Abstract
Rationale: Existing studies of risk factors for physical impairments in acute lung injury (ALI) survivors were potentially limited by single-center design or relatively small sample size.
Objectives: To evaluate risk factors for three measures of physical impairments commonly experienced by survivors of ALI in the first year after hospitalization.
Methods: A prospective, longitudinal study of 6- and 12-month physical outcomes (muscle strength, 6-minute-walk distance, and Short Form [SF]-36 Physical Function score) for 203 survivors of ALI enrolled from 12 hospitals participating in the ARDS Network randomized trials. Multivariable regression analyses evaluated the independent association of critical illness–related variables and intensive care interventions with impairments in each physical outcome measure, after adjusting for patient demographics, comorbidities, and baseline functional status.
Measurements and Main Results: At 6 and 12 months, respectively, mean (± SD) values for strength (presented as proportion of maximum strength score evaluated using manual muscle testing) was 92% (± 8%) and 93% (± 9%), 6-minute-walk distance (as percent-predicted) was 64% (± 22%) and 67% (± 26%), and SF-36 Physical Function score (as percent-predicted) was 61% (± 36%) and 67% (± 37%). After accounting for patient baseline status, there was significant association and statistical interaction of mean daily dose of corticosteroids and intensive care unit length of stay with impairments in physical outcomes.
Conclusions: Patients had substantial impairments, from predicted values, for 6-minute-walk distance and SF-36 Physical Function outcome measures. Minimizing corticosteroid dose and implementing existing evidence-based methods to reduce duration of intensive care unit stay and associated patient immobilization may be important interventions for improving ALI survivors’ physical outcomes.
Keywords: acute lung injury, exercise test, muscle strength, risk factors, follow-up studies
At a Glance Commentary
Scientific Knowledge on the Subject
Acute lung injury (ALI) survivors frequently experience new physical impairments, lasting long after their intensive care unit (ICU) stay. Prior studies have been single-center and/or had smaller sample sizes that potentially limited generalizability and/or statistical power to evaluate a wide range of potential patient and ICU-related risk factors for a spectrum of physical impairments commonly reported after ALI.
What This Study Adds to the Field
After accounting for patient baseline factors associated with worse physical outcomes, there are significant associations and statistical interaction of mean daily dose of systemic corticosteroids and ICU length of stay with ALI survivors’ impairments in muscle strength, 6-minute-walk test, and Short Form-36 Physical Function scores. Further investigation is necessary to elucidate the interplay between corticosteroid dose and duration of ICU stay in their association with post-ALI physical impairment.
Survivors of critical illness and acute lung injury (ALI) frequently experience new, long-lasting physical impairments (1–7). To advance research and improve patient outcomes, clinicians and researchers need a better understanding of these impairments, their trajectories of recovery (8), and associated patient- and intensive care–related risk factors (9). Larger-sized, multicentered, follow-up studies, with careful retention of research participants and evaluation of impairments across the spectrum of physical outcome measures, including strength, function, and quality of life, are needed (9, 10). Together these data are important to better inform patients, families, and clinicians caring for patients during and after their critical illness.
Corticosteroids may be an important risk factor for physical impairments after critical illness. A landmark study by De Jonghe and coworkers (11) demonstrated that administration of corticosteroids was independently associated with in-hospital muscle weakness. However, there is uncertainty regarding this relationship because no association was observed between muscle weakness and duration or cumulative dose of corticosteroids in this study (11) or in subsequent research (1). Moreover, a systematic review demonstrated no consistent relationship between corticosteroids and neuromuscular abnormalities in critical illness (12), and recent detailed analyses of muscle wasting in mechanically ventilated patients also demonstrated no association (13, 14). The nature of any association between corticosteroids and physical impairments may be complex and may vary with corticosteroid dose, the physical outcome evaluated, and the trajectory of patient recovery. For example, there was a statistically significant association of corticosteroids at 28-day follow-up, but not at hospital discharge, in one study evaluating neuromyopathy (15). In another study evaluating 6-minute-walk distance, a significant association was found at 3-month, but not at 6- and 12-month, follow-up (2). To further elucidate the potential association of corticosteroid use and dosage with physical impairments, additional research is needed. Ideally such research will build on prior studies, with larger sample sizes and a multicentered design to permit variability in corticosteroid usage and dosing, and with evaluation of several physical outcome measures at different time points.
The ARDSNet Long-Term Outcomes Study (ALTOS) was designed to assess survivors of Acute Respiratory Distress Syndrome Network (ARDSNet) trials at 6 and 12 months after ALI. Recently, ARDSNet reported on two coenrolling randomized trials of aerosolized albuterol versus placebo (ALTA trial), and of early versus delayed enteral feeding (EDEN trial) factorialized with a trial of placebo versus an omega-3 fatty acid and antioxidant supplement (OMEGA trial). The ALTA and OMEGA trials were terminated early (n = 282 and 272, respectively) because of a lack of efficacy (16, 17), whereas the EDEN trial enrolled its full 1,000-patient sample size and demonstrated no significant difference in both short-term end points (e.g., ventilator-free days and mortality) and in 6- and 12-month physical outcomes (including patient-reported and performance-based outcome measures) (18–20). Hence, pooling patients from these trials, which shared similar eligibility criteria and were enrolled from the same study sites during a similar time period, allows for a unique multicentered evaluation of 6- and 12-month physical outcomes and related risk factors in a relatively large population of survivors of ALI.
Methods
Five of the 12 ARDSNet research centers, representing 12 hospitals, participated in prospective, longitudinal, in-person evaluation of trial survivors, with follow-up occurring between June 2008 and May 2012. The study was approved by the institutional review boards at all participating hospitals. Informed consent to participate in this follow-up study was obtained from each patient or their proxy when the patient was incapable of consent.
Study Population
The eligibility criteria of the ARDSNet trials have been previously reported (16–18), with major exclusion criteria including severe comorbid malnutrition, lung, liver, or neuromuscular diseases, or limitations in life support at time of eligibility. These ARDSNet trial participants were excluded from ALTOS follow-up if they had potential baseline cognitive impairment (ascertained via medical records review and/or patient/proxy interview), or were non-English speaking, homeless, or younger than 18 years old. All patients were managed with simplified versions of the ARDSNet treatment protocols for lung-protective ventilation (21) and fluid-conservative hemodynamic management (22), with blood glucose control targeted 80–150 mg/dl (with tighter control permitted), using institution-specific insulin protocols (18). Across all study sites participating in ALTOS, clinical practice regarding use of corticosteroids and neuromuscular blockers for patients with ALI was not standardized and three-quarters of sites, representing 75% of all evaluated patients in this analysis, did not routinely provide early mobility or rehabilitation services in the intensive care unit (ICU).
Study Procedures
Research personnel collected patient demographics and baseline functional and comorbidity status (including both the Charlson [23] and the Functional [24] Comorbidity Indices, the latter specifically used for predicting the Short Form [SF]-36 physical function domain score in ARDS survivors [25]), and daily medication use in the ICU after study enrollment for systemic corticosteroids and neuromuscular blocking agents. For data analysis, corticosteroids doses were converted to prednisone-equivalents using standard conversions (26). Research personnel underwent in-person training and annual in-person quality assurance reviews for conducting the standardized battery of physical measures in this study. These evaluations were performed, masked to treatment allocation, at 6 and 12 months after ALI onset and included the following measures, as previously described (19): (1) arm muscle area as a percentage of predicted value, based on upper arm anthropometric assessment (27, 28); (2) muscle strength as a percentage of maximum score, evaluated by manual muscle strength testing using the Medical Resource Council (MRC) sum score (29, 30), (range, 0–60; <48 designated as “ICU-acquired weakness” [11]); (3) hand grip strength as percentage of predicted value (31); (4) maximum inspiratory pressure as a percentage of predicted value (32, 33); (5) 4-m timed walking speed (in meters per second) (34–36); (6) 6-minute-walk test (6MWT) as a percentage of predicted value (37, 38); and (7) the Medical Outcomes Study SF-36 Physical Function domain score (SF-36 PF) as a percentage of predicted value (39). Published methods were used to minimize loss to follow-up (40–46), including conducting research visits at patients’ location of residence for those who were unable to attend a research clinic.
Primary Physical Outcome Measures
As primary outcomes for analytical purposes, we selected three a priori physical measures (from among the seven measures described previously) as representative long-term outcome measures (10): (1) manual muscle testing strength (a measure of overall extremity strength), (2) 6MWT (a measure of physical functioning), and (3) SF-36 PF (a measure of quality of life).
Statistical Methods
Descriptive statistics were performed, at 6 and 12 months, for the three primary outcomes. For both 6- and 12-month time points, Pearson correlation coefficients were calculated for each of the three primary outcomes versus the full set of seven tests in the physical battery, with only correlations at the 6-month time point presented given similarity with correlations observed at 12-month follow-up.
Separate linear regression models were used to evaluate the association between each of the three primary outcomes and an a priori set of patient baseline variables and ICU measures (i.e., “exposure” variables) (Table 1). Post hoc sensitivity analyses evaluated the randomized treatments used in the ARDSNet trials for any potential interaction with the significant ICU exposure variables and the physical outcome measures. The initial regression models separately evaluated associations between exposures and outcomes at each of the 6- and 12-month follow-up time points. However, given that these exposure-outcome associations did not vary significantly between the 6- and 12-month time points (evaluated using statistical interaction terms in the multivariable regression models), a simplified approach was used in which the bivariable associations at both 6 and 12 months were evaluated in the same linear regression model, using an indicator for time (i.e., 6 vs. 12 mo follow-up) and generalized estimating equations with an exchangeable correlation structure. Exposure variables with a potentially significant bivariable association (P < 0.20) were then included in multivariable linear regression models constructed in the same manner as the bivariable models.
Table 1:
Characteristics for 203 Patients with ALI*
| Characteristic | Mean (SD), Unless Otherwise Specified |
|---|---|
| Baseline status before hospital admission | |
| Age, yr | 48 (15) |
| Male, No. (%) | 100 (49) |
| Body mass index, kg/m2 | 31 (8) |
| Living independently at home, No. (%) | 187 (92) |
| Functional Comorbidity Index | 1.9 (1.5) |
| Charlson Comorbidity Index | 1.2 (1.6) |
| Specific comorbidities | |
| Psychiatric, No. (%) | 77 (38) |
| Substance abuse, No. (%) | 53 (26) |
| Pulmonary, No. (%) | 34 (17) |
| Rheumatologic, No. (%) | 28 (14) |
| Cardiac, No. (%) | 28 (14) |
| Neurologic, No. (%) | 17 (8) |
| Status while in intensive care unit after enrollment | |
| Pneumonia or sepsis as ALI risk factor, No. (%) | 177 (87) |
| APACHE III score | 85 (25) |
| Pao2/FiO2 ratio < 200, ever during first 3 d, No. (%) | 128 (80) |
| Brussels score: mean proportion of failing organs, % | 31 (13) |
| Cumulative 7-d fluid balance, L | −0.6 (7.6) |
| Mean daily 8 a.m. blood glucose, mg/dl | 126 (29) |
| Proportion of days with catecholamine use, % | 17 (23) |
| Any dialysis, No. (%) | 29 (16) |
| Any neuromuscular blocker, No. (%) | 54 (27) |
| Any systemic corticosteroids,† No. (%) | 85 (43) |
| Mean daily corticosteroid dose among those who received any dose, mg (prednisone-equivalent) | 52 (81) |
| Mechanical ventilation duration, d | 11 (9) |
| Intensive care unit length of stay, d | 14 (11) |
Definition of abbreviations: ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation.
Number of unknown or missing data: Charlson Comorbidity Index, 3; Functional Comorbidity Index, 3; APACHE III, 9; PaO2/FiO2 ratio, 42; cumulative 7-day fluid balance, 62; dialysis, 17; catecholamine, 3; dialysis, 17; neuromuscular blocker, 3; corticosteroids, 3.
Of those receiving any corticosteroids, 59% (n = 50) received a mean daily dose less than 40 mg of prednisone-equivalents.
In the multivariable regression models, a single statistical interaction was evaluated for ICU length of stay and mean daily corticosteroid dose with some evidence of statistical significance. Hence, this interaction term was retained in the multivariable models. Linearity of the association of each continuous exposure variable with each primary outcome was separately assessed using locally weighted scatterplot smoothing (LOWESS plots). Standard regression diagnostics were assessed for all multivariable models, including evaluating the effect of outlier values of mean corticosteroid doses by sensitivity analyses that removed such values. These sensitivity analyses demonstrated no important change from the primary results and thus were not reported. If two covariates were highly correlated, only one was used in the multivariable models, with variance inflation factors confirming no multicollinearity in the final models (47). To illustrate the effect of the most important ICU-related risk factors (as determined from the multivariable models) on the primary physical outcomes, estimates of outcome measures were presented for a range of risk factor values occurring for a prototypical patient that had mean values for all continuous exposure variables and mode values for all binary exposures. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). A two-sided P value less than 0.05 was considered statistically significant.
Results
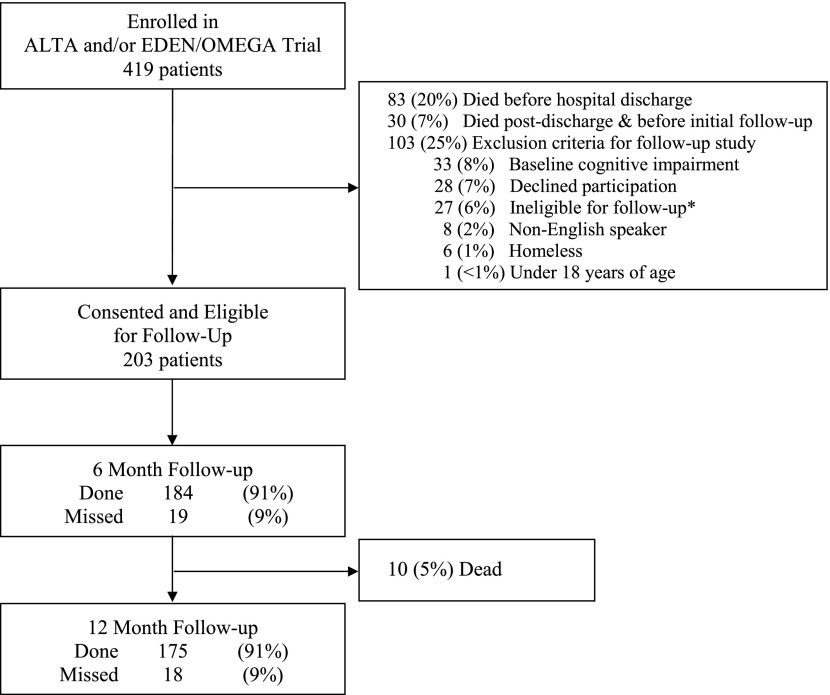
At the 12 hospital study sites participating in ALTOS, 419 patients were enrolled in the ARDSNet trials. Of these patients, 20% died before hospital discharge, 7% died after discharge and before initial follow-up, and 25% met exclusion criteria (Figure 1). For the 203 survivors who consented and were eligible for follow-up in this study (29 in ALTA, 33 in EDEN/OMEGA, 123 in EDEN, and 18 in ALTA plus EDEN/OMEGA), the mean (± SD) age was 48 (± 15) years, 49% were male, and 92% lived independently at home before hospitalization, with 80% ever having a Pao2/FiO2 ratio less than 200 during the first 3 days after enrollment (Table 1). Systemic corticosteroids were received by 85 (43%) patients on 660 (25%) of 2,596 ICU days evaluated in this study. In these patients, 71 (84%) received corticosteroids by Day 3 after ICU admission, with a mean (SD) daily dose of 52 (± 81) mg of prednisone-equivalents and duration of use of 8 (± 8) days. Among all days of steroid use, 89% occurred while patients were receiving mechanical ventilation. Mean (SD) duration of mechanical ventilation and intensive care stay were 11 (± 9) and 14 (± 11) days, respectively.
Figure 1.
Patient flow diagram. *One site joined the follow-up study late; hence, 27 patients enrolled into the ALTA and/or EDEN/OMEGA trials from that site were ineligible for follow-up.
Physical Outcomes at 6 and 12 Months: Mean Values and Correlations between Physical Measures
At 6-month follow-up, the mean (± SD) muscle strength, as a percentage of maximum MRC sum-score, was 92% (± 8%), with 8% (13 of 169) having ICU-acquired weakness, and the mean (± SD) percent-predicted values for 6MWT and SF-36 PF were 64% (± 22%) and 61% (± 36%), respectively. There was relatively small improvement observed in mean physical outcome measures between 6 and 12 months (Table 2). Correlations between the three primary physical outcomes and all seven measures in the physical test battery found the strongest correlations (0.52–0.56; P < 0.001) between the 6MWT, 4-m timed walk speed, and SF-36 PF (Table 2).
Table 2:
Summary of Physical Outcomes and Correlations with All Physical Measures at 6 Months
| Physical Outcome | Strength (% of Maximum MMT Score) (n = 191)* | 6-Minute-Walk Test (% Predicted) (n = 183)* | SF-36 Physical Function (% Predicted) (n = 200)* |
|---|---|---|---|
| 6-month, mean (SD) | 92 (8)† | 64 (22)‡ | 61 (36) |
| 12-month, mean (SD) | 93 (9)† | 67 (26)‡ | 67 (37) |
| Correlation (P value) of measure at 6-mo§ |
|||
| Arm muscle area, % predicted§ | 0.11 (0.177) | −0.06 (0.483) | −0.04 (0.643) |
| Strength, % of maximum MMT score | 1.00 (0) | 0.32 (<0.001) | 0.44 (<0.001) |
| Hand grip strength, % predicted | 0.40 (<0.001) | 0.28 (<0.001) | 0.32 (<0.001) |
| Maximal inspiratory pressure, % predicted | 0.14 (0.079) | 0.40 (<0.001) | 0.35 (<0.001) |
| 4-meter timed walk speed, m/s | 0.38 (<0.001) | 0.52 (<0.001) | 0.56 (<0.001) |
| 6-minute-walk test, % predicted | 0.32 (<0.001) | 1.00 (0) | 0.54 (<0.001) |
| SF-36 Physical Function, % predicted | 0.44 (<0.001) | 0.54 (<0.001) | 1.00 (0) |
Definition of abbreviations: MMT = manual muscle testing for muscle strength using Medical Research Council scale (30); SF-36 = Short Form-36.
N represents the number of unique patients with an outcome measure at either 6 or 12 months. The number of missing values for 6 and 12 months, respectively: MMT, 22, 31; 6-minute-walk test, 25, 34; and SF-36 PF, 3, 16.
Frequency of intensive care unit–acquired weakness (i.e., strength score <80% of maximum) and mean (SD) MMT scores at 6 and 12 months, respectively were 8% (13 of 169) and 6% (10 of 160), and 55 (5) and 55 (5) meters. The proportion of patients with muscle strength assessments who had a missing score for an extremity at 6 and 12 months is 0.6% (1 of 169) and 1.9% (3 of 160), respectively.
Mean (SD) walk distances at 6 and 12 months, respectively, were 368 m (139) and 388 m (159).
Sample sizes for correlation analyses by primary outcome: (1) for MMT: 156 for arm muscle area, 169 for MMT, 167 for grip, 159 for maximum inspiratory pressure, 161 for 4-m timed walk speed, 156 for 6-minute-walk test, 165 for SF-36 physical function; (2) for 6-minute-walk test: 148 for arm muscle area, 156 for MMT, 155 for grip, 148 for maximum inspiratory pressure, 153 for 4-m timed walk speed, 158 for 6-minute-walk test, 154 for SF-36; (3) for SF-36 physical function: 153 for arm muscle area, 165 for MMT, 164 for grip, 156 for maximum inspiratory pressure, 157 for 4-m timed walk speed, 154 for 6-minute-walk test, 197 for SF-36 physical function.
Associations of Patient Characteristics and Primary Physical Outcome Measures
In evaluating the linearity of the association of each continuous variable with each primary outcome (see Statistical Methods), corticosteroids was the only variable with a nonlinear relationship. Across each of the three primary outcomes, there was a change in the slope of the linear relationship occurring at an average daily dose of approximately 40 mg of prednisone-equivalents (Figure 2). Hence, to permit appropriate modeling of this continuous exposure variable, the regression models for each of the primary outcomes included a linear spline term for mean corticosteroid dose, using a “knot” at 40 mg (48). Separate sensitivity analyses conducted with a “knot” at 30 and 50 mg were similar to the primary results using a “knot” at 40 mg and thus are not reported.
Figure 2.
Scatterplot of the physical outcomes versus mean corticosteroid dose. The solid line in each panel represents the change in mean value of the physical outcome measure as a function of mean daily dose of corticosteroid (prednisone-equivalent dose in milligrams) using locally weighted scatterplot smoothing (LOWESS). The x axis of each panel is truncated at 300 mg, excluding a single patient with an average dose greater than 300 mg. 6MW = 6-minute-walk test; MRC = Medical Resource Council.
Bivariable associations of the patient characteristics with each of the primary outcomes are reported in Table 2, with multivariable regression model results reported in Table 3. In the multivariable regression models, after adjusting for age and sex (by including age and sex in the regression models and/or use of predicted values that adjusted for age and sex), comorbidity, and baseline functional status, two ICU-related exposure variables (modeled with their interaction as described in Statistical Methods) were significantly associated with worse outcomes across the primary physical measures: ICU length of stay and mean corticosteroid dose up to 40 mg/day of prednisone-equivalent. There was no significant association noted for use of neuromuscular blockers. Across the three primary physical outcomes, each additional week of ICU stay (in patients not receiving corticosteroids) was associated with a 1.33–4.59% decrease in the percent maximum muscle strength score or percent predicted 6MWT and SF-36 PF scores (P = 0.016 to < 0.001) (Table 4). Moreover, using an example ICU length of stay of 14 days, each 10-mg increase in mean daily prednisone-equivalent dose (up to 40 mg) was associated with a 2.52% (P = 0.032) and 4.08% (P = 0.005) decrease in percent predicted for 6MWT and SF-36 PF, respectively, with no significant change in outcomes for dose increases above a mean daily dose of 40 mg of prednisone-equivalents (Table 4).
Table 3:
Bivariable Associations of Physical Outcomes and Patient Characteristics*
| Risk Factor | Strength (% of Maximum MMT Score) (n = 191) | P Value | 6-Minute-Walk Test (% Predicted) (n = 183) | P Value | SF-36 Physical Function (% Predicted) (n = 200) | P Value |
|---|---|---|---|---|---|---|
| Age, yr | −0.16 (−0.22 to −0.10) | <0.001 | −0.13 (−0.37 to 0.10) | 0.259 | −0.40 (−0.73 to −0.08) | 0.016 |
| Male | 4.13 (2.01 to 6.25) | <0.001 | 0.81 (−5.97 to 7.60) | 0.814 | 6.81 (−2.81 to 16.43) | 0.165 |
| Body mass index, kg/m2 | −0.09 (−0.25 to 0.07) | 0.271 | −0.23 (−0.63 to 0.16) | 0.248 | −0.65 (−1.24 to −0.06) | 0.030 |
| Living independently at home | 4.51 (0.28 to 8.73) | 0.037 | 33.04 (17.49 to 48.59) | <0.001 | 35.67 (20.50 to 50.85) | <0.001 |
| Functional Comorbidity Index | −0.47 (−1.29 to 0.35) | 0.258 | −3.89 (−6.44 to −1.34) | 0.003 | −7.69 (−10.84 to −4.54) | <0.001 |
| Charlson Comorbidity Index | −0.18 (−0.87 to 0.51) | 0.612 | −2.98 (−5.32 to −0.64) | 0.013 | −3.10 (−5.64 to −0.56) | 0.017 |
| Psychiatric comorbidity | −2.06 (−4.26 to 0.15) | 0.067 | −6.31 (−13.15 to 0.52) | 0.070 | −15.45 (−24.88 to −6.01) | 0.001 |
| Substance abuse comorbidity | −0.77 (−3.40 to 1.85) | 0.564 | −2.56 (−9.78 to 4.65) | 0.486 | −7.69 (−18.05 to 2.66) | 0.145 |
| Pulmonary comorbidity | −1.30 (−4.28 to 1.67) | 0.390 | −16.77 (−25.59 to −7.94) | <0.001 | −7.71 (−19.60 to 4.19) | 0.204 |
| Rheumatologic comorbidity | −2.00 (−4.78 to 0.77) | 0.156 | −2.65 (−11.00 to 5.71) | 0.534 | −10.31 (−22.64 to 2.02) | 0.101 |
| Cardiac comorbidity | −1.60 (−4.69 to 1.48) | 0.309 | −2.24 (−12.15 to 7.68) | 0.658 | −8.77 (−21.42 to 3.89) | 0.175 |
| Neurologic comorbidity | −1.54 (−6.01 to 2.94) | 0.501 | −2.86 (−17.12 to 11.39) | 0.694 | −6.73 (−22.15 to 8.70) | 0.393 |
| Pneumonia or sepsis as ALI risk factor | 1.84 (−2.58 to 6.26) | 0.415 | −1.19 (−11.23 to 8.86) | 0.817 | 0.83 (−13.53 to 15.18) | 0.910 |
| APACHE III score | −0.03 (−0.07 to 0.00) | 0.073 | −0.04 (−0.18 to 0.10) | 0.532 | −0.12 (−0.33 to 0.09) | 0.252 |
| Pao2/FiO2 ratio <200, ever during first 3 d | −0.04 (−2.57 to 2.48) | 0.972 | −1.11 (−8.65 to 6.43) | 0.773 | 6.12 (−5.52 to 17.75) | 0.303 |
| Brussels score: mean proportion of failing organs | −0.08 (−0.16 to 0.00) | 0.053 | −0.13 (−0.38 to 0.12) | 0.318 | −0.27 (−0.63 to 0.09) | 0.140 |
| Cumulative 7-d fluid balance, L | −0.09 (−0.24 to 0.07) | 0.266 | 0.05 (−0.42 to 0.51) | 0.844 | −0.07 (−0.85 to 0.71) | 0.865 |
| Mean daily 8 a.m. blood glucose, mg/dl | 0.00 (−0.03 to 0.03) | 0.844 | 0.02 (−0.07 to 0.11) | 0.715 | −0.08 (−0.23 to 0.07) | 0.273 |
| Proportion of days with catecholamine use | −4.16 (−10.35 to 2.03) | 0.187 | −0.05 (−13.54 to 13.44) | 0.994 | −14.32 (−31.41 to 2.76) | 0.100 |
| Any dialysis | 0.73 (−1.84 to 3.30) | 0.576 | 0.27 (−8.97 to 9.52) | 0.954 | 3.60 (−10.67 to 17.88) | 0.621 |
| Any neuromuscular blocker | −2.45 (−5.04 to 0.14) | 0.064 | −1.44 (−8.95 to 6.06) | 0.706 | −5.46 (−16.01 to 5.10) | 0.349 |
| Any corticosteroids | −2.97 (−5.2 to −0.75) | 0.009 | −7.51 (−14.04 to −0.98) | 0.024 | −15.13 (−24.53 to −5.73) | 0.002 |
| Mean corticosteroid dose† (per 10 mg of prednisone-equivalent increase in mean dose when mean < 40 mg) | −0.29 (−1.07 to 0.49) | 0.465 | −3.40 (−5.65 to −1.15) | 0.003 | −3.98 (−7.06 to −0.90) | 0.011 |
| Mean corticosteroid dose† (per 10 mg of prednisone-equivalent increase in mean dose when mean ≥ 40 mg) | −0.50 (−1.0 to 0.00) | 0.052 | 0.83 (−0.19 to 1.85) | 0.109 | −0.04 (−1.04 to 0.96) | 0.944 |
| Mechanical ventilation duration, per week | −1.26 (−2.25 to −0.26) | 0.013 | −1.14 (−3.59 to 1.30) | 0.360 | −3.24 (−6.50 to 0.00) | 0.050 |
| Intensive care unit length of stay, per week | −1.42 (−2.23 to −0.62) | 0.001 | −1.76 (−3.60 to 0.09) | 0.062 | −3.44 (−6.11 to −0.77) | 0.012 |
Definition of abbreviations: ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation; MMT = manual muscle testing for muscle strength using Medical Research Council scale (30); SF-36 = Short Form-36.
Values represent the average increase (or decrease) in the physical outcome measure for a one-unit increase in a continuous exposure, or the average increase (or decrease) for presence (vs. absence) of a binary exposure.
For regression modeling, mean corticosteroid dose was calculated for all patients, across all patient days, with a dose of zero used for days in which no corticosteroid was given.
Table 4:
Multivariable Associations of Physical Outcomes and Patient Characteristics
| Risk Factor | Strength, % of Maximum MMT Score (n = 191) | P Value | 6-minute-walk Test, % Predicted (n = 183) | P Value | SF-36 Physical Function, % Predicted (n = 200) | P Value |
|---|---|---|---|---|---|---|
| Age, yr | −0.16 (−0.21 to −0.10) | <0.001 | −0.23 (−0.56 to 0.10) | 0.165 | ||
| Male | 4.25 (2.41 to 6.09) | <0.001 | 4.34 (−4.93 to 13.61) | 0.359 | ||
| Body mass index, kg/m2 | −0.45 (−1.09 to 0.19) | 0.171 | ||||
| Living independently at home | 3.44 (−0.83 to 7.72) | 0.115 | 27.88 (11.13 to 44.64) | 0.001 | 23.93 (7.88 to 39.98) | 0.003 |
| Functional Comorbidity Index | −1.36 (−3.94 to 1.22) | 0.303 | −6.43 (−10.86 to −2.01) | 0.004 | ||
| Charlson Comorbidity Index | −1.56 (−4.24 to 1.13) | 0.256 | −0.65 (−3.43 to 2.14) | 0.650 | ||
| Psychiatric comorbidity | −0.37 (−2.11 to 1.37) | 0.677 | −1.63 (−8.79 to 5.54) | 0.656 | −3.53 (−14.17 to 7.11) | 0.515 |
| Substance abuse comorbidity | −10.91 (−20.53 to −1.30) | 0.026 | ||||
| Pulmonary comorbidity | −11.04 (−19.60 to −2.49) | 0.011 | ||||
| Rheumatologic comorbidity | −0.18 (−2.82 to 2.45) | 0.893 | 2.69 (−9.83 to 15.20) | 0.674 | ||
| Cardiac comorbidity | 12.34 (−0.42 to 25.10) | 0.058 | ||||
| APACHE III score | 0.02 (−0.01 to 0.05) | 0.113 | ||||
| Brussels score: mean proportion of failing organs | −0.04 (−0.12 to 0.03) | 0.256 | 0.13 (−0.30 to 0.56) | 0.561 | ||
| Proportion of days with catecholamine use | −3.23 (−9.41 to 2.95) | 0.305 | −6.60 (−25.85 to 12.65) | 0.502 | ||
| Any neuromuscular blocker | −2.23 (−4.67 to, 0.22) | 0.074 | ||||
| Mean corticosteroid dose* (per 10 mg of prednisone-equivalent increase in mean dose when mean <40 mg) at ICU LOS = 14† | −0.15 (−0.81 to 0.52) | 0.666 | −2.52 (−4.31 to −0.19) | 0.032 | −4.08 (−6.95 to −1.21) | 0.005 |
| Mean corticosteroid dose* (per 10 mg of prednisone-equivalent increase in mean dose when mean ≥40 mg) at ICU LOS = 14† | −0.12 (−0.42 to 0.18) | 0.421 | 0.69 (−0.40 to 1.78) | 0.216 | 0.45 (−0.84 to 1.75) | 0.494 |
| ICU LOS among patients with no corticosteroid use, per week† | −1.33 (−1.93 to −0.74) | <0.001 | −2.81 (−5.09 to −0.53) | 0.016 | −4.59 (−8.04 to −1.14) | 0.009 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit; LOS = length of stay.
For regression modeling, mean corticosteroid dose was calculated for all patients, across all patient days, with a dose of zero used for days in which no corticosteroid was given.
Multivariable regression model includes statistical interaction term for ICU length of stay and mean corticosteroid dose (using linear spline with a knot at 40 mg of prednisone-equivalents). The interaction term coefficient (95% confidence interval) for each physical outcome measure for mean corticosteroid dose less than 40 mg and greater than or equal to 40 mg, respectively, are as follows: (1) MMT strength: 0.46 (0.20 to 0.72; P < 0.001) and −0.25 (−0.34 to −0.16; P < 0.001); (2) 6-minute-walk test: 0.97 (0.01 to 1.93; P = 0.048) and −0.58 (−1.57 to 0.41; P = 0.249); and (3) SF-36 Physical Function: 0.71 (−0.94 to 2.35; P = 0.399) and −0.40 (−0.81 to 0.01; P = 0.057).
Across each of the three physical outcomes, there was statistical interaction between ICU length of stay and steroid dose (Table 4), indicating that the negative effect of corticosteroids (up to a mean daily dose of 40 mg of prednisone-equivalents) was greatest in patients with a shorter ICU length of stay. For example, using a prototypical patient from this multicenter cohort with an ICU length of 5 versus 15 days, use of systemic corticosteroids (modeled as 30 vs. 0 mg of mean daily prednisone-equivalent dose) was associated with an absolute decrease in percent-predicted SF-36 PF score of 15% versus 12%, respectively (Table 5).
Table 5:
Estimated Effects of Mean Corticosteroid Dose and ICU LOS with Physical Outcomes for a Prototypical Patient at 6 Months after ALI*
| Mean Steroid Dose (mg) | ICU LOS |
||
|---|---|---|---|
| 5 d | 15 d | ||
| Strength, % of maximum MMT score | 0 | 93 | 91 |
| 30 | 91 | 91 | |
| 6-minute-walk test, % predicted | 0 | 72 | 68 |
| 30 | 61 | 61 | |
| SF-36 Physical Function, % predicted | 0 | 74 | 67 |
| 30 | 59 | 55 | |
Definition of abbreviations: ALI = acute lung injury; ICU = intensive care unit; LOS = length of stay; MMT = manual muscle testing for muscle strength using Medical Research Council scale; SF-36 = Short Form-36.
Estimates were calculated using multivariable linear regression (as described in Table 4) with an interaction term for ICU length of stay and mean corticosteroid dose (using linear spline with a knot at 40 mg of prednisone-equivalents) for a prototypical patient from this multicenter ALI cohort with mean values for all continuous covariates and mode values for all binary covariates.
Post hoc sensitivity analyses demonstrated no material change in study results with (1) exclusion of the 17 patients with neurologic comorbidity; (2) inclusion of a treatment indicator for the antioxidant supplement received by 29 patients in the OMEGA trial and for the aerosolized albuterol intervention received by 19 patients in the ALTA trial or the interactions of each of these randomized interventions with blood glucose levels or with each of the corticosteroid and the ICU length of stay exposure variables; (3) inclusion of mean daily dose of propofol, benzodiazepines, and opioids in the multivariable model; and (4) evaluation for a statistical interaction of study center with the corticosteroid and ICU length of stay exposures.
Discussion
In this 1-year, multicenter, longitudinal, follow-up study (the ALTOS study) of 203 survivors of ALI from the ARDSNet ALTA and EDEN/OMEGA trials, patients demonstrated substantial impairments in percent-predicted values for 6MWT and SF-36 PF physical outcome measures, with ICU length of stay and mean daily dose of systemic corticosteroids significantly associated with these impairments.
The results for the muscle strength outcome measure revealed less impairment than for the 6MWT and SF-36 PF measure. This finding reflects that factors other than strength alone contribute to impairments in physical functioning (6MWT) and quality of life (SF-36 PF), including issues of muscle endurance, cardiopulmonary function, and psychological status (2, 49, 50). Moreover, this result may also reflect that the ordinal MRC sum-score, used to evaluate muscle strength, has a ceiling effect that does not occur with the 6MWT and SF-36 outcome measures.
There are important similarities and differences in the physical outcomes reported in this study versus prior longitudinal, follow-up evaluations of survivors of ALI. The 6MWT and SF-36 PF results reported herein were remarkably similar to those reported by Herridge and coworkers (2) in their landmark single-center study conducted 10 years previously. However, the physical outcomes in this study were modestly less impaired than those reported by Fan and coworkers (1) in their single-center study conducted 5 years previously. Because pre-ALI baseline physical test values cannot be obtained for participants, 6MWT and SF-36 PF tests are typically compared with matched normal values (1–3). Consequently, the 6- and 12-month impairments reported in this study and in prior research (1–3) may reflect some amount of pre-ALI impairment. However, the survivors evaluated in our study were relatively young (mean age, 48 yr) with 92% living independently at home before ALI. Moreover, as further evidence of physical impairments being ICU-acquired, a prior report on this cohort indicated that over 1-year follow-up, 56% of EDEN survivors required new institutionalization post-ALI and/or physical rehabilitation, with only 48% of previously employed survivors returning to work (20). In addition, in mechanically ventilated patients, there is clear evidence of early and rapid muscle wasting (13, 14), especially in patients with more severe oxygenation impairment (e.g., ALI) and multiorgan failure (13). Existing cohort studies with prospective measurement of pre-ICU status also have clearly demonstrated the occurrence of important new post-ICU physical impairments (4–6). Hence, these physical impairments after an ICU stay are important findings.
The significant associations of ICU length of stay and corticosteroid dose with impaired physical outcomes in 183 survivors of ALI from our multicenter study builds on prior research conducted in both general populations of mechanically ventilated and ICU patients (11, 51, 52) and in patients with ALI. For instance, in studies of patients with ALI specifically, a single-center study of 83 survivors at 6- and 12-month follow-up demonstrated an independent negative association for the use of any corticosteroids with 6MWT, but this association did not reach statistical significance (P ≥ 0.14), possibly because of a smaller sample size than our study (2). Similarly, in another single-center study of 136 survivors of ALI, Fan and coworkers (1) demonstrated that the duration of bed rest, which closely paralleled ICU length of stay in that study, was significantly and independently associated with decreased muscle strength at 12 months and was marginally associated at 6 months (1). Similar to this prior research (1), in our study, we believe that ICU length of stay also approximates duration of bed rest because most of our patients were in study sites not providing early mobility or rehabilitation services in the ICU, a finding consistent with (1) data from control groups in US trials of ICU rehabilitation (53–55), (2) a US national survey that reported only 10% of hospitals had criteria for initiating physical therapy in the ICU (56), and (3) large-scale international point prevalence studies of ICU mobility (57, 58).
Importantly, the duration of bed rest is clearly associated with physical impairments (59), and is a modifiable risk factor for such impairments (53, 54, 60, 61). More specifically, both a metaanalysis of randomized trials and studies of routine ICU clinical practice have demonstrated that early rehabilitation decreases ICU length of stay (60, 62, 63). Hence, early rehabilitation may have a direct benefit on physical impairment, plus an indirect benefit from reducing ICU length of stay, thus making length of stay a modifiable risk factor for physical impairment. In addition, randomized trials have demonstrated other evidence-based practices that consistently reduce ICU length of stay, including using less sedation and standardized ventilator liberation practices (64–68).
This study also demonstrates new evidence of potential interplay between mean daily dose of corticosteroids and ICU length of stay, demonstrating that the magnitude of the corticosteroid effect is attenuated with larger doses and with a longer ICU stay. This finding builds on prior research. For instance, in a secondary analysis of the ARDS Network’s randomized trial of methylprednisolone (15, 69), corticosteroids were significantly associated with neuromyopathy within the first 28 days after randomization, but not with its cumulative incidence over the entire hospital stay. Moreover, a landmark study demonstrated that in-hospital muscle weakness was independently associated with receipt of corticosteroids, but not with its duration of use or cumulative dosage (11). When evaluated together, our results and prior research provide new insights into understanding the association between corticosteroids and post-ICU physical impairments. These findings may be explained by the cumulative probability of ICU-acquired physical complications being so high after a long hospital stay that it becomes difficult to detect an effect uniquely attributable to corticosteroid use in the ICU. Alternatively, there may be competing risks (e.g., an effect of corticosteroids on duration of mechanical ventilation) that complicate the understanding of the direct association between corticosteroids and physical outcomes (70). Further mechanistic and clinical investigation of these findings is required, ideally conducted within the setting of any future masked randomized trial evaluating the use of corticosteroids in ALI (69, 71), to control for bias arising from differences in patient and ICU-factors associated with use of corticosteroids. Such studies could provide important insights through specifically evaluating the effect of corticosteroids on muscles, including assessment of potential mediation via their effect on adrenal function and other aspects of endocrine function (51).
These data on the use of corticosteroids and their dose–response relationship with physical impairments, in addition to recent data on corticosteroids’ association with ICU delirium (72) and other in-hospital adverse effects (52), should help inform clinicians when considering indications for corticosteroid use and weighing the risks versus potential benefits of corticosteroid use and associated dosing and duration of use. Given substantial practice variability in corticosteroid prescribing in the ICU setting (52, 73), clinicians should critically evaluate the indications for corticosteroid use, and their dose and duration, because corticosteroid may be a potentially modifiable risk factor for physical impairments after hospital discharge.
In considering our study, there are important strengths, including detailed collection of patient baseline status, daily collection of relevant ICU-related exposure variables (e.g., daily corticosteroid dose), and use of a detailed battery of performance-based and patient-reported physical tests, ranging across the span of muscle strength, physical function, and quality of life, at 6- and 12-month follow-up, with low rates of missed visits. However, this study also has potential limitations. First, as an observational study, we cannot assess causality of the associations of ICU length of stay and corticosteroids with post-ALI physical impairments. However, our results are consistent with detailed ultrasound, histologic, and biochemical muscle evaluations in mechanically ventilated ICU patients, which demonstrate early and rapid muscle wasting that is progressive with increasing ICU length of stay (13, 14), and with preclinical data on the effect of corticosteroids on muscles (74, 75). Patient characteristics and the medical indications for administering corticosteroids may confound the association with physical complications. Consequently, our analyses evaluated relevant factors potentially related to corticosteroid administration, including preexisting pulmonary and rheumatologic comorbidities and use of catecholamines (as a marker of septic shock). Data on the exact indication for corticosteroid use and residual confounding remain limitations of the existing analyses. Detailed evaluations of physical outcomes in any future randomized trial of corticosteroids would be invaluable in helping assess causality and confirming the associations observed in this study and prior research. Second, the results may not be generalizable to all ALI or ICU survivors because the survivors were relatively young (mean age, 48 yr) and the ARDSNet trials had exclusion criteria, including severe malnutrition, lung, liver, or neuromuscular disease, and primarily included patients with pneumonia and nonpulmonary sepsis. However, generalizability is aided by the marked similarity in survivors’ age in our study versus prior research (1, 2) and by the multicenter nature of the study (12 hospitals from five study centers). Third, the absence of physical measures before the onset of ALI, because of the nonfeasible nature of such measurement within the context of clinical trials, and the lack of short-term physical outcome measures at ICU or hospital discharge are important limitations,. Finally, despite the relatively large sample size, the study is underpowered for detecting additional potential statistical interactions.
In conclusion, in this 1-year, multicenter, longitudinal follow-up study, survivors of ALI demonstrated substantial impairments in 6MWT and SF-36 PF physical outcome measures at 6- and 12-month follow-up. ICU length of stay (a proxy for duration of bed rest in this study) and mean corticosteroid dose were important, potentially modifiable risk factors for these physical impairments, whereas use of neuromuscular blockers was not associated with such impairments. These findings, in the context of the prior literature, suggest that minimizing corticosteroid usage, dose, and duration, and implementing existing evidence-based strategies to reduce ICU length of stay and associated bed rest may be important changes in clinical practice to improve ALI survivors’ frequent and long-lasting physical impairments.
Acknowledgments
Acknowledgment
The authors thank all patients and their proxies who participated in the study. They acknowledge our dedicated research staff, including the following who assisted with data collection, training/quality assurance, and/or data management: Lindsay Anderson, Ellen Caldwell, Nancy Ciesla, William Flickinger, Jacqueline Flynn, Jonathan Gellar, Stephanie Gundel, John Keenan, Christopher Mayhew, Melissa McCullough, Jessica McCurley, Mardee Merrill, Laura Methvin, Kristin Sepulveda, Kelly Swanson, Elizabeth Vayda, and Cassie Wicken.
Investigators and Research Staff from NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network Sites that Participated in this Follow-up Study: University of Washington, Harborview (*L. Hudson, S. Gundel, C. Hough, M. Neff, K. Sims, A. Ungar, T. Watkins); Johns Hopkins University (*R. Brower, H. Fessler, D. Hager, P. Mendez-Tellez, D. Needham, K. Oakjones); Johns Hopkins Bayview Medical Center (J. Sevransky, A. Workneh); University of Maryland (C. Shanholtz, D. Herr, H. Howes, G. Netzer, P. Rock, A. Sampaio, J. Titus); Union Memorial Hospital (P. Sloane, T. Beck, D. Highfield, S. King); Washington Hospital Center (B. Lee, N. Bolouri); Vanderbilt University (*A.P. Wheeler, G.R. Bernard, M. Hays, S. Mogan, T.W. Rice); Wake Forest University (*R.D. Hite, A. Harvey, P.E. Morris, Mary Ragusky); Intermountain Medical Center (*A. Morris, *C. Grissom, A. Austin, S. Barney, S. Brown, J. Ferguson, H. Gallo, T. Graydon, E. Hirshberg, A. Jephson, N. Kumar, M. Lanspa, R. Miller, D. Murphy, J. Orme, A. Stowe, L. Struck, F. Thomas, D. Ward,); LDS Hospital (P. Bailey, W. Beninati, L. Bezdjian, T. Clemmer, S. Rimkus, R. Tanaka, L. Weaver); McKay Dee Hospital (C. Lawton, D. Hanselman); Utah Valley Regional Medical Center (K. Sundar, W. Alward, C. Bishop, D. Eckley, D. Harris, T. Hill, B. Jensen, K. Ludwig, D. Nielsen, M. Pearce). Clinical Coordinating Center: Massachusetts General Hospital and Harvard Medical School (*D. Schoenfeld, N. Dong, M. Guha, E. Hammond, P. Lazar, R. Morse, C. Oldmixon, N. Ringwood, E. Smoot, B.T. Thompson, R. Wilson). National Heart, Lung and Blood Institute: A. Harabin, S. Bredow, M. Waclawiw, G. Weinmann. Data and Safety Monitoring Board: R. G. Spragg (chair), A. Slutsky, M. Levy, B. Markovitz, E. Petkova, C. Weijer. Protocol Review Committee: J. Sznajder (chair), M. Begg, L. Gilbert-McClain E. Israel, J. Lewis, S. McClave, P. Parsons.
*Principal investigator.
Footnotes
Supported by the NHLBI, which funded this follow-up study (R01HL091760, R01HL091760-02S1, and R01HL096504); the Johns Hopkins Institute for Clinical and Translational Research (UL1 TR 000424-06); and the ALTA and EDEN/OMEGA trials (contracts for sites participating in this study: HSN268200536170C, HHSN268200536171C, HHSN268200536173C, HHSN268200536174C, HSN268200536175C, and HHSN268200536179C).
Author Contributions: All authors contributed to the conception and/or design of this study. D.M.N., V.D.D., P.E.M., C.L.H., J.C.J., P.A.M.-T., and R.O.H. contributed to the acquisition of data. All authors contributed to the analysis and interpretation of data. D.M.N. drafted the manuscript, and all authors critically revised it for important intellectual content and approved the final version to be submitted.
Originally Published in Press as DOI: 10.1164/rccm.201401-0158OC on April 9, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. Physical complications in acute lung injury survivors: a 2-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 8.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Netzer G. The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med. 2012;33:327–338. doi: 10.1055/s-0032-1321982. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 12.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 13.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 14.Wollersheim T, Woehlecke J, Krebs M, Hamati J, Lodka D, Luther-Schroeder A, Langhans C, Haas K, Radtke T, Kleber C, et al. Dynamics of myosin degradation in intensive care unit-acquired weakness during severe critical illness. Intensive Care Med. 2014;40:528–538. doi: 10.1007/s00134-014-3224-9. [DOI] [PubMed] [Google Scholar]
- 15.Hough CL, Steinberg KP, Taylor Thompson B, Rubenfeld GD, Hudson LD. Intensive care unit-acquired neuromyopathy and corticosteroids in survivors of persistent ARDS. Intensive Care Med. 2009;35:63–68. doi: 10.1007/s00134-008-1304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β₂-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, et al. NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 22.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 23.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 24.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Groll DL, Heyland DK, Caeser M, Wright JG. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil. 2006;85:574–581. doi: 10.1097/01.phm.0000223220.91914.61. [DOI] [PubMed] [Google Scholar]
- 26.New York: McGraw-Hill; 2006. Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's the pharmacological basis of therapeutics, 11th ed. [Google Scholar]
- 27.Frisancho AR. Ann Arbor, MI: The University of Michigan Press; 1990. Anthropometric standards for the assessment of growth and nutritional status. [Google Scholar]
- 28.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. National Health and Nutrition Examination Survey: anthropometry procedures manual. [Google Scholar]
- 29.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36:1038–1043. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medical Research Council. London: Her Majesty's Stationary Office; 1976. Aids to the investigation of the peripheral nervous system. [Google Scholar]
- 31.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 32.Harik-Khan RI, Wise RA, Fozard JL The Baltimore Longitudinal Study of Aging. Determinants of maximal inspiratory pressure. Am J Respir Crit Care Med. 1998;158:1459–1464. doi: 10.1164/ajrccm.158.5.9712006. [DOI] [PubMed] [Google Scholar]
- 33.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 34.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 38.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Kosinski M, Dewey JE. Lincoln, RI: QualityMetric Incorporated; 2000. How to score Version 2 of the SF-36 Health Survey. [Google Scholar]
- 40.Needham DM, Dennison CR, Dowdy DW, Mendez-Tellez PA, Ciesla N, Desai SV, Sevransky J, Shanholtz C, Scharfstein D, Herridge MS, et al. Study protocol: The Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2006;10:R9. doi: 10.1186/cc3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. J Clin Epidemiol. 2007;60:757–765. doi: 10.1016/j.jclinepi.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tansey CM, Matté AL, Needham D, Herridge MS. Review of retention strategies in longitudinal studies and application to follow-up of ICU survivors. Intensive Care Med. 2007;33:2051–2057. doi: 10.1007/s00134-007-0817-6. [DOI] [PubMed] [Google Scholar]
- 43.Hall EA, Zuniga R, Cartier J, Anglin MD, Danila B, Ryan T, Mantius K. 2nd ed. Los Angeles, CA: UCLA Integrated Substance Abuse Programs; 2003. Staying in touch: a fieldwork manual of tracking procedures for locating substance abusers in follow-up studies. [Google Scholar]
- 44.Roary MC, Hill MN, Bone LR, Levine DM. Innovative strategies that dispel the myths about urban young black men with high blood pressure. Cardiovasc Rev Rep. 2000;21:129–137. [Google Scholar]
- 45.Coday M, Boutin-Foster C, Goldman Sher T, Tennant J, Greaney ML, Saunders SD, Somes GW. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Ann Behav Med. 2005;29:55–65. doi: 10.1207/s15324796abm2902s_9. [DOI] [PubMed] [Google Scholar]
- 46.Hunt JR, White E. Retaining and tracking cohort study members. Epidemiol Rev. 1998;20:57–70. doi: 10.1093/oxfordjournals.epirev.a017972. [DOI] [PubMed] [Google Scholar]
- 47.Belsley DA, Kuh E, Welsch RE. New York: Wiley; 1980. Regression diagnostics: identifying influential data and sources of collinearity. [Google Scholar]
- 48.Harrell FE. New York: Springer; 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. [Google Scholar]
- 49.Weisman IM, Zeballos RJ. Clinical exercise testing. Clin Chest Med. 2001;22:679–701, viii. doi: 10.1016/s0272-5231(05)70060-5. [DOI] [PubMed] [Google Scholar]
- 50.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharshar T, Bastuji-Garin S, De Jonghe B, Stevens RD, Polito A, Maxime V, Rodriguez P, Cerf C, Outin H, Touraine P, et al. Hormonal status and ICU-acquired paresis in critically ill patients. Intensive Care Med. 2010;36:1318–1326. doi: 10.1007/s00134-010-1840-6. [DOI] [PubMed] [Google Scholar]
- 52.Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:1052–1064. doi: 10.1164/rccm.201401-0058OC. [DOI] [PubMed] [Google Scholar]
- 53.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 55.Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, Brower RG, Needham DM. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25:254–262. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Hodgin KE, Nordon-Craft A, McFann KK, Mealer ML, Moss M.Physical therapy utilization in intensive care units: results from a national survey Crit Care Med 200937561–566.quiz 566–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, Kaltwasser A, Mende H, Rothaug O, Schuchhardt D, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 58.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, Denehy L. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 59.Brower RG.Consequences of bed rest Crit Care Med 20093710 SupplS422–S428. [DOI] [PubMed] [Google Scholar]
- 60.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, Hermans G, Decramer M, Gosselink R. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 62.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 63.Lord RK, Mayhew CR, Korupolu R, Mantheiy EC, Friedman MA, Palmer JB, Needham DM. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41:717–724. doi: 10.1097/CCM.0b013e3182711de2. [DOI] [PubMed] [Google Scholar]
- 64.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 65.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 66.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 67.Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 70.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C.Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications Med Care 2010486, SupplS96–S105. [DOI] [PubMed] [Google Scholar]
- 71.Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 72.Schreiber MP, Colantuoni E, Bienvenu OJ, Neufeld KJ, Chen KF, Shanholtz C, Mendez-Tellez PA, Needham DM.Corticosteroids and transition to delirium in patients with acute lung injury Crit Care MedIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karir V, Cooke CR, Andersson L, Caldwell E, Rubenfeld GD. Practice variability in the assessment and treatment of critical illness-related corticosteroid insufficiency. J Crit Care. 2010;25:363, e9–e363, e14. doi: 10.1016/j.jcrc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Horinouchi H, Kumamoto T, Kimura N, Ueyama H, Tsuda T. Myosin loss in denervated rat soleus muscle after dexamethasone treatment. Pathobiology. 2005;72:108–116. doi: 10.1159/000084113. [DOI] [PubMed] [Google Scholar]
- 75.Di Giovanni S, Mirabella M, D’Amico A, Tonali P, Servidei S. Apoptotic features accompany acute quadriplegic myopathy. Neurology. 2000;55:854–858. doi: 10.1212/wnl.55.6.854. [DOI] [PubMed] [Google Scholar]