Abstract
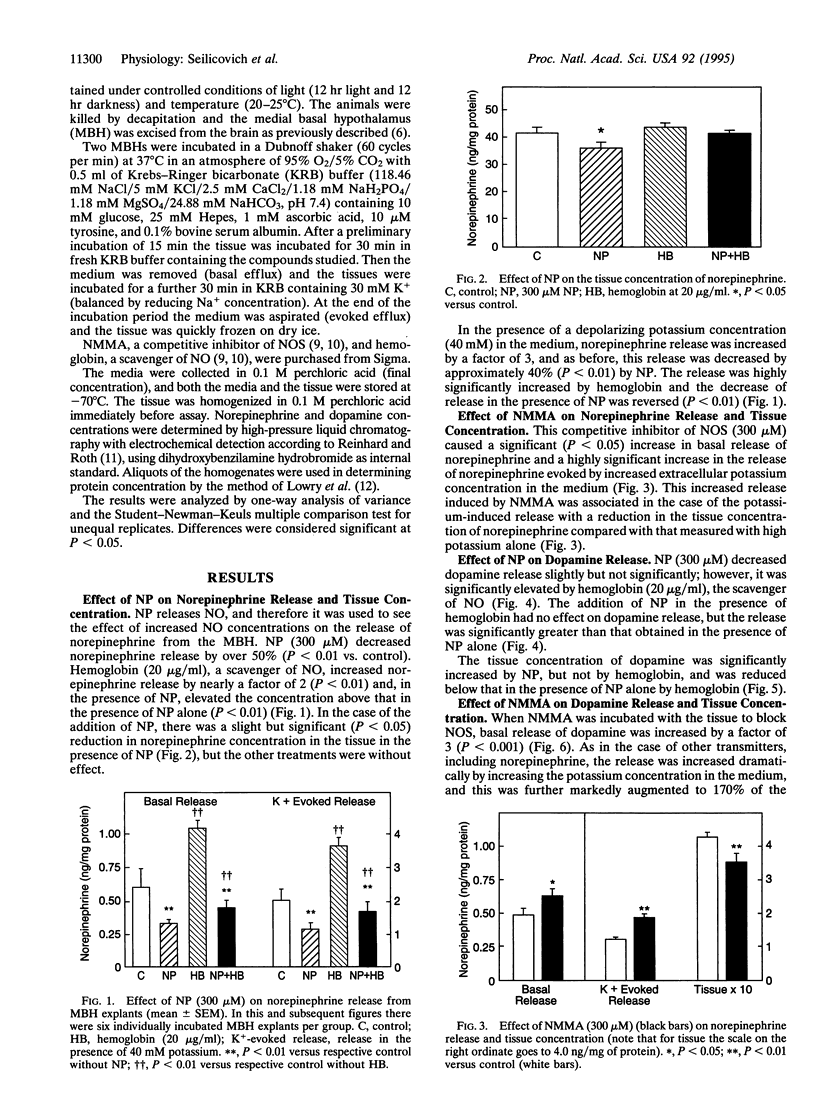
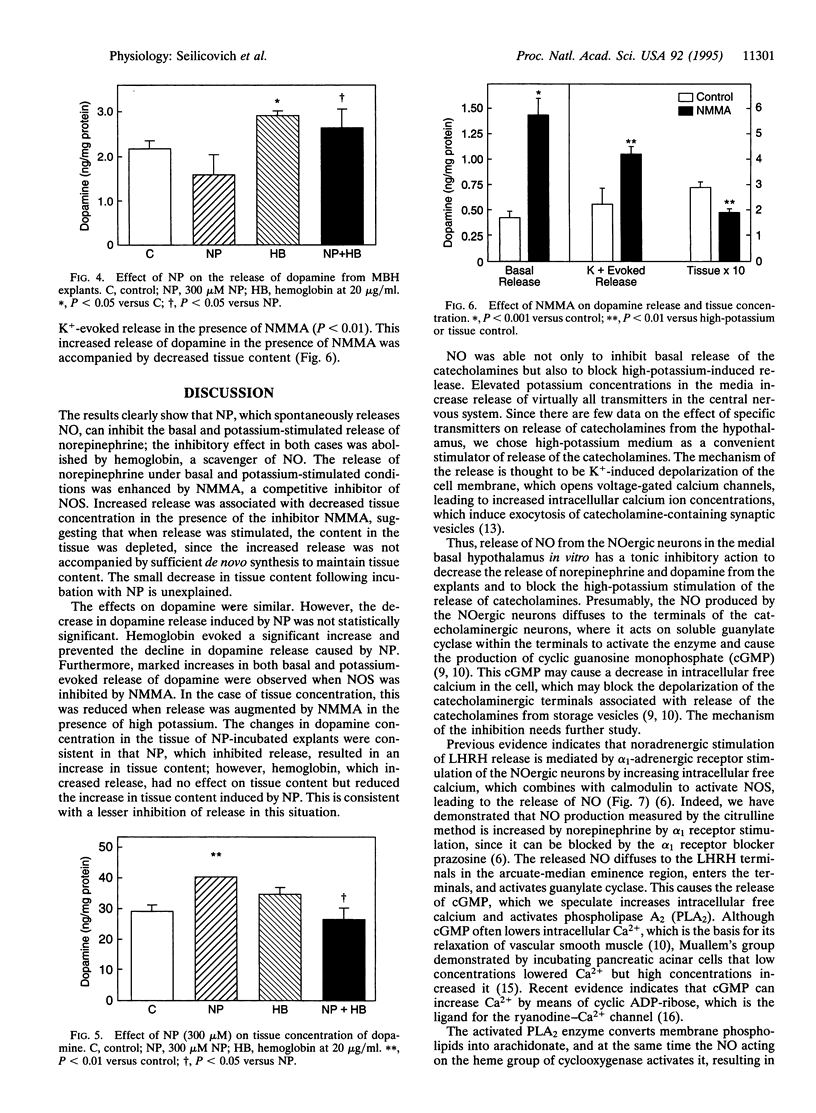
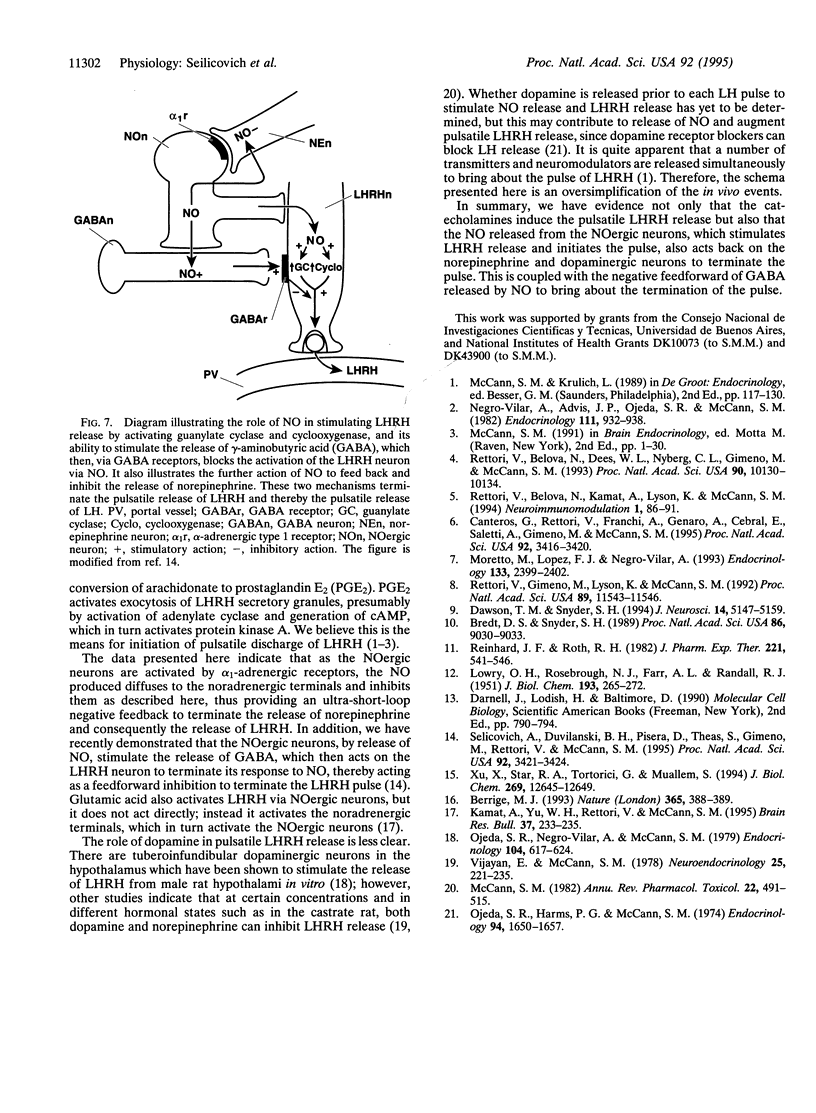
Previous research indicates that norepinephrine and dopamine stimulate release of luteinizing hormone (LH)-releasing hormone (LHRH), which then reaches the adenohypophysis via the hypophyseal portal vessels to release LH. Norepinephrine exerts its effect via alpha 1-adrenergic receptors, which stimulate the release of nitric oxide (NO) from nitricoxidergic (NOergic) neurons in the medial basal hypothalamus (MBH). The NO activates guanylate cyclase and cyclooxygenase, thereby inducing release of LHRH into the hypophyseal portal vessels. We tested the hypothesis that these two catecholamines modulate NO release by local feedback. MBH explants were incubated in the presence of sodium nitroprusside (NP), a releaser of NO, and the effect on release of catecholamines was determined. NP inhibited release of norepinephrine. Basal release was increased by incubation of the tissue with the NO scavenger hemoglobin (20 micrograms/ml). Hemoglobin also blocked the inhibitory effect of NP. In the presence of high-potassium (40 mM) medium to depolarize cell membranes, norepinephrine release was increased by a factor of 3, and this was significantly inhibited by NP. Hemoglobin again produced a further increase in norepinephrine release and also blocked the action of NP. When constitutive NO synthase was inhibited by the competitive inhibitor NG-monomethyl-L-arginine (NMMA) at 300 microM, basal release of norepinephrine was increased, as was potassium-evoked release, and this was associated in the latter instance with a decrease in tissue concentration, presumably because synthesis did not keep up with the increased release in the presence of NMMA. The results were very similar with dopamine, except that reduction of potassium-evoked dopamine release by NP was not significant. However, the increase following incubation with hemoglobin was significant, and hemoglobin, when incubated with NP, caused a significant elevation in dopamine release above that with NP alone. In this case, NP increased tissue concentration of dopamine along with inhibiting release, suggesting that synthesis continued, thereby raising the tissue concentration in the face of diminished release. When the tissue was incubated with NP plus hemoglobin, which caused an increase in release above that obtained with NP alone, the tissue concentration decreased significantly compared with that in the absence of hemoglobin, indicating that, with increased release, release exceeded synthesis, causing a fall in tissue concentration. When NO synthase was blocked by NMMA, the release of dopamine, under either basal or potassium-evoked conditions, was increased. Again, in the latter instance the tissue concentration declined significantly, presumably because synthesis did not match release. Therefore, the results were very similar with both catecholamines and indicate that NO acts to suppress release of both amines. Since both catecholamines activate the release of LHRH, the inhibition of their release by NO serves as an ultra-short-loop negative feedback by which NO inhibits the release of the catecholamines, thereby reducing the activation of the NOergic neurons and decreasing the release of LHRH. This may be an important means for terminating the pulses of release of LHRH, which generate the pulsatile release of LH that stimulates gonadal function in both male and female mammals.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Cell signalling. A tale of two messengers. Nature. 1993 Sep 30;365(6445):388–389. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteros G., Rettori V., Franchi A., Genaro A., Cebral E., Faletti A., Gimeno M., McCann S. M. Ethanol inhibits luteinizing hormone-releasing hormone (LHRH) secretion by blocking the response of LHRH neuronal terminals to nitric oxide. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A., Yu W. H., Rettori V., McCann S. M. Glutamic acid induces luteinizing hormone releasing hormone release via alpha receptors. Brain Res Bull. 1995;37(3):233–235. doi: 10.1016/0361-9230(94)00280-e. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCann S. M. Physiology and pharmacology of LHRH and somatostatin. Annu Rev Pharmacol Toxicol. 1982;22:491–515. doi: 10.1146/annurev.pa.22.040182.002423. [DOI] [PubMed] [Google Scholar]
- Moretto M., López F. J., Negro-Vilar A. Nitric oxide regulates luteinizing hormone-releasing hormone secretion. Endocrinology. 1993 Nov;133(5):2399–2402. doi: 10.1210/endo.133.5.8104781. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A., Advis J. P., Ojeda S. R., McCann S. M. Pulsatile luteinizing hormone (LH) patterns in ovariectomized rats: involvement of norepinephrine and dopamine in the release of LH-releasing hormone and LH. Endocrinology. 1982 Sep;111(3):932–938. doi: 10.1210/endo-111-3-932. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Negro-Vilar A., McCann S. M. Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979 Mar;104(3):617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Roth R. H. Noradrenergic modulation of serotonin synthesis and metabolism. I. Inhibition by clonidine in vivo. J Pharmacol Exp Ther. 1982 Jun;221(3):541–546. [PubMed] [Google Scholar]
- Rettori V., Belova N., Dees W. L., Nyberg C. L., Gimeno M., McCann S. M. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V., Belova N., Kamat A., Lyson K., Gimeno M., McCann S. M. Blockade by interleukin-1-alpha of nitricoxidergic control of luteinizing hormone-releasing hormone release in vivo and in vitro. Neuroimmunomodulation. 1994 Jan;1(1):86–91. doi: 10.1159/000097095. [DOI] [PubMed] [Google Scholar]
- Rettori V., Gimeno M., Lyson K., McCann S. M. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilicovich A., Duvilanski B. H., Pisera D., Theas S., Gimeno M., Rettori V., McCann S. M. Nitric oxide inhibits hypothalamic luteinizing hormone-releasing hormone release by releasing gamma-aminobutyric acid. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3421–3424. doi: 10.1073/pnas.92.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan E., McCann S. M. The effect of systemic administration of dopamine and apomorphine on Plasma LH and prolactin concentrations in conscious rats. Neuroendocrinology. 1978;25(4):221–235. doi: 10.1159/000122744. [DOI] [PubMed] [Google Scholar]
- Xu X., Star R. A., Tortorici G., Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J Biol Chem. 1994 Apr 29;269(17):12645–12653. [PubMed] [Google Scholar]


