Abstract
Complete remission (CR) is the gold standard for assessing outcomes following chemotherapy for acute myelogenous leukemia (AML). “CRp,” a response criterion defined as fulfillment of all criteria for CR except platelet count recovery to ≥100 × 109/L, is associated with inferior outcomes following chemotherapy. The prognostic importance of CRp before allogeneic stem cell transplantation (allo-SCT) remains unknown. We analyzed a cohort of AML (n=334) and myelodysplastic syndrome (MDS; n=10) patients to determine the prognostic significance of achieving CR versus CRp before allo-SCT. At time of transplantation, 266 patients were in CR (CR1 and ≥CR2) and 78 in CRp (CR1p and ≥CR2p). Median follow-up was 38 months (3–131 months). Overall survival, progression-free survival, and nonrelapse mortality (NRM) were most favorable in patients transplanted in CR (CR1 or ≥CR2) compared with CRp (CR1p or ≥CR2p). Achieving CR is therefore associated with improved posttransplantation outcomes compared with achieving CRp and is a significant prognostic factor that needs to be considered when evaluating AML/MDS patients for clinical trials and allo-SCT.
Keywords: CRp, Platelet recovery, AML, MDS, Transplant
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative option for patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). However, the optimal candidates for allo-SCT and the ideal timing of the transplantation have not been agreed upon universally Numerous prognostic factors can help identify patients who are at a high risk for relapse and patients who will benefit most from allo-SCT [1,2]. For example, pre- and postinduction therapy characteristics have been shown to correlate with relapse and mortality outcomes. Although pretherapy characteristics, primarily cytogenetic abnormalities and genetic mutations, are often used for patient selection, data obtained during and after induction chemotherapy have also been shown to provide valuable prognostic information. In 1 study, patients who required more chemotherapy courses to achieve complete remission (CR) were shown to have a shorter disease-free interval than patientswho required fewer treatment courses [3]. The prognostic value of peripheral blood (PB) parameters, specifically platelet count, in the setting of allo-SCT remains unknown.
Many patients achieve a morphologic complete remission, clearing the bone marrow (BM) of detectable leukemia cells, but have incomplete recovery of hematopoiesis. Achieving disease remission without platelet recovery (ie, PB platelets <100 × 109 platelets/L), frequently referred to as CRp (or CRi), is reported as a form of partial remission in patients receiving induction chemotherapy and is correlated with inferior disease outcomes compared with patients who achieve hematologic recovery [4–7]. Whether or not outcomes following allo-SCT are adversely affected in patients with CRp is yet to be defined. Here, we describe a retrospective analysis we conducted to assess the prognostic significance of achieving CR (vs CRp) at the time of allo-SCT.
MATERIALS AND METHODS
Patient Selection
The study cohort comprised 344 patients with AML (n = 334) or high-risk MDS (n = 10) transplanted at The University of Texas M.D. Anderson Cancer Center between July 1995 and December 2007. Patients were categorized as CR (ie, <5% BM blasts and ≥100 × 109 platelets/L of PB following chemotherapy) or CRp (ie, <5% BM blasts but no platelet recovery at time of allo-SCT, which was at least 28 days after chemotherapy). We classified patients as first CR or CRp based on response following induction chemotherapy or equal to or greater than second CR (≥CR2) or CRp (≥CR2p) based on response following salvage chemotherapy. A minimum of 28 days from the last chemotherapy to the start of the preparative regimens had elapsed before the patients were assigned to the aforementioned disease status categories. All protocols, including this retrospective analysis, were approved by the institutional review board of the M.D. Anderson Cancer Center. Patients provided written informed consent for their treatment. Patients were treated in accordance with the Declaration of Helsinki.
Preparative regimens were classified as myeloablative (n=233) or reduced-intensity conditioning (RIC) (n = 111). The myeloablative regimens were primarily busulfan-based, including oral and intravenous (i.v.) busulfan (50%; n = 173), or total body irradiation (TBI) based (2%; n = 7). The RIC regimens primarily included fludarabine and melphalan (21%; n = 73) or were busulfan based (6%; n=21). Sixty-one percent of patients had received matched-sibling donor transplants (n = 208), and 34% (n = 117) received matched unrelated donor transplants. Patients received a tacrolimus-based regimen (n = 341), a cyclosporine-based regimen (n = 1), or methotrexate alone (n = 1) for graft-versus-host disease (GVHD) prophylaxis. One patient received a syngeneic stem cell transplantation (SCT) and did not receive GVHD prophylaxis. Antithymocyte globulin (ATG) was administered to those who received grafts from unrelated donors.
Cytogenetic risk categories were defined as follows: good-risk cytogenetics included patients with translocation (t)(8;21); inversion (inv)(16), or t(16;16); deletion (del)(9q); or t(15;17). Intermediate-risk cytogenetics included patients with a normal karyotype; −Y; del (5q); loss of 7q; t(9;11); +11; del(11q); abnormality (12p);+13; del(20q); or+21. Poor-risk cytogenetics included patients with a complex karyotype (≥3 abnormalities); inv(3) or t(3;3); t(6;9); t(6;11); −7; +8 (sole abnormality); or +8 with 1 other abnormality other than t(8;21), t(9;11), inv(16), or t(16;16); t(11;19) (q23;p13.1) [8].
Statistical Analysis
The primary outcome measures were overall survival (OS), progression-free survival (PFS), and nonrelapse mortality (NRM). All outcomes were evaluated from the transplantation date. PFS was defined as the time to disease progression or death. Actuarial OS and PFS times were estimated using the Kaplan-Meier method [9]. We estimated cumulative NRM incidence considering disease progression and death in relapse or with persistent disease as competing risks. Weused the Cox proportional hazards model to evaluate prognostic factors for OS, PFS, and NRM. Prognostic factors considered included disease status at the time of allo-SCT, patient age, patient gender, donor type, stem cell source, cytogenetics, and conditioning regimen. Statistical significance was defined at the 0.05 level. All the statistical analyses were performed using Stata version 9.0 software (StataCorp. 2005, College Station, TX).
RESULTS
Patient Characteristics
Patient characteristics are presented in Table 1. The median age was 49 years (range: 6–73) and 54% were male (n = 186). The median time from the end of chemotherapy to allo-SCT was 66 days (range: 29–303). Disease status at SCT was CR1 in 48% of patients (n = 164), ≥CR2 in 30% (n = 102), CR1p in 14% (n = 48), and ≥CR2p in 9% (n = 30). Of the 10 patients with MDS, 6 patients were in CRp. Cytogenetic data were available for 92% (n = 315) of patients. The AML subtypes, according to World Health Organization classification, included M0-M2 (n = 136), M3 (n = 4), M4-M5 (n = 91), M6-M7 (n = 17), and unclassified (n = 96). The distribution of cytogenetics according to disease status is shown in Table 2. The proportion of patients with poor-risk cytogenetics was comparable between the CR and CRp patients, 37% and 31% (P = .30), respectively. The proportion of patients with poor-risk cytogenetics was also comparable within each subgroup, CR1 (38%) versus CR1p (42%) (P = .50) and ≥CR2 (13%) versus ≥CR2p (20%) (P = .20). The source of stem cells was BM in 40% of patients (n = 137) and PB in 60% (n = 207). The median times to transplantation for patients in CR and CRp were 62 and 89 days, respectively. Successful engraftment was seen in 98% of patients.
Table 1.
Patient Characteristics
| Number (%) | |
|---|---|
| Gender | |
| Male | 186 (54) |
| Female | 158 (46) |
| Age (years) | |
| >50 | 163 (47) |
| ≤50 | 181 (53) |
| Disease status at transplantation | |
| CR1 | 164 (48) |
| ≥CR2 | 102 (30) |
| CR1p | 48 (14) |
| ≥CR2p | 30 (9) |
| Cytogenetics | |
| Good | 20 (6) |
| Intermediate | 194 (56) |
| Poor | 101 (29) |
| Unknown | 29 (8) |
| Donor type | |
| Matched sibling | 208 (61) |
| MUD | 117 (34) |
| 1 Ag MM related | 14 (4) |
| 1 Ag MM unrelated | 5 (1) |
| Stem cell source | |
| Bone marrow | 137 (40) |
| Peripheral blood | 207 (60) |
| Number of prior chemotherapy courses | |
| ≤2 | 125 (36) |
| >2 | 209 (61) |
| Unknown | 10 (3) |
CR1 indicates first complete remission; ≥CR2, equal to or more than second complete remission; CR1p, CR1 without platelet recovery; ≥CR2p, equal to or more than CR2 without platelet recovery; MUD, matched unrelated donor; MM, mismatched.
Table 2.
Distribution of Cytogenetics and Age According to Response Group
| Patient parameter | CR1 n = 164 |
CR1p n = 48 |
≥CR2 n = 102 |
≥CR2p n = 30 |
|---|---|---|---|---|
| Cytogenetics | ||||
| Good | 1% | 2% | 17% | 3% |
| Intermediate | 57% | 50% | 57% | 60% |
| Poor | 38% | 42% | 13% | 20% |
| Unknown | 4% | 6% | 14% | 17% |
| Age (years) >50 | 47% | 71% | 34% | 57% |
CR1 indicates first complete remission; CR1p, CR1 without platelet recovery; ≥CR2, equal to or greater than second complete remission; ≥CR2p, equal to or greater than CR2 without platelet recovery.
Prognostic Factors for OS and PFS
After a median follow-up of 38 months in surviving patients (range: 3–131 months), a total of 153 deaths occurred in this cohort. The actuarial overall survival was 57% (95% confidence interval [CI] 51–62) and PFS 50% (95% CI 44–55) at 36 months posttransplantation. Almost one-third of the patients (n = 95) experienced disease progression. In univariate analysis, disease status at time of SCT was the only significant predictor of OS and PFS (Table 3). Compared with patients in CR, patients in CRp had significantly worse OS (39% vs 62%, P < .001) and PFS (36% vs 54%, P = .001) at 36 months posttransplantation (Table 3). None of the additional factors evaluated were shown to be significant predictors of OS, or PFS including gender, age (>50 vs ≤50 years), graft type (matched related versus other), graft source (peripheral blood vs bone marrow), preparative regimen (RIC vs myeloablative), and cytogenetics (poor risk vs good/intermediate risk).
Table 3.
Impact of Platelet Recovery on Overall Survival, Progression-Free Survival, and Nonrelapse Mortality for All Patients, CR1 Patients, and ≥CR2 Patients at Time of Stem Cell Transplantation
| Patient Group | OS HR (P value) |
PFS HR (P value) |
NRM HR (P value) |
|---|---|---|---|
| CRp versus CR | 2 (P < .001) | 1.7 (P=.001) | 1.7 (P =.03) |
| CR1p versus CR1 | 2.1 (P =.002) | 1.7 (P =.02) | 2.3 (P =.01) |
| ≥CR2p versus ≥CR2 | 1.9 (P =.02) | 1.7 (P =.05) | 1.1 (P =.9) |
OS indicates overall survival; PFS, progression-free survival; NRM, nonrelapse mortality; HR, hazards ratio; CRp, remission without platelet recovery; CR, complete remission; CR1p, CR1 without platelet recovery; CR1, first complete remission; ≥CR2p, equal to or greater than second complete remission without platelet recovery; ≥CR2, equal to or greater than second complete remission. Outcomes are compared at the median follow-up time: 36 months for the CR versus CRp and CR1 versus CR1p groups, and at 60 months for the ≥CR2 versus ≥CR2p groups.
Subgroup analyses evaluating the impact of platelet recovery separately in CR1 and ≥CR2 patients showed consistent results. At 36 months, OS was higher for patients receiving transplants while in CR1 (64%) compared with those receiving transplants in CR1p (45%) (hazard ratio [HR] = 2.1; P = .002) (Tables 3 and 4 and Figure 1A). PFS was also higher for patients receiving transplants in CR1 (54%) compared with those receiving transplants in CR1p (38%) (HR=1.7; P=.02) (Tables 3 and 4, Figure 1B).
Table 4.
36-Month Outcomes by Pretransplantation Disease Status
| Pretransplant Disease Status | OS (95% CI) | PFS (95% CI) | NRM (95% CI) |
|---|---|---|---|
| CR1 (n 5 164) | 64% (56–72) | 54% (45–61) | 18% (12–25) |
| ≥CR2 (n 5 102) | 58% (47–67) | 54% (44–63) | 27% (19–38) |
| CR1p (n 5 48) | 45% (30–59) | 38% (24–52) | 30% (19–47) |
| ≥CR2p (n 5 30) | 31% (15–49) | 32% (16–50) | 24% (13–46) |
OS indicates overall survival; PFS, progression-free survival; NRM, nonrelapse mortality; CR1, first complete remission; ≥CR2, equal to or greater than second complete remission; CR1p, CR1 without platelet recovery; and≥CR2p, equal to or greater than second complete remission without platelet recovery.
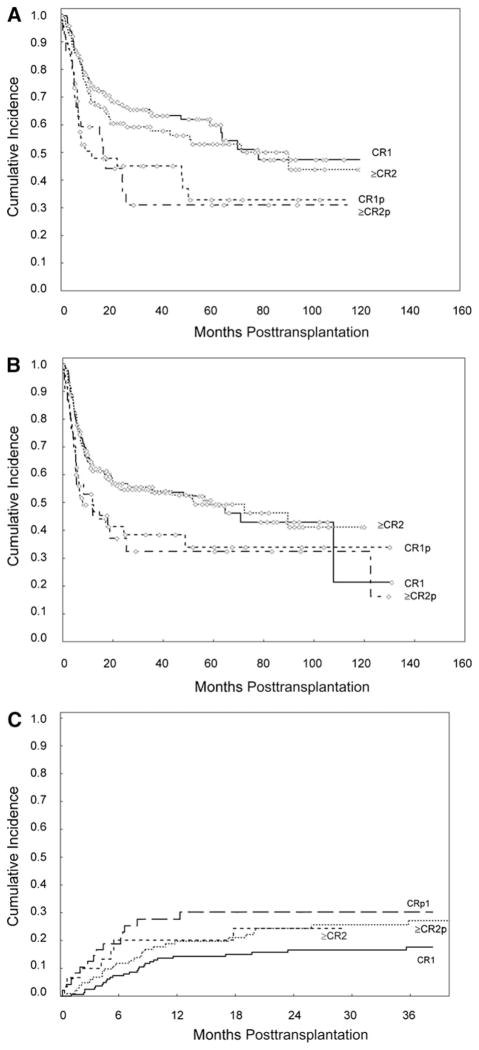
Figure 1.
Kaplan-Meier survival analysis. Cumulative incidence of (A) overall survival (OS), (B) progression-free survival (PFS), and (C) nonrelapse mortality (NRM) by pretransplantation remission status. Data reflects preparative regimens used between July 1995 and December 2007. CR1, first complete remission; ≥CR2, equal to or greater than second complete remission; CR1p, CR1 without platelet recovery; and ≥CR2p, equal to or greater than second complete remission without platelet recovery.
Similar results were found in patients who received stem cell transplants while in ≥CR2 or ≥CR2p. At a median follow-up of 60 months (range: 5–130 months), OS was higher for patients receiving transplants while in ≥CR2 (53%) compared with those receiving transplants in ≥CR2p (31%) (HR = 1.9; P = .02) (Table 4 and Figure 1A). PFS at 60 months was also higher for patients receiving transplants in ≥CR2 (49%) compared with those receiving transplants in ≥CR2p (32%) (HR = 1.7; P = .05) (Table 4 and Figure 1B). None of the additional prognostic factors evaluated (listed previously had a significant impact on OS or PFS in subgroup analyses.
Prognostic Factors for NRM
A total of 82 patients died of nonrelapse causes including graft failure (n = 4), infection (n = 13), pneumonia (n = 8), acute GVHD (aGVHD) (n = 12), chronic GVHD (cGVHD) (n = 21), organ failure (n = 11), veno-occlusive disease (n = 2), secondary malignancy (n = 2), acute myocardial infarction (n = 1), hemorrhage (n = 1), and unknown causes while in remission (n = 7). In univariate analysis, platelet recovery was the only significant predictor of NRM at 36 months, which was significantly higher for patients receiving transplants in CRp (28%; 95% CI 19–40) in comparison with patients receiving transplants in CR (21%; 95% CI 17–27); (HR = 1.7; P = .03) (Table 3). Subgroup analyses showed that the effect of platelet recovery was more pronounced for patients in CR1 (HR = 2.3, P = .01) than in patients in ≥CR2 (HR = 1.1, P = .90) (Table 3). The estimated cumulative incidence of NRM at 36 months was 18% for patients in CR1; 30% for those in CR1p; 27% for those in ≥CR2; and 24% for those in ≥CR2p (Table 4 and Figure 1C).
DISCUSSION
Many patients with AML/MDS achieve a morphologic CR with<5% BM blasts, but fail to fully recover hematopoiesis. In this report, we demonstrate that failure to achieve full platelet recovery (<100 × 109 platelets/ L) before allo-SCT is associated with worse outcomes for patients with AML/MDS. The requirement for platelet recovery to define CR has been challenged. Some studies have proposed that achieving ≥100×109 platelets/Lmay not be as critical with newer AML therapies, and that outcomes following allo-SCT would not be compromised [10,11]. Our results indicate that patients fulfilling classical criteria forCR, including a requirement for platelet recovery at the time of transplantation, have better OS, PFS, and NRM rates than those transplanted in CRp. We show that in the allo-SCT setting, achieving a CRp is associated with better outcomes than a lack of response to therapy (data not shown), but worse outcomes compared with achieving a CR. Unlike previous reports [10,11], our study included patients in CR1 as well as patients in disease status ≥CR2, making our conclusions broadly applicable even to patients who are being considered for allo-SCT in the salvage setting.
Although it is conceivable that in some patients platelet recovery may eventually be reached with longer waiting periods before allo-SCT, in practice, such delays before making therapeutic decisions are not feasible and may cause adverse outcomes. We defined CRp after a waiting period of at least 28 days in an attempt to minimize selection bias, because most patients likely to recover platelet counts will have achieved full recovery by this time point. Use of the minimum 28-day time point to assess platelet recovery would act to potentially improve the prognosis in the CRp group, because some patients without disease may have subsequently achieved later platelet recovery.
We acknowledge that a clear limitation of our study is that patients in CRp are oftentimes excluded from allo-SCT, because it may be a surrogate marker of other morbid conditions, such as infections. This may have caused a selection bias in the CRp patients analyzed in this study that may have had a better performance status than CRp patients who were excluded from receiving allo-SCT. If such bias did occur here, it could have contributed to better outcomes for the patients in CRp. This bias would actually strengthen our conclusions that patients in CRp have significantly worse outcomes compared with patients in CR. We also recognize that our results should be cautiously interpreted in the setting of MDS, given the small number of MDS patients included.
Worse outcomes were seen in CRp patients irrespective of the intensity of the preparative regimen used. This fact would suggest that patients in CRp be considered for novel therapies, including posttransplantation maintenance [12].
In summary, our results indicate that in patients with AML or MDS, achieving a CR, including a platelet count of ≥100 × 109/L in PB, is associated with improved survival and NRM compared with CRp. Therefore, achievement of CR and CRp is a valuable and efficient prognostic marker that needs to be considered in the evaluation of novel transplantation treatments.
Acknowledgments
Salary for Dr. Alatrash at the time this research was conducted was provided in part by the M.D. Anderson Barbara Rattay Advanced Scholars Program.
Footnotes
Financial disclosure: The authors have nothing to disclose.
References
- 1.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 2.Nevill TJ, Hogge DE, Toze CL, et al. Predictors of outcome following myeloablative allo-SCT for therapy-related myelodysplastic syndrome and AML. Bone Marrow Transplant. 2008;42:659–666. doi: 10.1038/bmt.2008.226. [DOI] [PubMed] [Google Scholar]
- 3.Keating MJ, Smith TL, Gehan EA, et al. Factors related to length of complete remission in adult acute leukemia. Cancer. 1980;45:2017–2029. doi: 10.1002/1097-0142(19800415)45:8<2017::aid-cncr2820450806>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 28:1766–1771. doi: 10.1200/JCO.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estey E, Garcia-Manero G, Giles F, Cortes J, O’Brien S, Kantarjian H. Clinical relevance of CRp in untreated AML. Blood. 2005;106:Abstract 541. [Google Scholar]
- 6.Etienne A, Charbonnier A, Prebet T, et al. Impact of CRi on the outcome of elderly patients with untreated acute myeloid leukemia (AML) Blood. 2008:Abstract 2988. [Google Scholar]
- 7.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 11.Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 12.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]