain Lang. 2003;86:9–22. doi: 10.1016/S0093-934X(02)00526-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedmann N., Taranto G., Shapiro L.P., Swinney D. The leaf fell (the leaf): The online processing of unaccusatives. Linguist. Inq. 2008;39:355–377. doi: 10.1162/ling.2008.39.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koring L., Mak P., Reuland E. The time course of argument reactivation revealed: Using the visual world paradigm. Cognition. 2012;123:361–379. doi: 10.1016/j.cognition.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Kamide Y., Scheepers C., Altmann G.T. Integration of syntactic and semantic information in predictive processing: Cross-linguistic evidence from German and English. J. Psycholinguist. Res. 2003;32:37–55. doi: 10.1023/A:1021933015362. [DOI] [PubMed] [Google Scholar]
- 16.Olson D.R., Filby N. On the comprehension of passive and active sentences. Cogn. Psychol. 1972;3:361–381. doi: 10.1016/0010-0285(72)90013-8. [DOI] [Google Scholar]
- 17.Knoeferle P. Comparing the Time-course of Processing Initially Ambiguous and Unambiguous German SVO/OVS Sentences in Depicted Events. In: van Gompel R., Fischer M., Murray W., Hill R., editors. Eye Movement Research: A Window on Mind and Brain. Elsevier; Oxford, UK: 2007. pp. 517–531. [Google Scholar]
- 18.Knoeferle P., Crocker M.W., Scheepers C., Pickering M.J. The influence of the immediate visual context on incremental thematic role-assignment: Evidence from eye-movements in depicted events. Cognition. 2005;95:95–127. doi: 10.1016/j.cognition.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Meyer A.M., Mack J.E., Thompson C.K. Tracking passive sentence comprehension in agrammatic aphasia. J. Neurolinguist. 2012;25:31–43. doi: 10.1016/j.jneuroling.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stromswold K., Eisenband J., Norland E., Ratzan J. Tracking the Acquisition and Processing of English Passives: Using Acoustic cues to Disambiguate Actives and Passives; Paper Presented at the CUNY Conference on Sentence Processing; New York, NY, USA. 21–23 March 2002. [Google Scholar]
- 21.Hanne S., Sekerina I.A., Vasishth S., Burchert F., de Bleser R. Chance in agrammatic sentence comprehension: What does it really mean? Evidence from eye movements of German agrammatic aphasic patients. Aphasiology. 2011;25:221–244. doi: 10.1080/02687038.2010.489256. [DOI] [Google Scholar]
- 22.Brookshire R.H., Nicholas L.E. Verification of active and passive sentences by aphasic and nonaphasic subjects. J. Speech Hear. Res. 1980;23:878–893. doi: 10.1044/jshr.2304.878. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira F. The misinterpretation of noncanonical sentences. Cogn. Psychol. 2003;47:164–203. doi: 10.1016/S0010-0285(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 24.Caplan D., Alpert N., Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J. Cogn. Neurosci. 1998;10:541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- 25.Friederici A.D., Fiebach C.J., Schlesewsky M., Bornkessel I.D., von Cramon D.Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- 26.Hirotani M., Makuuchi M., Rüschemeyer S.A., Friederici A.D. Who was the agent? The neural correlates of reanalysis processes during sentence comprehension. Hum. Brain Mapp. 2011;32:1775–1787. doi: 10.1002/hbm.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makuuchi M., Bahlmann J., Anwander A., Friederici A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. USA. 2009;106:8362–8367. doi: 10.1073/pnas.0810928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santi A., Grodzinsky Y. Broca’s area and sentence comprehension: A relationship parasitic on dependency, displacement or predictability? Neuropsychologia. 2012;50:821–832. doi: 10.1016/j.neuropsychologia.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friederici A.D. The brain basis of language processing: From structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shachar M., Hendler T., Kahn I., Ben-Bashat D., Grodzinsky Y. The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychol. Sci. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shachar M., Palti D., Grodzinsky Y. Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Grodzinsky Y. The neurology of syntax: Language use without Broca’s area. Behav. Brain Sci. 2000;23:1–21. doi: 10.1017/S0140525X00002399. [DOI] [PubMed] [Google Scholar]
- 33.Santi A., Grodzinsky Y. Working memory and syntax interact in Broca’s area. Neuroimage. 2007;37:8–17. doi: 10.1016/j.neuroimage.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 34.Shetreet E., Friedmann N., Hadar U. The neural correlates of linguistic distinctions: Unaccusative and unergative verbs. J. Cogn. Neurosci. 2010;22:2306–2315. doi: 10.1162/jocn.2009.21371. [DOI] [PubMed] [Google Scholar]
- 35.Kinno R., Kawamura M., Shioda S., Sakai K.L. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum. Brain Mapp. 2008;29:1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Z., Zhou X. Conflict control during sentence comprehension: fMRI evidence. Neuroimage. 2009;48:280–290. doi: 10.1016/j.neuroimage.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama S., Miyamoto T., Riera J., Kim J., Akitsuki Y., Iwata K., Yoshimoto K., Horie K., Sato S., Kawashima R. Cortical mechanisms involved in the processing of verbs: An fMRI study. J. Cogn. Neurosci. 2006;18:1304–1313. doi: 10.1162/jocn.2006.18.8.1304. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama S., Okamoto H., Miyamoto T., Yoshimoto K., Kim J., Iwata K., Hyeonjeong J., Uchida S., Ikuta N., Sassa Y., et al. Cortical activation in the processing of passive sentences in L1 and L2: An fMRI study. Neuroimage. 2006;30:570–579. doi: 10.1016/j.neuroimage.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama S., Watanabe J., Iwata K., Ikuta N., Haji T., Usui N., Taira M., Miyamoto T., Nakamura W., Sato S., et al. Is Broca’s area involved in the processing of passive sentences? An event-related fMRI study. Neuropsychologia. 2007;45:989–996. doi: 10.1016/j.neuropsychologia.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Wartenburger I., Heekeren H.R., Burchert F., de Bleser R., Villringer A. Grammaticality judgments on sentences with and without movement of phrasal constituents: An event-related fMRI study. J. Neurolinguist. 2003;16:301–314. doi: 10.1016/S0911-6044(03)00028-9. [DOI] [Google Scholar]
- 41.Hoshi H. Theta-role assignment, passivization, and excorporation. J. East Asian Ling. 1994;3:147–178. doi: 10.1007/BF01736125. [DOI] [Google Scholar]
- 42.Meltzer-Asscher A., Schuchard J., den Ouden D.B., Thompson C.K. The neural substrates of complex argument structure representations: Processing “alternating transitivity” verbs. Lang. Cogn. Process. 2012 doi: 10.1080/01690965.2012.672754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson C.K., Bonakdarpour B., Fix S., Blumenfeld H.K., Parrish T.B., Gitelman D.R., Mesulam M.-M. Neural correlates of verb argument structure processing. J. Cogn. Neurosci. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson C.K., Bonakdarpour B., Fix S. Neural mechanisms of verb argument structure processing in agrammatic aphasic and healthy age-matched listeners. J. Cogn. Neurosci. 2010;22:1993–2011. doi: 10.1162/jocn.2009.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bornkessel I., Zysset S., Friederici A.D., von Cramon D.Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 46.Friederici A.D., Rüschemeyer S.A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 47.Thompson C.K., Meltzer-Asscher A. Neurocognitive Mechanisms of Verb Argument Structure Processing. In: Bachrach A., Roy I., Stockall L., editors. Structuring the Argument. John Benjamins; Amsterdam, The Netherlands: 2013. in press. [Google Scholar]
- 48.Bastiaanse R., Edwards S. Word order and finiteness in Dutch and English Broca’s and Wernicke’s aphasia. Brain Lang. 2004;89:91–107. doi: 10.1016/S0093-934X(03)00306-7. [DOI] [PubMed] [Google Scholar]
- 49.Grodzinsky Y., Piñango M.M., Zurif E., Drai D. The critical role of group studies in neuropsychology: Comprehension regularities in Broca’s aphasia. Brain Lang. 1999;67:134–147. doi: 10.1006/brln.1999.2050. [DOI] [PubMed] [Google Scholar]
- 50.Linebarger M.C., Schwartz M.F., Saffran E.M. Sensitivity to grammatical structure in so-called agrammatic aphasics. Cognition. 1983;13:361–392. doi: 10.1016/0010-0277(83)90015-X. [DOI] [PubMed] [Google Scholar]
- 51.Luzzatti C., Toraldo A., Guasti M.T., Ghirardi G., Lorenzi L., Guarnaschelli C. Comprehension of reversible active and passive sentences in agrammatism. Aphasiology. 2001;15:419–441. doi: 10.1080/02687040143000005. [DOI] [Google Scholar]
- 52.Kielar A., Meltzer-Asscher A., Thompson C.K. Electrophysiological responses to argument structure violations in healthy adults and individuals with agrammatic aphasia. Neuropsychologia. 2012;50:3320–3337. doi: 10.1016/j.neuropsychologia.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson C.K., den Ouden D.B., Bonakdarpour B., Garibaldi K., Parrish T.B. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. 2010;48:3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caplan D., Hildebrandt N., Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119:933–949. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- 55.Caplan D., Waters G., Kennedy D., Alpert N., Makris N., Dede G., Michaud J., Reddy A. A study of syntactic processing in aphasia II: Neurological aspects. Brain Lang. 2007;101:151–177. doi: 10.1016/j.bandl.2006.06.226. [DOI] [PubMed] [Google Scholar]
- 56.Dronkers N.F., Wilkins D.P., van Valin R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Rogalski E., Cobia D., Harrison T.M., Wienecke C., Thompson C.K., Weintraub S., Mesulam M.-M. Anatomy of language impairments in primary progressive aphasia. J. Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson S.M., Dronkers N.F., Ogar J.M., Jang J., Growdon M.E., Agosta F., Henry M.L., Miller B.-.L., Gorno-Tempini M.L. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J. Neurosci. 2010;15:16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thothathiri M., Kimberg D.Y., Schwartz M.F. The neural basis of reversible sentence comprehension: Evidence from voxel-based lesion symptom mapping in aphasia. J. Cogn. Neurosci. 2012;24:212–222. doi: 10.1162/jocn_a_00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Just M.A., Carpenter P.A., Keller T.A., Eddy W.F., Thulborn K.R. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 61.Caplan D., Chen E., Waters G. Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex. 2008;44:257–275. doi: 10.1016/j.cortex.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meltzer J.A., McArdle J.J., Schafer R.J., Braun A.R. Neural aspects of sentence comprehension: Syntactic complexity, reversibility, and reanalysis. Cereb. Cortex. 2010;20:1853–1864. doi: 10.1093/cercor/bhp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bornkessel-Schlesewsky I., Schlesewsky M., von Cramon D.Y. Word order and Broca’s region: Evidence for a supra-syntactic perspective. Brain Lang. 2009;111:125–139. doi: 10.1016/j.bandl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Bornkessel-Schlesewsky I., Grewe T., Schlesewsky M. Prominence vs. aboutness in sequencing: A functional distinction within the left inferior frontal gyrus. Brain Lang. 2012;120:96–107. doi: 10.1016/j.bandl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Bornkessel-Schlesewsky I., Schlesewsky M. The role of prominence information in the real-time comprehension of transitive constructions: A cross-linguistic approach. Lang. Linguist. Compass. 2009;3:19–58. doi: 10.1111/j.1749-818X.2008.00099.x. [DOI] [Google Scholar]
- 66.Grewe T., Bornkessel I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. The emergence of the unmarked: A new perspective on the language-specific function of Broca’s area. Hum. Brain Mapp. 2005;26:178–190. doi: 10.1002/hbm.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grewe T., Bornkessel I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. Linguistic prominence and Broca’s area: The influence of animacy as a linearization principle. Neuroimage. 2006;32:1395–1402. doi: 10.1016/j.neuroimage.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 68.Fiebach C.J., Schlesewsky M., Lohmann G., von Cramon D.Y., Friederici A.D. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Hum. Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christensen K.R. Interfaces, syntactic movement, and neural activation: A new perspective on the implementation of language in the brain. J. Neurolinguist. 2008;21:71–103. doi: 10.1016/j.jneuroling.2007.01.002. [DOI] [Google Scholar]
- 70.Moro A., Tettamanti M., Perani D., Donati C., Cappa S.F., Fazio F. Syntax and the brain: Disentangling grammar by selective anomalies. Neuroimage. 2001;13:110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- 71.Tettamanti M., Rotondi I., Perani D., Scotti G., Fazio F., Cappa S.F., Moro A. Syntax without language: Neurobiological evidence for cross-domain syntactic computations. Cortex. 2009;45:825–838. doi: 10.1016/j.cortex.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 72.Davis S.W., Kragel J.E., Madden D.J., Cabeza R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb. Cortex. 2012;22:232–242. doi: 10.1093/cercor/bhr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park D., Reuter-Lorenz P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu. Rev. Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyler L.K., Shafto M.A., Randall B., Wright P., Marslen-Wilson W.D., Stamatakis E.A. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caplan D. Task effects on BOLD signal correlates of implicit syntactic processing. Lang. Cogn. Process. 2010;25:866–901. doi: 10.1080/01690961003672447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooke A., Zurif E.B., DeVita C., Alsop D., Koenig P., Detre J., Gee J., Pinãngo M., Balogh J., Grossman M. Neural basis for sentence comprehension: Grammatical and short-term memory components. Hum. Brain Mapp. 2002;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies M. The Corpus of Contemporary American English, 2008. [(accessed on 1 August 2011)]. Available online: http://corpus.byu.edu/coca/
- 78.Jaeger T.F. Categorical data analysis: Away from ANOVAs (transformation or not) and towards logit mixed models. J. Mem. Lang. 2008;59:434–446. doi: 10.1016/j.jml.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baayen R.H., Davidson D.J., Bates D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008;49:390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- 80.Thompson C.K., Kielar A. Neural bases of sentence processing: Evidence from neurolinguistic and neuroimaging studies. In: Goldrick M., Ferreira V., Miozzo M., editors. The Oxford Handbook of Language Production. Oxford University Press; New York, NY, USA: 2014. [Google Scholar]