A major pharmaceutical success story of the late 20th century was the introduction of statins into clinical medicine. This class of drugs (the firefighters) has contributed to reduced morbidity and mortality from atherosclerotic cardiovascular disease by lowering total cholesterol levels, particularly LDL cholesterol (the arsonists), and also by raising HDL cholesterol levels, albeit less dramatically1. Triglyceride levels may also be reduced. The success of this approach in older adults has also led to the controversial proposal to begin statin therapy even earlier in younger individuals with elevated lipid levels2. Other benefits have also been attributed to statins, including effects on inflammatory and immune responses after myocardial infarction3. Despite this success story, statins are not a panacea. Some patients suffer adverse reactions such as myositis that preclude their taking optimal doses and in some instances prevent statin use altogether. Much less commonly, statins can lead to rhabdomyolysis, especially in combination with drugs such as gemfibrozil4. More recently, the FDA mandated a change in labelling which warns consumers that statins can lead to poorer control of blood glucose levels in patients with type 2 diabetes5. Alternative approaches to the treatment of elevated cholesterol levels, for example therapy with niacin or drugs that influence cholesterol metabolism such as ezetimibe, have either failed in randomized clinical trials6 or fallen out of favor because of relatively weak strength of evidence7.
In this issue of Circulation, Chatterjee et al. describe an additive or alternative experimental approach to statin therapy8. Sphingosine is an unsaturated 18-carbon alcohol that forms the backbone of sphingolipids, which are essential for normal cell membrane structure and function. Sphingolipid metabolites include ceramide and sphingosine 1-phosphate (S1P), signaling molecules considered to have opposing effects, ceramide being pro-apoptotic and S1P being pro-survival9. The ceramide biosynthetic pathway includes the transformation of ceramide into glucosylceramide and lactosylceramide by specific transferase enzymes (Figure 1). It has been known for several decades that these glycosphingolipids (GSLs) are present in human plasma10,11. GSLs such as lactoceramide are also found in excess in atherosclerotic plaque intima, and have been implicated in aortic smooth muscle cell proliferation12.
Figure 1.
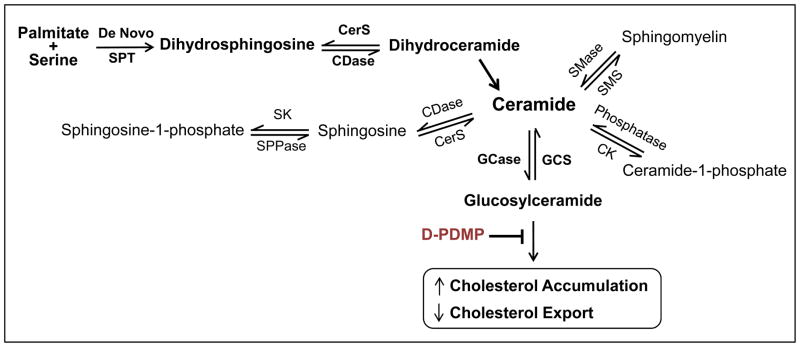
Shown in schematic format is the pathway leading to glucosylceramide. De novo synthesis of ceramide begins with the condensation of palmitate and serine to form 3-keto-dihydrosphingosine (not depicted). The latter is reduced to dihydrosphingosine followed by acylation to yield dihydroceramide, which in turn generates ceramide via the action of desaturases. Ceramide is at the hub of several pathways, as it can be transformed into sphingomyelin, sphingosine and sphingosine 1-phosphate, ceramide 1-phosphate or glucosylceramide. As noted in the text, glucosylceramide (and lactylceramide) foster cellular cholesterol accumulation and reduce cholesterol export. D-DMPP inhibits these actions of glucosylceramide, retarding cholesterol accumulation and enhancing cholesterol export via transporter molecules such as SR-B1, CD36 and ABCA1. CDase, ceramidase; CerS, (dihydro)ceramide synthase; CK, ceramide kinase; GCase, glucosyl ceramidase; GCS, glucosyl ceramide synthase; SK, sphingosine kinase; SMase, sphgomyelinase; SMS, sphingomyelin synthase; SPPase, sphingosine phosphate phosphatase; SPT, serine palmitoyl transferase.
Inhibitors of GSL synthesis which exhibit a profound effect on lipid metabolism have been developed. Among these is D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-PDMP) which blocks the synthesis of the GSL glucosylceramide8. Chaterjee et al. fed a high fat, high cholesterol diet to male mice deficient in Apolipoprotein E (Apo E−/−)8. This diet resulted in increased aortic wall thickening and extensive aortic calcium deposits, which were prevented by daily oral gavage of 10 mg/kg D-PDMP. Increased arterial stiffness was also prevented by D-PDMP. Similarly, progressive aortic atherosclerosis and coronary artery plaque deposition were reduced/prevented by D-PDMP treatment. In these mice D-PDMP decreased glycosyltransferase activity in the aorta, reduced levels of oxidized LDL and triglycerides, and increased the expression of transporter genes involved in cholesterol efflux. The mRNA for HMG-CoA reductase increased, which would be expected to augment cholesterol synthesis, but total plasma cholesterol was decreased. This may in part be associated with an increase in the mRNA of molecules involved in cholesterol efflux such as the scavenger receptor class B type 1 (SR-B1), CD36 and the ATP-binding cassette transporter A1 (ABCA1) reported in the paper. However, the authors do not show any measurements of either the protein levels or activities of these moieties, nor do they discuss the possible mechanisms involved. They also examined atherosclerosis in New Zealand white rabbits fed a similar diet and treated or not with D-PDMP, which had the same effect as in ApoE−/− mice. Similar findings were reported by Bietrix et al. in APOE*3 Leiden and LDL receptor−/− mice using the iminosugar N-(5′-adamantane-1′-yl-methoxy)-pentyl-1-deoxynoijirimycin (AMP-DNM), which is also a glucosylceramide inhibitor13.
The mechanisms whereby GSL synthesis inhibition influences cholesterol metabolism and atherosclerosis have been previously investigated. For example, in human foam cells and macrophages, the GSL lactoceramide resulted in cholesterol accumulation14,15. This observation led to a “molecular trap” hypothesis which states that increased cholesterol sequestration can be stimulated by GSLs in late endosomes/lysosomes14. Subsequently Glaros et al. reported that PDMP enhanced cholesterol export from cholesterol laden macrophage foam cells via ABCA115. As noted above, Chatterjee et al. also described an increase in the mRNA for this transporter in response to D-PDMP treatment. Other transporters similarly affected included the aforementioned CD36 and SR-B18. D-PDMP also enhanced cholesterol metabolism via the LDL receptor pathway as evidenced by increases in LDL receptor protein mass and elevated expression of the sterol regulatory element binding protein (SREBP2)8. In vascular smooth muscle cells, PDMP has also been reported to enhance IL-1β stimulation of nitrite thereby providing an additional mechanism whereby this GSL may be of benefit in atherosclerotic vessels.
The first rate-limiting step in the sphingolipid biosynthetic pathway is the formation of 3-ketosphinganine from serine and palmitoyl CoA catalyzed by serine palmitoyltransferase. Myriocin, a natural product isolated from the plant Isari sinclairii, inhibits this enzyme and exhibits immunosuppressive activity. Chemical derivatization of myriocin yielded FTY720, an S1P1and 3 receptor agonist, which is an FDA-approved drug for the treatment of multiple sclerosis in humans. It became of interest to determine if FTY720 could retard the development of atherosclerosis. In two studies, one in LDL receptor deficient mice16 and another in ApoE null mice17, FTY720 was indeed reported to significantly reduce atherosclerosis. Subsequently, Poti et al reported that KRP-203, a selective S1P receptor type 1 agonist, ameliorated atherosclerosis in LDL receptor−/− mice without affecting lipid levels18. In the latter study there was extensive reduction of inflammatory markers.
In a more recent report, Wang et al. studied ApoeR61h/h mice that express a form of mouse apoE that closely resembles the native mouse protein at 5% of normal plasma levels19. These ApoeR61h/h mice, also known as HypoE mice20, were bred to mice deficient in SR-B1. The HypoE allele predisposes to diet-induced hyperlipidemia20 that causes occlusive coronary atherosclerosis and myocardial infarction when the resulting ApoeR61h/h/SRB1−/− mice are fed a diet rich in fat and cholesterol. Wang et al. then asked whether FTY720 placed in the drinking water could retard atherosclerotic obstruction of coronary arteries as well as aortic root atherosclerosis19. In the ApoeR61h/h/SRB1−/− mice, mortality was high during 4 weeks of high fat, high cholesterol feeding compared with animals fed the same diet plus FTY720 (34 vs 5%, P < 0.02).
Like the study of Poti et al.18, there was no reduction in serum lipids, nor were any effects on coronary or aortic root atherosclerosis observed. Similar to the results reported by Poti et al. there was also a marked alteration of immune responses. Thus, Wang et al. noted reduced numbers of T and B cells, and an increased proportion of Tregs19. A remarkable new finding was that in addition to enhancing longevity, FTY720 surprisingly and substantially improved left ventricular function measured by serial echocardiography that could have been a consequence of immunosuppression19. This observation suggests that S1P1 agonism could be useful in the treatment of heart failure.
In summary, the report by Chatterjee et al. emphasizes the importance of biochemical arson as a key element in the pathogenesis of atherosclerosis, and the current paper and other recent published data provide evidence that manipulation of immune system responses can damp down the flames responsible for these conflagrations.
Acknowledgments
I thank Robert Raffai, PhD for helpful comments and Norman Honbo, MA for preparing Figure 1.
Funding Sources: R01 HL 090606 from the National Heart, Lung and Blood Institute to JSK.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active comparitor trials. Euro J Prevent Cardiol. 2013;20:641–657. doi: 10.1177/2047487313480435. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Glass CK, Witztum JL. Evidence mandating earlier and more aggressive treatment of hypercholesterolemia. Circulation. 2008;118:672–677. doi: 10.1161/CIRCULATIONAHA.107.753152. [DOI] [PubMed] [Google Scholar]
- 3.Patel MJ, Blazing MA. Inflammation and atherosclerosis: Disease modulating therapies. Curr Treat Opt Cardiovasc Med. 2013;15:681–695. doi: 10.1007/s11936-013-0268-z. [DOI] [PubMed] [Google Scholar]
- 4.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: Mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N, Taskinen M-R. Statins are diabetogenic – Myth or reality? Atheroscler Suppl. 2012;13:1–10. doi: 10.1016/j.atherosclerosissup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Tariq SM, Sidhu MS, Toth PP, Boden WE. HDL hypothesis: where do we stand now? Curr Atheroscler Rep. 2014;16:398. doi: 10.1007/s11883-014-0398-0. [DOI] [PubMed] [Google Scholar]
- 7.Monroe AK, Gudzune KA, Sharma R, Chelladurai Y, Ranashinghe PD, Ansari MT, Robinson KA. Comparative effectiveness Review No 132. Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2014. Combination therapy versus intensification of statin monotherapy: An update. (prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2012-00007). AHRQ Publication No. 14-EHCo13-EF. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 8.Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, Kass D, Berkowitz D, Renehan M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in Apo E−/− mice and rabbits fed a high fat and cholesterol diet. Circulation. 2014;129:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1- phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svennerholm E, Svennerholm L. The separation of neutral blood serum glycolipid by TLC. Biochim Biophys Acta. 1963;70:432–438. doi: 10.1016/0006-3002(63)90773-x. [DOI] [PubMed] [Google Scholar]
- 11.Dawson G, Kruski AW, Scanu AM. Distribution of glycosphingolipids in the serum lipoproteins of normal human subjects and patients with hypo-and hyperlipidemias. J Lipid Res. 1976;17:125–131. [PubMed] [Google Scholar]
- 12.Chatterjee SB, Dey S, Shi WY, Thomas K, Hutchins GM. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology. 1997;7:57–65. doi: 10.1093/glycob/7.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Bietrix F, Lombardo E, van Roomen CPAA, Ottenhoff R, Vos M, Rensen PCN, Verhoeven AJ, Aerts JM, Groen AK. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2010;30:931–93. doi: 10.1161/ATVBAHA.109.201673. [DOI] [PubMed] [Google Scholar]
- 14.Puri V, Jefferson J, Singh RD, Wheatley CL, Marks DL, Pagano RE. Sphingolipid storage Induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J Biol Chem. 2003;278:20961–20970. doi: 10.1074/jbc.M300304200. [DOI] [PubMed] [Google Scholar]
- 15.Glaros EN, Woojin SK, Quinn CM, Wong J, Gelissen I, Jessup W, Garner B. Glycosphingolipid accumulation inhibits cholesterol efflux via the ABCA1/Apolipoprotein A-1 pathway. J Biol Chem. 2005;280:24515–24523. doi: 10.1074/jbc.M413862200. [DOI] [PubMed] [Google Scholar]
- 16.Keul P, Tolle M, Lucke S, von Wunck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The sphingosine -1-posphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 17.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor- deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 18.Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer J-R. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice. Arterioscler Thromb Vasc Biol. 2013;33:1505–1512. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Kim RY, Imhof I, Honbo N, Luk FS, Li K, Kumar N, Zhu Bo-Q, Eberle D, Ching D, Karliner JS, Raffai RL. The immunosuppresant FTY720 prolongs survival in a mouse model of diet-induced coronary atherosclerosis and myocardial infarction. J Cardiovasc Pharmacol. 2014;63:132–143. doi: 10.1097/FJC.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffai RL, Weisgraber KH. Hypomorphic apolipoprotein E mice: a new model of conditional gene repair to examine apolipoprotein E-mediated metabolism. J Biol Chem. 2002;277:11064–11068. doi: 10.1074/jbc.M111222200. [DOI] [PubMed] [Google Scholar]


