Abstract
Background
Dengue fever is the most common identifiable cause of acute febrile illness among travelers returning from South America, South Central Asia, Southeast Asia, and the Caribbean. Although the characteristic exanthem of dengue fever occurs in up to 50% of patients, few descriptions of it are found in the dermatology literature, and discussions of how to distinguish the dengue exanthem from other infectious disease entities are rare. Chikungunya fever is an emerging infectious disease now seen in returning US tourists and should be considered in the differential diagnosis of dengue fever in the appropriate patient.
Objective
The purpose of our study was to report two cases of dengue fever among returning US tourists, provide a review of dengue fever, offer an extensive differential diagnosis of dengue fever, and raise awareness among dermatologists of chikungunya fever.
Methods
This study includes clinical findings of two returning travelers, one who traveled to Mexico and the other to Thailand, complemented by a discussion of both dengue fever and its differential diagnosis.
Limitations
Limited to 2 case reports.
Conclusion
Dengue fever should be considered in the differential diagnosis of fever and rash in the returning traveler. Dermatologists should be aware of the distinctive exanthem of dengue fever. Recognition of the dengue fever rash permits a rapid and early diagnosis, which is critical, as dengue fever can progress to life-threatening dengue hemorrhagic fever or dengue shock syndrome.
We describe two cases of dengue fever in returning travelers. One patient was from San Francisco and traveled to Mexico; the other patient was from New York City and traveled to Thailand.
CASE 1
A previously healthy 32-year-old woman from San Francisco, Calif, developed headache, fever, extreme fatigue, and a flushing macular erythema over the chest 2 days after returning from a vacation in Central Mexico. Three days later, she developed a generalized morbilliform eruption studded with pinpoint petechiae and islands of sparing.
Initial laboratory evaluations indicated a white blood cell count of 1.5 × 109/L (normal 3.4-10 × 109/L), a hematocrit of 48.9% (normal 36%-46%), a platelet count of 37 × 109/L (normal 140-450 × 109/L), an aspartate aminotransferase of 124 U/L (normal 16-41 U/L), an alanine aminotransferase of 87 U/L (normal 11-54 U/L), and a serum sodium of 137 mmol/L (normal 134-143 mmol/L).
Based on a history of travel to an endemic area and the presenting characteristic rash, accompanied by neutropenia, thrombocytopenia, hemoconcentration, and elevation of liver transaminases, a presumptive diagnosis of dengue fever was made. This clinical impression was confirmed with serologic testing. Initial dengue virus titers were IgM 11.78 index and IgG less than 0.5 index; convalescent titers drawn 2 weeks later were IgM 12.36 index (reference range <0.90) and IgG 2.51 index (reference range<0.90) (antibody titers obtained through an enzyme-linked immunosorbent assay, reported as a ratio of the patient's results compared to a control index). Further laboratory evaluations included negative serologies for Epstein-Barr virus IgM and parvovirus B19 IgG and IgM, a negative group-A streptococcal throat culture, and a negative thick and thin blood smear for malaria. With supportive care, the rash resolved and laboratory values normalized within 1 week.
CASE 2
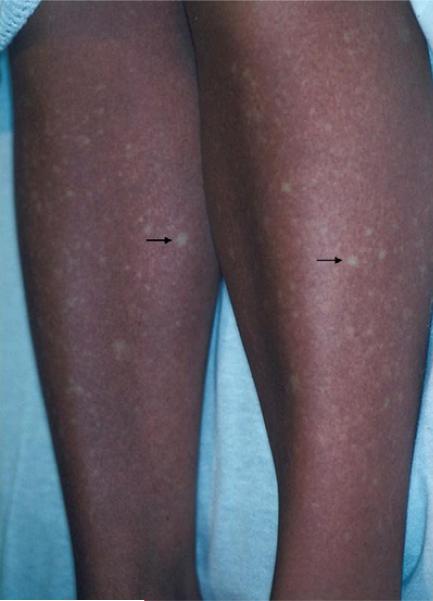
A previously healthy 21-year-old female college student presented to a hospital in New York City with a generalized rash 2 days after returning to the United States after spending 1 month in Thailand. Two days before returning to the United States, she developed the sudden onset of high fever, myalgias, and severe fatigue. On examination, she had a diffuse morbilliform eruption with petechiae and islands of sparing (Fig 1). When she first developed symptoms in Thailand, she thought she had sunburn.
Fig 1.
Dengue fever: Pinpoint petechiae and islands of sparing (arrows) on background of erythema.
Laboratory evaluation included a white blood cell count of 1.9 × 109/L (normal 3.54-9.06 × 109/L), a hematocrit of 34.2% (normal 35.4%-44.4%), a platelet count of 91 × 109/L (normal 165-415 × 109/L), an aspartate aminotransferase of 988 U/L (normal 12-38 U/L), an alanine aminotransferase of 753 U/L (nl 7-41 U/L), and a serum sodium of 140 mmol/L (normal 136-146 mmol/L).
A preliminary diagnosis of dengue fever was made based on her travel history, rash morphology, and laboratory abnormalities. The clinical impression was confirmed with acute serologies positive for anti-dengue IgM antibodies. Convalescent titers performed 1 month later were positive for anti-dengue IgG antibodies at 1:10,240 (titer obtained through enzyme-linked immunosorbent assay performed at serial dilutions; Diagnostic Laboratory of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention [CDC], San Juan, Puerto Rico). Further evaluation revealed negative serologies for Borrelia burgdorferi, coxsackievirus, parvovirus B19, Epstein-Barr virus, and cytomegalo-virus; a negative group-A streptococcal throat culture; and a negative thick and thin blood smear for malaria. With supportive care, the rash resolved and laboratory values normalized within 4 days.
DISCUSSION
Dengue virus, of the family Flaviviridae, is a single-stranded RNA virus transmitted by the mosquitoes Aedes aegypti and Aedes albopictus. There are 4 serotypes of the dengue virus (DEN 1-4). Infection with one strain provides lifelong immunity to that strain, but only a few months of immunity to other strains.
Infection with the dengue virus can cause a spectrum of 3 clinical syndromes: classic dengue fever, dengue hemorrhagic fever, and dengue shock syndrome. World Health Organization criteria exist for classification of dengue into these 3 clinical categories (Table I)1; however, many find these categories difficult to use in practice and there is significant overlap between the categories.2 For example, although a positive tourniquet test (the presence of ≥ 20 petechiae/sq in after inflation of a blood pressure cuff) is one of the 4 criteria required to make the diagnosis of dengue hemorrhagic fever, a recent review suggests that a positive tourniquet test result is neither sensitive nor specific to distinguish between classic dengue fever and dengue hemorrhagic fever.2 Therefore, in practice, clinicians often use modified criteria in making diagnoses. Dengue hemorrhagic fever often occurs in patients with immunity to one serotype who become infected with a different serotype.3
Table I.
World Health Organization criteria for dengue fever, dengue hemorrhagic fever, and dengue shock syndrome
| DF | DHF* | DSS |
|---|---|---|
| An acute febrile illness with ≥2 of the following manifestations: | All of the following must be present: | All 4 criteria for DHF must be met, plus evidence of circulatory failure manifested by: |
| Fever or history of acute fever, lasting 2-7 d | ||
| Headache | Bleeding, evidenced by at least one of the following: | Rapid and weak pulse, |
| Retro-orbital pain | and | |
| Myalgia | Positive tourniquet test result† | Narrow pulse pressure |
| Arthralgia | Petechiae, ecchymoses, or purpura | or |
| Rash | Bleeding from the mucosa, GI tract, injection sites, or other locations | Hypotension for age (systolic pressure < 80 mm Hg for those < age 5 y, or <90 mm Hg for those > age 5 y) |
| Hemorrhagic manifestations | ||
| Leukopenia | Hematemesis or melena | |
| AND | Thrombocytopenia (≤ 100,000 cells/mm3) | Cold clammy skin and restlessness |
| Supportive serology | ||
| OR | Evidence of plasma leakage caused by increased vascular permeability, manifested by at least one of the following: | |
| Occurrence at the same location and time as other confirmed cases of DF | ||
| Laboratory criteria | Increase in hematocrit ≥ 20% above average for age, sex, and population | |
| Isolation of dengue virus ≥ 4-Fold change in antibody titers | ||
| Decrease in hematocrit after volume-replacement treatment ≥ 20% of baseline | ||
| Demonstration of dengue virus antigen | ||
| Detection of dengue virus genomic sequence | Signs of plasma leakage such as pleural effusion, ascites, and hypoproteinemia |
Adapted from the World Health Organization.1
DF, Dengue fever; DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; GI, gastrointestinal.
DHF classified according to severity. Grade I: fever and nonspecific constitutional symptoms; only hemorrhagic manifestation is a positive tourniquet test and/or easy bruising. Grade II: same as grade I but includes spontaneous bleeding. Grade III: Circulatory failure manifested by a rapid, weak pulse and narrowing of the pulse pressure or hypotension. Grade IV: Profound shock with undetectable blood pressure or pulse. Grades III and IV define DSS.
Tourniquet test is performed by inflating a blood pressure cuff on the upper aspect of arm to a point midway between systolic and diastolic pressures for 5 minutes. Test result is positive when ≥ 20 petechiae/2.5 cm2 are observed.
The incidence of dengue fever has increased dramatically during the past 60 years.3 Dengue fever is now endemic in many tropical and subtropical regions, including Central America, South America, the Caribbean, Sub-Saharan Africa, South Central Asia, and Southeast Asia.4 It is estimated that approximately 50 to 100 million dengue infections occur annually worldwide.5 Factors hypothesized to be responsible for the increase in the incidence of dengue include rapid urbanization, population growth, an increase in nonbiodegradable products that can serve as sites for mosquito larvae proliferation, air travel,4 and global warming.6
The increased incidence of dengue fever is reflected in the dramatic increase in cases of dengue fever among travelers to endemic areas. In a recent report of surveillance data among 17,353 ill returning travelers from 6 continents between 1996 and 2004, dengue was the most common identifiable cause of a systemic febrile illness in returning travelers from the Caribbean, South America, South Central Asia, and Southeast Asia.7 Of note, malaria was the most common identifiable cause of a systemic febrile illness in returning travelers from Central America and Sub-Saharan Africa.7 Travelers may spread dengue fever within their native country on return. An outbreak of dengue fever that occurred in Hawaii from 2001 to 2002 was attributed to travelers initially infected in French Polynesia. Although Aedes aegypti is the primary vector of dengue fever, Aedes albopictus, a prevalent mosquito found in Hawaii, was responsible for this epidemic.8
The clinical presentation of classic dengue fever, illustrated in part by the two patients described in this article, is characterized by the sudden onset of high fever, severe headache, retro-orbital pain, fatigue, severe myalgias, arthralgias, and a sequential cutaneous eruption. Typical laboratory abnormalities include leukopenia, thrombocytopenia, elevated transaminases, hemoconcentration, and hyponatremia. It is important for clinicians to recognize that the incubation time for dengue fever is between 3 to 14 days. Therefore, dengue fever is unlikely to be the diagnosis in patients with symptoms occurring more than 14 days after returning from an endemic area.
The characteristic exanthem of dengue fever is estimated to occur in 50% to 82% of patients with dengue fever.9,10 The initial rash involves a flushing erythema of the face, neck, and chest that typically occurs within the first 24 to 48 hours of the onset of symptoms and is thought to be the result of capillary dilatation. The sunburn reported by patient 2 likely represented this initial eruption. The subsequent rash, seen 3 to 5 days later, is characterized by a generalized morbilliform eruption with petechiae and islands of sparinge“white islands in a sea of red”9,11eand is thought to be an immune response to the virus. Some patients display only the initial rash and recover completely; others develop the more generalized eruption. Findings on routine skin biopsy specimen are nonspecific.12
When confronted with a febrile returning traveler who has a rash similar to that seen with dengue fever, the differential diagnosis is quite broad. However, it can be organized by the stage of the rash. The initial flushing erythema of the chest, head, and neck in association with fever can be seen in the early stages of many viral and bacterial infections (Table II). The generalized morbilliform eruption in association with a fever can be seen in the later stage of viral exanthems and bacterial infections (Table III). In those patients who develop dengue hemorrhagic fever, the differential diagnosis of viral hemorrhagic fevers should be considered, which includes Marburg/Ebola hemorrhagic fever, hantavirus hemorrhagic fever, Kyasanur Forest disease/Omsk hemorrhagic fever, Colorado tick fever, Lassa fever, Crimean-Congo hemorrhagic fever, and leptospirosis.38 Malaria is included in the differential diagnosis because it is a common cause of fever in a returning traveler; however, malaria is rarely accompanied by a rash.39 It is important to note that patients infected with one vectorborne disease may be simultaneously infected with another. Indeed, several cases of patients infected with malaria and dengue fever have recently been reported.40,41
Table II.
Differential diagnosis of classic dengue fever–associated flushing erythema
| Disease | Presenting symptoms | Exanthem | Timing from symptoms to rash | Distinguishing features |
|---|---|---|---|---|
| Chikungunya fever | Fever, arthralgia, myalgia, HA, ± vomiting, diarrhea | Flushing erythema on face and upper aspect of chest.13 | 24 h | Asia, Africa, Indian Ocean |
| Sandfly fever | Fever, HA, malaise, retro-orbital pain | Flushing scarlatiniform erythema on face and neck. Very rare reports of subsequent rash (urticaria, erythema-multiforme, morbilliform). Lack of later rash helps distinguish from dengue fever.14 | Concomitant | Mediterranean, Asia, Africa |
| Scarlet fever | Fever, pharyngitis, ± vomiting, abdominal pain, ± convulsions | Initially on neck/chest. Rapid development of 1- to 2-mm papules on erythematous background (sandpaper). Linear petechiae (Pastia's lines) in the skin folds. Subsequent membranous desquamation of palms/soles. White, then red, strawberry tongue.15 | 12-48 h | ASO titer positive, leukocytosis |
| Toxic shock syndrome | Fever, hypotension | Generalized erythroderma or scarlatiniform rash. Erythema/edema of palms/soles. Strawberry tongue, conjunctival injection. Desquamation of palms/soles 1-2 wk after disease onset.16 | Concomitant | Staphylococcal or streptococcal infection |
| Kawasaki disease | Fever, LAD, oropharyngeal change, conjunctival injection | Polymorphic, including flushing macular erythema, nonpruritic erythematous plaques, erythema marginatum, pustules. Perineal involvement. Strawberry tongue, conjunctival hyperemia. Edema, erythema, then desquamation of palms/soles.17 | Concomitant | ± Cardiac abnormalities |
| Erythema infectiosum (parvovirus B19) | Fever, HA, rhinorrhea | Initial macular fiery erythema of cheeks with circumoral pallor. In 1-4 d, morbilliform reticulated eruption on extremities (spares palms/soles); recurring stage (rash on exposure to trigger).18 | 1-2 d | Arthritis in 80% of adults |
ASO, Antistreptolysin O; HA, headache; LAD, lymphadenopathy.
Table III.
Differential diagnosis of classic dengue fever–associated morbilliform eruption
| Disease | Presenting symptoms | Exanthem | Timing from symptoms to rash | Distinguishing features |
|---|---|---|---|---|
| Infectious mononucleosis (EBV, CMV) | Fever, malaise, pharyngitis, LAD | Polymorphic, including generalized macular erythema ± petechiae, urticaria, scarlatiniform, erythema multiforme-like. Rash is rare (3%-10%).19,20 Eyelid edema. Pinhead petechiae at junction of soft/hard palate (Forschheimer's spots). | Within first week of illness | |
| Roseola infantum (HHV 6) | Fever, LAD ± convulsions | Mostly discrete pink macules, occasional papules. Often starts on the back. Fully developed rash usually involves trunk, nape of neck, proximal limbs ± scalp. Rare for face involvement. Total duration 24-72 h. Nonpruritic. Not followed by desquamation or pigment change.21 | As fever subsides | Age 6-36 mo; reactivation in immunocompromised hosts; associated with DRESS syndrome (controversial)22 |
| Measles | Fever, cough, coryza | Initial erythematous papules on face; within 1-2 d, spreads to trunk. Small bright red spots with blue-white center on buccal mucosa–often appear at beginning stage of exanthem (Koplick spots).23 | 1-7 d | |
| Rubella | Fever, eye pain, LAD | Initial “brilliant” generalized erythema on face, which fades within 24 h. Then, pale rose-pink macules appear on face and scalp that spread down trunk and extremities. Macules coalesce on lower aspect of back/buttocks. Forschheimer's spots.24 | 2-5 d | |
| Enterovirus | Fever ± pharyngitis | Polymorphic, including diffuse macular or morbilliform eruption. ±Vesicles, petechiae, purpura.25 | Varies | Associated with myocarditis |
| Secondary syphilis | Fever, HA, pharyngitis, myalgia, weight loss, LAD | Polymorphic: macules/papules/psoriasiform papules. Often diffuse discrete red to red-brown papules involving palms and soles. ±Condyloma lata. ±Moth-eaten alopecia.26 | Weeks to months after initial chancre | Serology for RPR or VDRL |
| Typhoid fever | Fever, vomiting, diarrhea, HA | Rose spots: 2- to 4-mm pink grouped papules on trunk or generalized erythema “erythema typhosum.”27 | 2-4 wk | Rose spot cultures may be Salmonella typhi+ |
| Chikungunya fever | Fever, arthralgia, myalgia, HA, LAD, vomiting, diarrhea | Erythematous macules and papules on the trunk and extremities with islands of sparing. ±Petechiae. Face spared. Burning sensation in pinna.13 | 3-5 d | Asia, Africa, Indian Ocean |
| West Nile virus | Fever, seizures, ascending flaccid paralysis | Ill-defined erythematous-to-pink macules on trunk, and proximal extremities with pruritus and dysesthesia. Spares face, palms, soles, mucous membranes. Resolve without scaling.28 | 3-7 d | Asia, Africa, Europe, United States29 |
| O'nyong-nyong fever | Fever, arthritis, LAD | Pruritic morbilliform eruption. Initially discrete, then confluent. Starts on face, spreads to trunk and extremities. Favors neck, chest, back, flanks, inner aspect of thighs and arms. Does not desquamate.30 | 4 d, but varies | Sub-Saharan Africa |
| Mayaro virus | Fever, HA, myalgia, arthralgia | Erythematous macules and papules with some areas of confluence. Extremities and trunk31 ± hand involvement. Relative sparing of face. Rash more common in children (89%) than adults (53%).32 | As fever subsides | South America |
| Sindbis virus | Fever, fatigue, arthralgia/arthritis | Generalized morbilliform eruption. Reports of vesicles on pressure points (palms/soles).33 | 3-4 d | Europe, Africa, Asia, Australia |
| Ross River disease | Fever, fatigue, arthralgia/arthritis | Most commonly maculopapular on trunk and limbs. ± Palm/sole/face/scalp involvement. Reports of vesicular or purpuric. Rash seen in about 50% of affected persons.34 | 3-4 d | Australia, Papua New Guinea, Fiji, Samoa |
| Leptospirosis | Acute phase: fever, HA, myalgia, pharyngitis. Immune phase: organ failure |
Acute phase: generalized morbilliform rash, most prominent on trunk.35 Immune phase: hemorrhage, bleeding, jaundice. Jaundice with history of water exposure differentiates from dengue fever.36 |
Concomitant | History of exposure to fresh water |
| Acute retro-viral syndrome (HIV) | Fever, fatigue, HA, pharyngitis, myalgias, LAD | Macules and papules on trunk and upper arms.37 ±Palms and soles. | Concomitant |
CMV, Cytomegalovirus; DRESS, drug reaction with eosinophilia and systemic symptoms; EBV, Epstein-Barr virus; HA, headache; HHV, human herpesvirus; LAD, lymphadenopathy; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory test.
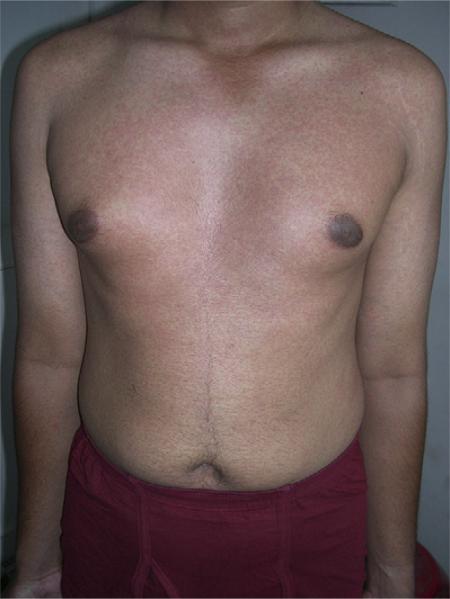
As illustrated in Tables II and III, the differential diagnosis of dengue fever is broad. However, it is imperative to exclude chikungunya fever, as not only can its clinical presentation be almost indistinguishable from that of dengue fever, but the largest epidemic to date of chikungunya fever is currently occurring in islands of the southwest Indian Ocean (further discussion below). The initial clinical manifestations of chikungunya fever include the sudden onset of high fever, arthralgia, myalgia, headache, and a flushing erythema on the face and upper aspect of chest. Nausea and vomiting can accompany the systemic symptoms. As the disease progresses, the systemic symptoms persist, but the rash develops into an eruption characterized by erythematous macules and papules on the trunk and extremities with islands of sparing and sometimes petechiae (Fig 2).13 As described herein, the systemic symptoms and cutaneous features of chikungunya fever are very similar to dengue fever.
Fig 2.
Chikungunya fever: morbilliform rash with islands of sparing. Photograph courtesy of Zawar Vijay, MD, Nashik, India.
Clinical features favoring chikungunya fever over dengue fever include a more rapid onset of symptoms, more severe rash, worse conjunctival injection, shorter febrile period,42 and fewer signs of easy bleeding.13 Although both conditions are associated with severe arthralgias (the alternative name for dengue fever is “breakbone fever”), patients with chikungunya fever are more likely to contort themselves into characteristic postures because of severe joint pain (the word “chikungunya,” originating from the Makonde language, means contorted or bent).13 In addition, the rash associated with drug hypersensitivity may rarely present with a morbilliform eruption with petechiae and islands of sparing, and should be included on the differential diagnosis of dengue and chikungunya virus infections (M. E. G., personal observation).
Chikungunya virus, a member of the genus Alphavirus in the family Togaviridae, is transmitted both by Aedes aegypti and Aedes albopictus. Chikungunya virus is found endemically in Asia, Africa, and the Indian Ocean, whereas dengue virus is also found in the Americas and the Caribbean. As mentioned previously, the largest epidemic to date of chikungunya fever is currently occurring in islands of the southwest Indian Ocean, including the popular travel destination, Mauritius.43,44 A new mutation in the chikungunya virus, producing a novel viral variant, is thought to be responsible for the outbreak. It is proposed that a mutation in the E1 envelope gene occurred between the spring and fall of 2005. Modification of the E1 envelope could allow for greater infectivity of the virus into the mosquito, or for greater virulence in human beings.45
Armed with the knowledge of the current chikungunya epidemic, its typical clinical presentation, and how it differs from that of dengue fever, the dermatologist becomes integral in recognizing this epidemic as it makes its way to the shores of the United States. In fact, the first cases of chikungunya fever in returning US tourists were reported in the September 29, 2006 issue of the Morbidity and Mortality Weekly Report published by the CDC.46
On the horizon are improved treatments for dengue fever. Thus far, strategies for controlling dengue fever have primarily focused on prevention of the disease through mosquito eradication programs. However, there are ongoing efforts to develop novel antiviral agents against the dengue virus and to produce a vaccine against the virus. Potential targets for antivirals include the dengue virus proteins NS5 polymerase and NS3 protease.47 The development of an anti-dengue vaccine has been relatively challenging as the vaccine ideally should be a tetravalent vaccine effective against all 4 strains of the virus. There has been some alarm that if the vaccine conferred incomplete protection, the vaccine could actually predispose recipients to dengue hemorrhagic fever or dengue shock syndrome when infected with a serotype to which recipients did not have full immunity. Despite these obstacles, there are two tetravalent anti-dengue vaccines currently in clinical trials.48
Increased physician awareness of the nuances of the dengue fever exanthem can help to narrow the differential diagnosis in the international traveler, student, returning tourist, or migrant worker who presents with fever and rash. Recognition of the distinctive rash of dengue fever facilitates a rapid diagnosis, which is critical, as dengue fever can progress to dengue hemorrhagic fever or dengue shock syndrome, both life-threatening conditions. All patients traveling to endemic areas should be educated about preventative measures, including using protective clothing and mosquito repellent containing N,N diethylmetatoluamide (DEET). Information for health care providers to report new cases of dengue fever can be found online at http://www.cdc.gov/ncidod/dvbid/dengue/index.htm. In addition, the CDC will help with diagnostic testing for dengue infection. Both acute and convalescent-phase serum samples should be sent through the state or territorial health department laboratories to the CDC's Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 1324 Calle Cañada, San Juan, Puerto Rico 00920-3860. Although the CDC does not require reporting of dengue fever cases,49 many states, such as Texas, do require reporting of dengue fever.50 State requirements should be consulted on a case-by-case basis.
Acknowledgments
Funding sources: None.
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.World Health Organization . Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. World Health Organization; Geneva: 1997. [Google Scholar]
- 2.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue hemorrhagic fever. Trop Med Int Health. 2006;11:1238–55. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324:1563–6. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler DJ. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. CABI International; Wallingford (UK: 1997. pp. 1–22. [Google Scholar]
- 5.Rigau-Perez JG, Clark GG, Gubler JG, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue hemorrhagic fever. Lancet. 1998;352:971–7. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 6.Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–4. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–30. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 8.Efflner PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, et al. Dengue fever, Hawaii, 2001-2002. Emerg Infect Dis. 2005;5:742–9. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117–22. doi: 10.1016/0738-081x(89)90034-5. [DOI] [PubMed] [Google Scholar]
- 10.Itoda I, Masuda G, Suganuma A, Imamura A, Atsushi A, Yamada K, et al. Clinical features of 62 imported cases of dengue fever in Japan. Am J Trop Med Hyg. 2006;75:470–4. [PubMed] [Google Scholar]
- 11.Radakavic-Fijan S, Graninger W, Muller C, Honigsmann H, Tanew A. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430–3. doi: 10.1067/mjd.2002.111904. [DOI] [PubMed] [Google Scholar]
- 12.Weedon D. Skin pathology. 2nd ed. Churchill Livingstone; Toronto: 2002. [Google Scholar]
- 13.Carey DE, Myers RM, DeRantiz CM, Jadhav M, Reuben R. The 1964 Chikungunya epidemic at Vellore, South India, including observations on concurrent Dengue. Trans R Soc Trop Med Hyg. 1969;63:434–45. doi: 10.1016/0035-9203(69)90030-3. [DOI] [PubMed] [Google Scholar]
- 14.Sabin AB, Philip CB, Paul JR. Phlebotomus fever. JAMA. 1944;125:693–9. [Google Scholar]
- 15.Bialecki C, Feder HM, Grant-Kels JM. The six classic childhood exanthems: a review and update. J Am Acad Dermatol. 1989;21:891–903. doi: 10.1016/s0190-9622(89)70275-9. [DOI] [PubMed] [Google Scholar]
- 16.James WD, Berger TG, Elston DM. Andrews’ diseases of the skin: clinical dermatology. 10th ed. Saunders Elsevier; Philadelphia: 2006. [Google Scholar]
- 17.Barron KS. Kawasaki disease in children. Curr Opin Rheumatol. 1998;19:29–37. doi: 10.1097/00002281-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Cherry JD. Parvovirus infections in children and adults. Adv Pediatr. 1999;46:245–69. [PubMed] [Google Scholar]
- 19.McCarthy JT, Hoagland RJ. Cutaneous manifestations of infectious mononucleosis. JAMA. 1964;187:153–4. doi: 10.1001/jama.1964.03060150077029. [DOI] [PubMed] [Google Scholar]
- 20.Contratto AW. Infectious mononucleosis: a study of one hundred and ninety-six cases. Arch Intern Med. 1944;73:449–59. [Google Scholar]
- 21.Letchner A. Roseola infantum: a review of fifty cases. Lancet. 1955;266:1163–5. doi: 10.1016/s0140-6736(55)92896-8. [DOI] [PubMed] [Google Scholar]
- 22.Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed] [Google Scholar]
- 23.Koplik H. The diagnosis of the invasion of measles from a study of the exanthema as it appears on the buccal mucous membranes. Arch Pediatr. 1896;13:918–22. [PubMed] [Google Scholar]
- 24.Wesselhoeft C. Rubella (German measles). N Engl J Med. 1947;236:943–50. doi: 10.1056/NEJM194706192362506. [DOI] [PubMed] [Google Scholar]
- 25.Galen WK. Cutaneous manifestations of enterovirus infections. In: Tyring SK, Yen-Moore A, editors. Mucocutaneous manifestations of viral diseases. Marcel Dekker Inc; New York: 2002. pp. 455–72. [Google Scholar]
- 26.Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161–4. doi: 10.1097/00007435-198010000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Marzano AV, Mercogliano M, Borghi A, Facchetti M, Caputo R. Cutaneous infection caused by Salmonella typhi. J Eur Acad Dermatol Venereol. 2003;17:575–7. doi: 10.1046/j.1468-3083.2003.00797.x. [DOI] [PubMed] [Google Scholar]
- 28.Giudice PD, Schuffenecker I, Zeller H, Grelier, Vandenbos F, Dellamonica P, et al. Skin manifestations of West Nile virus infection. Dermatology. 2006;211:348–50. doi: 10.1159/000088506. [DOI] [PubMed] [Google Scholar]
- 29.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 30.Shore H. O'nyong-nyong fever: an epidemic virus disease in East Africa. Trans R Soc Trop Med Hyg. 1961;55:361–73. doi: 10.1016/0035-9203(61)90017-7. [DOI] [PubMed] [Google Scholar]
- 31.Tesh RB, Watts DM, Russell KL, Damodaran C, Calampa C, Cabezas C, et al. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999;28:67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro FP, Freitas RB, deRosa JF, Gabbay YB, Mello WA, LeDuc JW. An outbreak of Mayaro virus disease in Belterra, Brazil: clinical and virological findings. Am J Trop Med Hyg. 1981;30:674–81. doi: 10.4269/ajtmh.1981.30.674. [DOI] [PubMed] [Google Scholar]
- 33.Laine M, Luukkainen R, Toivanen A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J Intern Med. 2004;256:457–71. doi: 10.1111/j.1365-2796.2004.01413.x. [DOI] [PubMed] [Google Scholar]
- 34.Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev. 2001;14:909–32. doi: 10.1128/CMR.14.4.909-932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karande S, Bhatt M, Kelkar A, Kulkarni M, De A, Varaiya A. An observational study to detect leptospirosis in Mumbai, India, 2000. Arch Dis Child. 2003;88:1070–5. doi: 10.1136/adc.88.12.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders EJ, Rigau-Perez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, et al. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1966. Am J Trop Med Hyg. 1999;61:399–404. doi: 10.4269/ajtmh.1999.61.399. [DOI] [PubMed] [Google Scholar]
- 37.Tindall B, Barker S, Donovan B, Barnes T, Roberts J, Kronenberg C, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945–9. [PubMed] [Google Scholar]
- 38.Weir MR, Weit TE. Flaviviridae. In: Tyring SK, Yen-Moore A, editors. Mucocutaneous manifestations of viral disease. Marcel Dekker Inc; New York: 2002. pp. 473–502. [Google Scholar]
- 39.Fairhurst RM, Wellems TE. Plasmodium species (Malaria). In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. Elsevier Inc; Philadelphia: 2005. pp. 3121–44. [Google Scholar]
- 40.Deresinski S. Concurrent Plasmodium vivax malaria and dengue. Emerg Infect Dis. 2006;12:1802. doi: 10.3201/eid1211.060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charrel RN, Brouqui P, Foucault C, de Lamballerie X. Concurrent dengue and malaria. Emerg Infect Dis. 2005;11:1153–4. doi: 10.3201/eid1107.041352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in a man in Thailand, 1962-1964: observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–71. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- 43.Paquet C, Quatresous I, Solet JL, Sissoko D, Renault P, Pierre V, et al. Chikungunya outbreak in Réunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill. 2006;11:E0602023. doi: 10.2807/esw.11.05.02891-en. [DOI] [PubMed] [Google Scholar]
- 44.Hochedez P, Jaureguiberry S, Debruyne M, Bossi P, Hausfater P, Brucker G, et al. Chikungunya infection in travelers. Emerg Infect Dis. 2006;12:1565–7. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks—the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 46.Chikungunya fever diagnosed among international travelers—United States, 2005-2006. MMWR Morb Mortal Wkly Rep. 2006;55:1040–2. [PubMed] [Google Scholar]
- 47.Keller TH, Chen YL, Knox JE, Siew PL, Lim SP, Ma NL. Finding new medicines for flaviviral targets. In: Bock G, Goode J, editors. New treatment strategies for dengue and other flaviviral diseases. Novartis Foundation Symposium 277. John Wiley; Chichester: 2006. pp. 102–19. [Google Scholar]
- 48.Stephenson JR. Developing vaccines against flavivirus diseases: past success, present hopes and future challenges. In: Bock G, Goode J, editors. New treatment strategies for dengue and other flaviviral diseases. Novartis Foundation Symposium 277. John Wiley. Chichester: 2006. pp. 193–205. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep. 1997;46:1–55. [PubMed] [Google Scholar]
- 50.Texas Department of State Health Services [September 18, 2007];Notifiable_conditions. Available from: URL: http://www.dshs.state.tx.us/idcu/investigation/forms/101A.pdf.