Abstract
From their initial ingression into the neural tube to the established, adult vascular plexus, blood vessels within the CNS are truly unique. Covered by a virtually continuous layer of perivascular cells and astrocytic endfeet and connected by specialized cell-cell junctional contacts, mature CNS blood vessels simultaneously provide nutritive blood flow and protect the neural mileu from potentially disruptive or harmful molecules and cells flowing through the vessel lumen. In this review we will discuss how the CNS vasculature acquires blood-brain barrier (BBB) properties with a specific focus on recent work identifying the cell types and molecular pathways that orchestrate barriergenesis.
The blood-brain barrier (BBB) is a physiological barrier that controls the extracellular environment of the central nervous system (CNS) and is critical to allow for proper neuronal function as well as protect the neural tissue from injury and disease. This barrier is not a single property but rather an amalgam of structural, cellular and physiological components possessed by CNS endothelial cells that allows these cells to tightly restrict the trafficking of molecules, proteins and cell types between the blood and the brain (Figure 1). These include: 1) specialized tight junctions (TJs) between brain endothelial cells that strictly limit movement of ions and molecules within the paracellular space, 2) unusually low rates of trans-cellular transport (e.g., transcytosis, lack of fenestrations), 3) expression of numerous uni- or bi-directional transporters that move required substances, like water-soluble amino acids and glucose, into the brain and transport out potentially toxic substances, and 4) low expression of leukocyte adhesion molecules (LAMs) that helps limit movement of immune cells from the blood into the brain. Thus CNS endothelial cells both contain unique BBB-specific properties (TJs and transporter expression) that confer a ‘tight’ barrier, but also lack peripheral, ‘leaky’ endothelial-specific properties (transcytosis, fenestra, LAMs). Although these are properties of the endothelial cells, transplant studies have demonstrated that they are induced by interactions with the CNS microenvironment in what is termed the neurovascular unit.
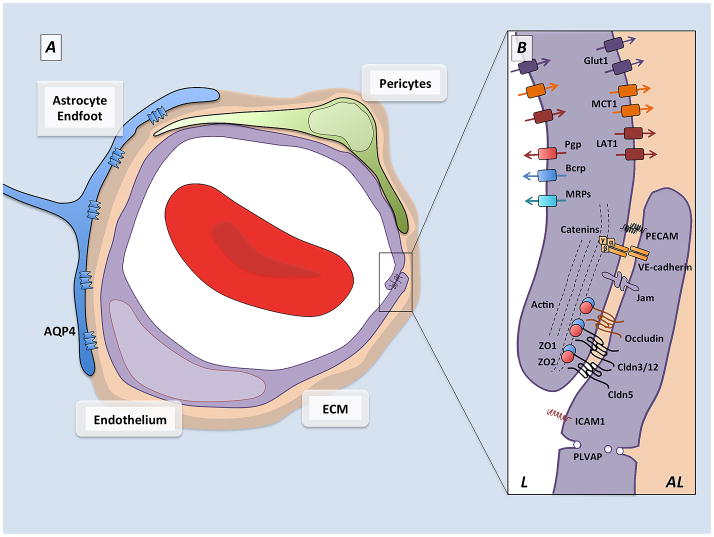
Figure 1. Schematic representation of the neurovascular unit.
A) Cellular components of the blood-brain barrier. Capillaries in the central nervous system are made up of endothelial cells (purple) which form the walls of the blood vessels, and their ablumenal surface is incompletely covered by a pericytes (green) which are embedded in the vascular extracellular matrix. Astrocytes (blue), a major glial cell population, extend cellular processes whose endfeet ensheath the blood vessels. Between the astrocytes and the vascular tube are two layers of extracellular matrix, the vascular extracellular matrix secreted by the endothelial cells and pericytes, and the glial matrix secreted by astrocytes.
B) Barrier components of the blood-brain barrier. Many of the properties of the BBB are manifested within the endothelial cells that make up the walls of the vessels. The endothelial cells are held together by tight junctions (TJs) which create tight paracellular barrier, and polarize the cells creating distinct lumenal (L) and ablumenal (AL) membrane compartments. The TJs are made up of transmembrane molecules including claudin family members, occludin and JAMs which are linked to the cytoskeleton and adherens junctions by cytoplasmic adaptors including ZO-1 and ZO-2. The endothelial cells undergo extremely low rates of transcytosis, mediated by low levels of PLVAP, limiting the transcellular movement of molecules and ions. These endothelial cells also express polarized transporters that determine the movement of many solutes across the endothelial cells. These include lumenal efflux transporters, such as Pgp and BCRP, which use ATP hydrolysis to actively transport a variety of small molecule substrates into the blood, as well as solute carriers such as Glut1, MCT1, and LAT1 which deliver specific nutrients (glucose, lactate and amino acids respectively) into the CNS. In addition endothelial cells express low levels of leukocyte adhesion molecules, including Icam1, which correlates with the low levels of CNS immune surveillance. These properties allow CNS endothelial cells to tightly regulate the movement of ions, molecules, and cells between the blood and the brain.
Emergence of BBB properties during neurovascular development and maturation is gradual. The earliest events are appearance of ‘tight’ properties including the formation of TJs between brain endothelial cells and expression of glucose transporter Glut-1. Ultrastructurally, some TJs are evident in blood vessels at their earliest stages of ingression into the rodent brain (embryonic day or E10-11) [1,2] and TJ protein occludin is expressed in patches along vessel lengths in E12 rodent brain [3]. Similarly, Glut-1 is expressed as early as E10 in mouse, which essentially coincides with vessel ingression into the neural tube [4]. The barrier functionality, however, at this stage is incomplete since BBB permeability studies using horseradish peroxidase indicate that the fetal BBB is permeable to HRP until ~E15 in rodents and E13 in chick [5,6]. At these early stages this leakage may be attributed to the fact that CNS vessels still have some peripheral ‘leaky’ properties including high levels of transcytosis and expression of LAMs [3]. Measurements of transendothelial resistance (TEER) in fetal and post-natal rat brain vasculature, an measure of BBB permeability, reveal gradual maturation in the BBB from late gestation to early post-natal stages [7]. This is likely due to a progressive tightening of junctional complexes caused by increased expression of TJ proteins and a reduction in transcellular transport. Indeed, ultrastructural and freeze fracture analysis in late embryonic and postnatal rodent observe the disappearance of interjunctional clefts (spaces between endothelial cells without components of the tight junctions), an increase in the density and complexity of tight junctions [1,8] and a decrease in evidence of vesicular trafficking in brain endothelial cells [3]. Co-incident with decreased paracellular and transcellular transport is loss of brain endothelial cell expression of LAMs that normally promote immune cell trafficking [3]. Much like TJ maturation, expression of efflux transporters that actively remove potentially toxic substances from the brain increases from pre- to postnatal stages [9]. Thus BBB development appears to be a complex multi-step process in which ‘tight’ properties are induced and then mature, and ‘leaky’ properties are inhibited.
Despite the importance of the BBB, several important questions remain: What are the cellular and molecular interactions that regulate the development and function of the BBB? Are each of the properties of the BBB regulated by similar or divergent mechanisms? Are these properties induced by differentiation signals during development or regulated maintenance signals throughout life? Here we will discuss what is known about the cellular and molecular regulation of BBB formation during development.
Cellular regulation of barriergenesis: Pericytes
Pericytes are an important perivascular cell type related to vascular smooth muscle cells (vSMCs) and are largely defined by their location with the vascular plexus (small-diameter capillaries) and close interaction with endothelial cells [10]. Pericytes are found at high density in CNS vasculature and their important role in neurovascular development is underscored by mouse models of pericyte-deficiency caused by genetic disruption of platelet-derived growth factor-B (PDGFB) signaling [11,12]. Endothelial cell-derived PDGFB is critical for recruitment of vSMCs and pericytes to the developing vasculature and their maintenance in adulthood. Reduction in pericyte coverage in PDGFB or PDGFrβ mutant mice causes a particularly severe phenotype in the fetal brain, including vessel hyperplasia and, occasionally, micro-hemorrhage [12,13]. Though in vitro work has implicated brain pericytes in blood brain barriergenesis [14,15], recent citations describing severe defects in BBB integrity in pericyte-deficient mutants both pre- and postnatally provides the most compelling evidence to date [3,16]. In the near absence of brain pericytes, as observed in PDGFrβ-null fetal brain, several BBB properties do not develop properly causing permeability to small and large molecular weight tracers, increased expression of LAMs and increased evidence of transcytosis in endothelial cells [3]. Increased vascular permeability in these mutants is not caused by lack of tight junctions between endothelial cells but rather may be connected to increased transcellular trafficking across the endothelial cell layer. Two important conclusions about blood brain barriergenesis can be drawn from analysis of PDGFrβ-null fetal brain. First, key features of the BBB are acquired as the vasculature develops suggesting that the immature brain, like the adult brain, needs protection from blood contents. Second, pericytes are needed in the developing brain vasculature for certain aspects of BBB maturation. Specifically, analysis of structure and gene expression of endothelial cells from PDGFrβ-null fetal brain suggests that pericytes are not required to induce BBB-specific ‘tight’ properties such as TJs and transporter expression, rather, they are required to stabilize the vasculature and inhibit the expression of ‘leaky’ properties, including transcytosis and leukocyte adhesion, normally associated with non-neural vessels.
As in development, pericytes are needed to maintain BBB properties in the mature, adult vasculature. To address this question, Armulik et al. circumvented the perinatal lethality of PDGFrβ-null and PDGFB-null mutants by using postnatally viable PDGFB mutants and generating mice that expressed one or two copies of the human PDGFB gene specifically in endothelial cells on the PDGFB-null background [16]. The result is viable mice that have moderate to significantly reduced pericyte coverage of adult vessels. Adult pericyte-deficient mutants have both increased vascular permeability and rates of trancytosis in endothelial cells. The authors also described defects in the association and polarization of astrocytic processes (“endfeet”) with the surface of the vasculature in adult pericyte-deficient mutants. Establishment of astrocytic-vascular interactions is a post-natal event [3]; this phenotype suggests that pericytes may be needed to attract or maintain astrocytic coverage of vessels. Consistent with a critical role for pericytes in BBB maintenance, progressive loss of pericytes in the aging brains of PDGFrβ-heterozygous mice leads to widespread vascular leak as well as significant loss of capillary density [17]. The resulting decrease in brain perfusion and toxicity causes neurodegeneration and cognitive defects. The connection between age-related pericyte loss and neurodegeneration in mice has sparked interest in a role for pericytes in the vascular pathology of neurodegenerative disorders like Alzheimer’s disease [18]. Indeed, recent work indicates that pericyte loss is a feature of the vascular pathology observed in the brains of Alzheimer’s patients [19].
A major question that remains is how pericytes interact with endothelial cells to both stimulate acquisition of BBB properties developmentally and help maintain these properties in mature neurovasculature. Key features of pericyte-mediated barrier properties appear to be sufficient coverage of and direct contact with the abluminal endothelial cell surface. Adult pericyte deficient mutants with severe to moderate loss of pericyte coverage (26% and 40%) both displayed BBB defects whereas mice with a small but significance decrease in pericyte coverage (76%) did not [16]. Possibly, there is threshold level of pericyte coverage, and thus pericyte-derived signals, required for barriergenesis and maintenance. In vitro studies using pericyte-endothelial cell co-cultures in which the two cells types are in contact displayed a greater decrease in permeability than non-contact co-cultures [15]. This may be due to the need for cell surface mediated signaling or perhaps pericyte-derived factors act best at short range. At the molecular level, what are possible pericyte-derived signals? Profiling analysis of blood vessel isolated from both embryonic and adult pericyte-deficient mice indicate that pericytes may regulate barrier integrity properties of endothelial cells through release of pro-vascular stability signals like angiopoiten-1 and suppression of angiopoiten-2 expression, related to vascular instability, by brain endothelial cells [3,16]. Also, pericytes suppress expression of several well-known leukocyte trafficking proteins by brain endothelial cells and in this way likely prevent unwanted movement of immune cells into the CNS [3]. Pericytes have recently been implicated in the post-natal and adult BBB defects observed in apolipoprotein E (ApoE) knockout mice [20]. Pericytes in ApoE-KO mice express excessive levels of both the pro-inflammatory cytokine cyclophilin A and, via NF-κB mediated signaling, MMP9. Both factors likely contribute to the decline in expression of TJ proteins by endothelial cells, BBB leakiness and subsequent neurodegeneration. APOE mutations are strongly linked to Alzheimer’s disease risk [21] and identification of specific signaling pathways in pericytes related to APOE dysfunction may lead to new therapeutic strategies. Though ApoE is implicated in post-natal and adult BBB maintenance, more studies are needed to determine if ApoE is involved in earlier stages of barriergenesis.
Cellular regulation of barriergenesis: Astrocytes
In the latter half of gestation in human development [22] and soon after birth in mice [3], astrocytic processes or endfeet ensheath the brain endothelium and, in this position, they are poised to aid to the maturation and maintenance of the BBB. Evidence of a role for astrocytes in barrier maturation is based on several in vitro studies showing that either co-culture with astrocytes or astrocyte-conditioned media induces BBB properties in cultured endothelial cells, specifically increased TEER and TJ organization and luminal polarization of transporters like Glut-1 and P-polyglycoprotein (P-pg) [23–27]. Furthermore, transplantation of astrocytes into non-neural tissue induces barrier properties in peripheral endothelial cells [28]. Taken together these data have supported a critical role for astrocytes in regulating BBB function. Recent evidence, however, suggests that barrier properties are formed during development well before astrocytes are generated and ensheath the vessels, indicating that astrocytes likely modulate BBB permeability maintenance rather than its induction during development. Recently, several astrocyte-derived factor(s) that regulate BBB properties in brain endothelial cells have been identified. For instance, sonic hedgehog (Shh) was shown to play an important role in barriergenesis and maintenance [29]. The authors showed that astrocytes, but not pericytes or endothelial cells, expressed Shh and disruption of hedgehog signaling in endothelial cells leads to BBB disruption in vivo. Though Shh likely has other sources in the mature brain, including neurons, the proximity of astrocyte-derived Shh to the endothelium makes it a potentially important source of this signal. Astrocytes also produce and cleave angiotensinogen into angiotensin, which binds and activates angiotensin receptors expressed by brain endothelial cells [30]. Angiotensin receptor signaling modifies TJ proteins like occludin to promote efficient organization of TJs. Astrocyte-derived retinoic acid (RA) has also been recently been implicated in inducing barrier properties in cultured human brain endothelial cells [31]. In addition, the authors showed that RA-biosynthetic enzymes are expressed by astrocytes and retinoic acid receptors localize to brain endothelial cells in human fetal brain tissue.
Astrocytic endfeet that contact the pericyte-endothelial layer are uniquely polarized with regard to their expression of specific proteins, including the water channel Aquaporin-4 (Aqp4). Localization of Aqp4 to astrocyte endfeet is an early event in astrocyte-endothelial contact [3], one that may require signals from pericytes [16]. Aqu4 does not appear to be required for BBB maturation or maintenance [32] rather it is involved in pathologic edema following brain injury [33].
Molecular regulation of barriergenesis
Cell types directly contacting endothelial cells during barriergenesis, especially perciytes, induce certain barrier properties (e.g., decreased trancytosis and LAM expression) but other important features appear to be under the control of signals coming directly from the brain itself. The appearance of specialized TJs and the expression of influx and efflux transporters are important events in barriergenesis that arise in the absence of pericytes, and prior to astrocyte generation, and likely depend on neural-derived signals.
Elegant mouse-chick and chick-quail chimera experiments provided the first evidence that neural tissue drives the acquisition of certain BBB characteristics in endothelial cells. Transplantation of avascular mouse or quail brain into chick chorio-allantoic membrane or coelomic cavity induces expression of BBB-specific proteins and the formation of TJs in non-neural endothelial cells [34,35]. Wnt ligands are strong candidates as a neural-derived BBB signal. Analysis of Wnt reporter mice suggests that canonical Wnt signaling is activated specifically in CNS endothelial cells as they invade the neural tissue, but not endothelial cells vascularizing peripheral tissues [36,37]. Disruption of Wnt signaling either by knocking out neural derived Wnt ligands (specifically Wnt7a and Wnt7b), by disrupting Wnt binding to Frizzled receptors, or by conditional depletion of beta-catenin in endothelial cells generate embryonic lethality with major CNS-specific angiogenesis defects [36,37]. Therefore, Wnts are uniquely required for normal growth of blood vessels into the brain making them one of the few known CNS-specific angiogenic factors. Treatment of brain endothelial cells with a canonical Wnt ligand, Wnt3a, leads to up-regulation of TJ proteins and the appearance of tight junctions between cultured endothelial cells [37,38]. Conditional deletion of β-catenin, a key component of the Wnt signaling pathway, in postnatal mouse brain endothelial cells leads to downregulation of the TJ protein claudin-3 and loss of BBB integrity [38]. Mice that lack the Wnt receptor Frizzled-4 display BBB defects, though notably only in the cerebellum [39]. Wnt signaling in brain endothelial cells is also involved in the early expression of the glucose transporter Glut-1. Brain capillaries in Wnt7a/Wnt7b double mutants and embryos with conditional inactivation of β-catenin in endothelial cells fail to up-regulate Glut-1 [36,37]. Expression of efflux transporter Ppg is also modulated by Wnt signaling in cultured brain endothelial cells [40]. Recently, a novel protocol for deriving endothelial cells with BBB-properties from human induced pluripotent stem cells utilized a method where neural and endothelial cells are co-differentiated [41]. The authors’ determined that Wnt ligands Wnt7a and Wnt7b were produced by neural cells in the cultures and Wnt signaling in the induced brain endothelial cells were key to acquisition of BBB traits, including expression transporters Glut-1 and Ppg. Wnt’s involvement in barriergenesis likely involves direct transcriptional regulation of proteins with BBB functions but also may function through downstream intermediates, like that of the recently described Wnt/β-catenin transcriptional targets DR6 and Troy [42]. Notably, DR6-KO embryos show defects in barriergenesis and adult mutants have reduced vascular density and BBB leakiness associated with reduced expression of the TJ protein ZO-1. Taken together these data suggest that Wnt is critical for driving angiogenesis in the CNS while also inducing BBB-specific ‘tight’ properties in endothelial cells including TJs and transporter expression. Loss of Gpr124, a g-protein coupled receptor expressed by brain endothelial cells, in mouse embryos leads to significant defects in forebrain angiogenesis and decreased expression of Glut-1 [43] similar to that observed during inhibition of Wnt signaling. The ligand for Gpr124 is neural-derived but has currently not been identified, and its relationship to Wnt signaling is still unknown.
Though there is considerable evidence that neural-derived Wnt ligands are key signals in barriergenesis, there are other signals coming from that brain that are involved in early establishment of the BBB. During mouse pre-natal development, Shh produced by neuronal progenitors/neural cells and Shh signaling in brain endothelial cells has a role in the initial stages of barriergenesis. Shh-KO embryos have reduced expression of TJ proteins in brain vasculature and endothelial cell-conditional knockouts of the Shh receptor Smoothened have brain vasculature that is abnormally leaky to serum proteins at pre-natal stages [29]. Interestingly, these mutant mice appear to have normal CNS angiogenesis suggesting that unlike Wnt, Shh regulates barrier properties after the vessels are formed. Similarly, inhibition of the RA signaling during fetal mouse development leads to reduced expression of TJ proteins and increased BBB permeability [31]. At this stage of mouse brain development, RA may come from the brain [44] itself or the meninges and CSF [45,46].
Concluding remarks
The complexity of signals involved in blood brain barriergenesis not only reflects the different properties of the BBB but also the temporal acquisition of these properties during pre- and post-natal brain development. Expression of different transporters, accumulation of TJ structures and proteins and decline in LAMs are not instantaneous events as blood vessels enter neural tissue. The induction of these properties appears to be orchestrated by a series of different cellular interactions occurring sequentially during brain vascular development (Figure 2). First, early BBB ‘tight’ properties like Glut-1 transporter expression and immature TJs between endothelial cells are induced by neural derived signals, like Wnt ligands, that also help drive endothelial cells into the neural tissues. Next, pericytes help stabilize the nascent vessels and inhibit ‘leaky’ properties in these vessels via suppression of transcellular pathways and expression of LAMs. Finally, full maturation of the BBB as defined by expression of an array of transporters and increased number and complexity of TJs seal off the CNS and BBB properties are maintained by pericytes, astrocytes and other neural cells throughout life, which appears to involve SHH, RA and AGT.
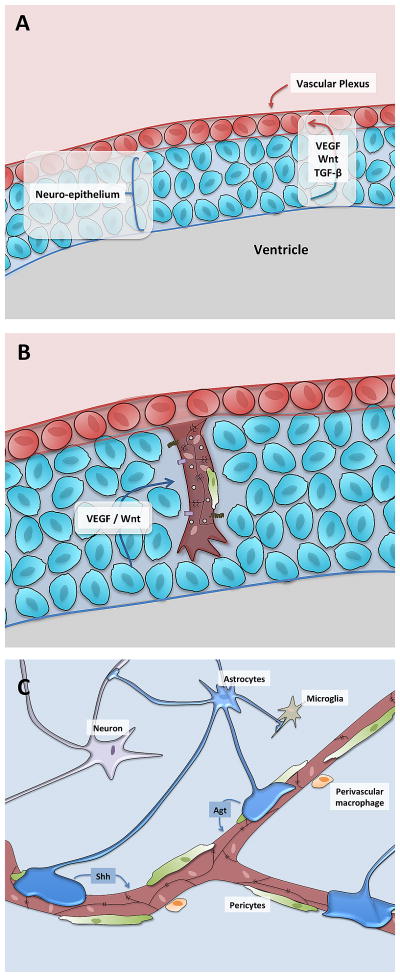
Figure 2. Schematic representation of BBB development.
A) During early development, endothelial cell progenitors (brown) form a vascular plexus surrounding the developing neural tissue (blue).
B) Angiogenic sprouts, consisting of endothelial tip and stalk cells, invade the neural tissue under the influence of general angiogenic factors (VEGF) as well as CNS-specific angiogenic factors (Wnt). These initial sprouts recruit pericytes (green) and are observed to have ‘tight’ properties including tight junction proteins as well as Glut1 expression, as well as ‘leaky’ properties including high rates of transcytosis and expression of leukocyte adhesion molecules (LAMs) such as Icam1.
C) Over the next several days and weeks under the influence of pericyte-derived factors, neural derived factors and astrocyte derived factors, the endothelial cell tight junctions mature, the cells start to increase expression of efflux transporters and lower their transcytosis and expression of LAMs.
Though the barrier is by no means fully mature in the developing brain, numerous studies reviewed here now indicate that the fetal brain vasculature efficiently restricts both trans- and paracellular movement. This suggests that the immature brain is at largely protected from molecular and cellular blood contents. This raises the question of why the developing brain, in which synaptic transmission is scarce and the potential for neuron “re-generation” is high, must be sequestered. One possibility is that morphogenic gradients of molecules like FGF, Wnt and BMPs, critical for normal brain development, could be disrupted by contamination of serum-derived growth factors. Also, proliferating neural progenitors have a limited capacity for neuron generation and neurotoxicity caused by exposure to blood contents at these early stages could significantly impact total neuronal output. Alternatively, precise extracellular composition may be required for neuronal function such that appropriate activity-dependent circuits are formed. Future work with animal and in vitro models of barriergenesis could shed light on these and other, unresolved questions including the identity of pericyte and astrocyte-derived signals that induce BBB properties in brain endothelial cells.
References
- 1.Stewart P, Hayakawa K. Early ultrastructural changes in blood-brain barrier vessels of the rat embryo. Brain Res Dev Brain Res. 1994;78:25–34. doi: 10.1016/0165-3806(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 2.Bauer H, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res. 1993;75:269–278. doi: 10.1016/0165-3806(93)90031-5. [DOI] [PubMed] [Google Scholar]
- **3.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. Using a pericyte-deficient mouse model, showed that perictyes were required for certain aspects of pre-natal barriergenesis including supression of endothelial trancytosis and LAMs. Provided a developmental timeline of BBB characteristics in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao T, Ishizawa A, Ogawa R. Observations of vascularization in the spinal cord of mouse embryos, with special reference to development of boundary membranes and perivascular spaces. Anat Rec. 1988;221:663–677. doi: 10.1002/ar.1092210212. [DOI] [PubMed] [Google Scholar]
- 5.Wakai S, Hirokawa N. Development of the blood-brain barrier to horseradish peroxidase in the chick embryo. Cell Tissue Res. 1978;195:195–203. doi: 10.1007/BF00236719. [DOI] [PubMed] [Google Scholar]
- 6.Risau W, Hallmann R, Albrecht U, Henke-Fahle S. Brain induces the expression of an early cell surface marker for blood-brain barrier-specific endothelium. EMBO J. 1986;5:3179–3183. doi: 10.1002/j.1460-2075.1986.tb04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- 9.Ek CJ, Wong A, Liddelow SA, Johansson PA, Dziegielewska KM, Saunders NR. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol Lett. 2010;197:51–59. doi: 10.1016/j.toxlet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- *12.Lindahl P, Johansson B, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. Seminal paper describing connection between PDGFB and pericyte recruitment, notably in the brain vasculature. [DOI] [PubMed] [Google Scholar]
- 13.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 14.Dente CJ, Steffes CP, Speyer C, Tyburski JG. Pericytes augment the capillary barrier in in vitro cocultures. J Surg Res. 2001;97:85–91. doi: 10.1006/jsre.2001.6117. [DOI] [PubMed] [Google Scholar]
- 15.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- **16.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. Highlights role for pericytes in maintinance of BBB integrity in adult mouse brain, specifially in supression of trans-cellular transport in endothelail cells and in astrocytic endfeet polarization on the surface of the endothelium. [DOI] [PubMed] [Google Scholar]
- *17.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. Connects decreased brain pericyte coverage of microvasculature in aged PDGFB and PDGFrβ heterzygous with vascular regression, loss of BBB integrity, and, ultimately neurodegeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006;59:673–679. doi: 10.1203/01.pdr.0000214975.85311.9c. [DOI] [PubMed] [Google Scholar]
- 23.Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehouck MP, Méresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 25.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- **28.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. First to provide in vivo evidence that astrocytes can induce barrier properties in non-CNS endothelial cells. [DOI] [PubMed] [Google Scholar]
- *29.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. Identified hedgehog signaling in endothelial cell as a critical pathway for both pre- and post-natal barriergenesis with astrocytes as a probable source of SHH in the post-natal brain. [DOI] [PubMed] [Google Scholar]
- *30.Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27:9032–9042. doi: 10.1523/JNEUROSCI.2088-07.2007. Shows that angiotensin II from astrocytes signals through the angiotensin receptor on endothelial cells to phosphorylate and stabilize the TJ protein occludin and increase barrier integrity. Also showed that in multiple sclerosis lesions, angiotensin II-expressing astrocytes are reduced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Mizee MR, Wooldrik D, Lakeman KA, van Het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, et al. Retinoic Acid Induces Blood-Brain Barrier Development. J Neurosci. 2013;33:1660–1671. doi: 10.1523/JNEUROSCI.1338-12.2013. Implicates retinoic acid signaling in induction of barrier properties in cultured human endothelial cells and in developing mouse brain. Also shows that barrier-promoting property of astrocyte conditioned media is at least in part from astrocyte-produced RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, Papadopoulos MC. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161:764–772. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 33.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- *34.Stewart P, Wiley M. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. One of the first studies suggesting that neural tissue has unique, barriergenesis inducing properties. [DOI] [PubMed] [Google Scholar]
- *35.Risau W, Hallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev Biol. 1986;117:537–545. doi: 10.1016/0012-1606(86)90321-0. Elegant developmental analysis of BBB maturation, specifially expression of BBB proteins, in mouse, quail and chick brain. Correlated changes in expression of these proteins with increasing impermeability of brain vasculature to horseradish peroxidase. [DOI] [PubMed] [Google Scholar]
- *36.Stenman J, Rajagopal J, Carroll T, Ishibashi M, McMahon J, McMahon A. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. First report identifying the critical role for Wnt signaling specifically in brain angiogenesis and Wnt7a and Wnt7b as the neural-derived ligands driving this process. [DOI] [PubMed] [Google Scholar]
- *37.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo C, Barres B. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. Corraborated a role for neural-derived Wnt ligands in brain angiogenesis, possibly through stimulation of endothelial cell proliferation. Also provide evidence that Wnt ligands stimulate expression of transporter Glut-1 in brain endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla C, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. Using both mouse genetics and cell culture approches, showed that Wnt ligands and Wnt siganling pathway components were sufficent to induce certain BBB properties in endothelial cells, notably expression of TJ proteins like claudin 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. Implicates Wnt receptor Frizzled4 in BBB maturation and maintinance specifically in the cerebellar vasculature and in the retinal vasculature, likely through maintinace of TJ proteins like claudin 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, Romero IA, Couraud PO, Weksler BB, Hladky SB, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106:1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. Provides novel protcol for co-specification of brain endothelail cells with BBB properties along with neural cells from human pluripotent stem cells. Provides evidence that Wnt ligands produced by neural cells differentiating alongside endothelial cells is part of induction of BBB properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RA, van Bruggen N, Watts RJ. Death receptors DR6 and TROY regulate brain vascular development. Dev Cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. Using genetic profiling of brain and non-brain endothelial cells, identified death receptors DR6 and TROY as enriched in brain endothelail cells and described novel role for the receptors in both barriergenesis and brain vascular development, possibly downstream of Wnt signaling. [DOI] [PubMed] [Google Scholar]
- 43.Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Drager UC. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev. 2000;95:283–289. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- 45.Siegenthaler J, Ashique A, Zarbalis K, Patterson K, Hecht J, Kane M, Folias A, Choe Y, May S, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonso MI, Martín C, Carnicero E, Bueno D, Gato A. Cerebrospinal fluid control of neurogenesis induced by retinoic acid during early brain development. Dev Dyn. 2011;240:1650–1659. doi: 10.1002/dvdy.22657. [DOI] [PubMed] [Google Scholar]