Abstract
Natural killer (NK) cells are believed to play a role in human immunodeficiency virus type 1 (HIV-1) disease progression, and NK cell levels are reduced in individuals with chronic HIV-1 infection. Interleukin (IL)–2 therapy results in an expansion of CD4+ T cells as well as NK cells; however, little is known about the detailed effects of IL-2 therapy on NK cells in HIV-1 infection in general and in early infection in particular. Here, we investigated the effects of combined IL-2 therapy and antiretroviral therapy (ART) on the number, frequency, phenotype, and interferon (IFN)–γ production of NK cells in individuals with early HIV-1 infection. Patients randomized to receive combined ART and IL-2 therapy predominantly expanded CD56dim NK cells, and the expansion was greater than in patients randomized to receive ART alone. Importantly, NK cell receptor expression and IFN-γ production were maintained over time. This reconstitution of NK cells may be useful in helping contain viremia if patients discontinue therapy or develop drug resistance.
Subcutaneous administration of low doses of interleukin (IL)–2 to HIV-1–infected patients elevates the numbers of CD4+ T cells [1–8], and IL-2 therapy has been shown to enhance recall responses to tetanus toxoid [4, 5]. In contrast, less is known about IL-2 therapy in HIV-1 infection with regard to innate immune cells, particularly early in infection. IL-2 therapy in HIV-1–infected subjects expands both NKT cells [9] and NK cells [5, 10–13]. However, NK cells consist of heterogeneous populations with different phenotypes and functions. As such, very little is known about the effects of combined antiretroviral therapy (ART) and IL-2 therapy on the different NK cell subsets, receptor expression, and function.
NK cells are an important source of chemokines in HIV-1–infected individuals and suppress HIV-1 entry and replication in vitro [14–16]. Epidemiological studies of killer immunoglobulin-like receptor (KIR) and human leukocyte antigen (HLA) haplotypes suggest that the combination of HLA-Bw480Ile and the NK cell receptor (NKR) KIR3DS1 has a protective effect on disease progression [17]. The effect of HIV-1 infection on the frequency and absolute number of NK cells remains unclear, partially because of the variation in definition of NK cells. Traditionally, NK cells have been defined as CD3−CD56+ lymphocytes, which have been further divided into CD56brightCD16− and CD56dimCD16+ subpopulations [18]. The frequency and absolute numbers of CD3−CD56+ NK cells is decreased in subjects with chronic HIV-1 infection [19–26], and the majority of studies have found that the CD56dim NK cell subpopulation is most affected [23–25]. Interestingly, a recent study of acutely infected patients reported an initial increase in the frequency of CD56dim NK cells [21]. In addition, the frequency of CD3−CD16+CD56− NK cells is increased in HIV-1–infected individuals with viremia [23, 25, 27–29] but is normal in HIV-1–infected subjects without viremia [27, 29].
In addition to changes in NK cell subsets, the pattern of NKR expression differs between HIV-1–infected individuals with and without viremia as well as between healthy and HIV-1–infected subjects [21, 26, 29–32]. However, longitudinal studies are required to demonstrate the causal relationship between changes in viral load and changes in NKR expression.
In the present longitudinal study, the main goal was to investigate the number, frequency, phenotype, and interferon (IFN)–γ production of NK cells in treatment-naive subjects with early HIV-1 infection who were randomized to initiate ART alone or to initiate combined ART and IL-2 therapy.
SUBJECTS, MATERIALS, AND METHODS
Study design and research subjects
Samples from early HIV-1–infected patients were obtained from the OPTIONS cohort. The outline of the study design is depicted in figure 1A. Thirty-two subjects were followed up for 1 year, all of whom were treatment naive at study entry. Of these subjects, 11 chose to continue not receiving treatment (hereafter, “untreated”), and 21 entered a trial investigating the effects of combined ART and IL-2 therapy. The latter 21 subjects initiated ART at study entry and were randomized to receive IL-2 either at an early or late stage. Early stage randomized subjects started IL-2 therapy once viral load had decreased to <500 copies/mL. These 10 subjects are referred to as “ART + IL-2.” IL-2 was given subcutaneously (7.5 million units, twice daily) for 5 days at 8-week intervals, with dose adjustments for toxicity. Late-stage randomized subjects continued receiving ART alone for 1 year before the initiation of IL-2 therapy. These 11 subjects are referred to as “ART alone.”
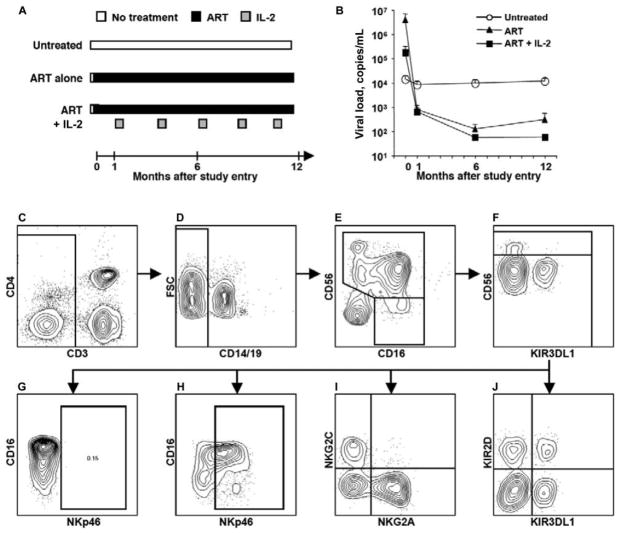
Figure 1.
Study design and gating strategy for flow cytometry. A, The 3 groups of HIV-1–infected patients examined. All 3 groups were treatment naive at study entry (month 0), when the first sample was collected. The first group elected not to initiate antiretroviral therapy (ART) and remained untreated throughout the study (untreated; n = 11). Note that this group was not randomized to remain untreated and is thus likely biased in relation to the 2 treated groups. The 2 other groups initiated ART (ART; n = 11) at study entry and were randomized to either continue receiving ART during the first year (ART alone) or to initiate interleukin (IL)–2 therapy once their viral load was <500 copies/mL (ART + IL-2). All subjects in these groups responded to ART. IL-2 was given twice daily for 5 days in 8-week intervals. Samples at week 24 and 48 were analyzed on average 6 weeks after the last cycle of IL-2 administration and on average 14 weeks after the first dose of IL-2. B, Mean viral load over time in subjects who remained untreated throughout the study (white circles), in subjects who initiated ART at study entry (black triangles), and in subjects who initiated ART at study entry, followed by IL-2 therapy (black squares). Error bars indicate SEs. Note that the viral load of the untreated group is significantly lower than those of the treated groups at study entry. C–J, Gating strategy for phenotyping of NK cells from HIV-1–infected individuals by use of 8-color flow cytometry. After gating on lymphocytes using forward and side light scatter (FSC and SSC, respectively), doublet cells were excluded using an FSC-area vs. FSC-height gate (data not shown). NK cells were defined by gating on CD3− cells (C), followed by exclusion of B cells and monocytes based on the expression of CD19 and CD14 (D), respectively. CD56+ NK cells were defined by a CD56+ gate among the CD3−CD14−CD19− cells (E). A small subset of cells, previously described as dysfunctional NK cells, were defined by gating on CD56−CD16+ cells among the CD3−CD14−CD19− cells. To define NK cells within this gate, CD11cbrightCD4low cells among the CD3−CD56−CD16+ cells were excluded (data not shown). CD56+ NK cells were subsequently split into CD56bright and CD56dim NK cells, based on the expression level of CD56 (F). Fluorescence minus one (FMO) controls were used to define the background fluorescence for each fluorochrome (G). An example of an FMO control for allophycocyanin-conjugated anti-NKp46 is shown. CD56dim NK cells were further phenotyped for NK cell receptor expression by use of combinations of different anti–NK cell receptor antibodies, including anti-NKp46 (H), anti-NKG2A and anti-NKG2C (I), and anti-KIR3DL1 and anti-KIR2D (J).
The first blood sample was drawn and cryopreserved at study entry (week 0), when all subjects were treatment naive and had viremia. On entering the study, blood samples were scheduled to be collected for cryopreservation at weeks 4, 12, 24, 48, and 72 after study entry. The initiation of IL-2 therapy was triggered by a reduction in viral load to <500 copies/mL, which is why subjects in the early stage randomized group did not initiate IL-2 at the same time after study entry. The actual median time from study entry to the second blood sample was 4 weeks (range, 4–8 weeks). The median time from study entry to the third and fourth blood sample was 24 weeks (range, 23–29 weeks) and 48 weeks (range, 45–56 weeks), respectively. In subjects receiving IL-2 therapy, the median time from the last dose of IL-2 to sampling was 6 weeks (range, 3–8 weeks) and from the first dose to sampling (at week 24) was 14 weeks (range, 11–20 weeks). The subject characteristics are presented in table 1 and the average viral loads in figure 1B. Peripheral blood from 10 healthy control subjects was obtained from the Stanford Blood Bank (n = 6) or from healthy volunteers after informed consent. The University of California, San Francisco, Committee for Human Research approved this study, and patients gave signed consent.
Table 1.
Subject characteristics.
| Characteristic | ART + IL-2a | ARTa | Untreatedb |
|---|---|---|---|
| Subjects, no. | 10 | 11 | 11 |
| HIV RNA load at week 0, log10 copies/mL | 4.1 (1.1) | 5.6 (1.0) | 3.8 (0.7) |
| CD4+ cell count at week 0, cells/μL | 596 (211) | 569 (188) | 700 (245) |
| Male, % | 90 | 100 | 91 |
| Age, years | 36 (7.4) | 36 (9.1) | 38 (5.5) |
| Estimated time from infection to treatment, median, weeks | 91 | 86 | NA |
| PI-containing ART regimen, % | 40 | 64 | NA |
NOTE. Data are mean (SD) values, unless otherwise indicated. NA, not applicable; PI, protease inhibitor.
Subjects in these groups were randomized to receive antiretroviral therapy (ART) alone or combined interleukin (IL)–2 therapy and ART.
Subjects in this group chose to remain untreated and thus were not randomized. The group is included as a reference but is not directly compared with the ART and ART + IL-2 groups.
Samples
Peripheral blood was collected by venipuncture in evacuated blood-collection tubes with acid citrate dextrose (Vacutainer; BD Diagnostics). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood and cryopreserved in fetal calf serum containing 10% dimethyl sulfoxide.
Cell culture
Frozen PBMCs were thawed, washed in R15 medium (RPMI 1640 [MediaTech] with 15% fetal calf serum [Gemini BioProducts], 2 mmol/L L-glutamine, 10 mmol/L HEPES, and 100 U/mL penicillin/streptomycin [Invitrogen]), counted, and plated at 4.0 × 105 cells/well in 96-well plates in R15 medium. We measured IFN-γ production by NK cells in 5 patients in each of the 3 different patients group. Cells were left untreated or stimulated with 50 ng/mL human recombinant IL-12 (PeProtech) for 24 h at 37°C in 5% CO2. Brefeldin A was added to a final concentration of 5 μg/mL for the last 5 h of culture, after which cells were harvested and analyzed for intracellular IFN-γ by flow cytometry.
Cell staining and flow cytometry
The first set of samples, including samples from 5 subjects from each treatment group, was stained with a common panel of antibodies (P1): energy-coupled dye (ECD)– conjugated anti-CD3 (Beckman Coulter), allophycocyanin (APC)–Cy7– conjugated anti-CD16, and phycoerythrin (PE)–Cy7– conjugated anti-CD56 (BD Biosciences). In addition, cells were stained with fluorescein isothiocyanate (FITC)– conjugated anti-KIR3DL1 (DX9) (BD Biosciences), PE-conjugated anti-NKG2C, allophycocyanin-conjugated anti-NKG2A (R&D Systems), FITC-conjugated anti-CD161, and FITC-conjugated anti-perforin (BD Biosciences). The second set of samples was stained with a common panel of antibodies (P2): ECD-conjugated anti-CD3, Alexa700-conjugated anti-CD4, APC-Cy7– conjugated anti-CD14 and anti-CD19, PE-Cy7– conjugated anti-CD56, and Pacific Blue– conjugated anti-CD16 (BD Biosciences). In addition to the common antibodies, NK cells were stained with antibodies directed against receptors, including: FITC-conjugated anti-KIR3DL1 (DX9), PE-conjugated anti-KIR2D (DX27), FITC-conjugated anti-CD94, APC-conjugated anti-CD161, FITC-conjugated anti-CD11c (BD Biosciences), PE-conjugated anti-NKG2C, APC-conjugated anti-NKG2A, PE-conjugated anti-NKp30, and APC-conjugated anti-NKp46 (R&D Systems). The anti– human CD94, NKG2A, and NKG2C antibodies were developed by a collaborative effort between L.L.L. and J. P. Houchins (R&D Systems). PBMCs were analyzed for intracellular per-forin content using a FITC-conjugated anti-perforin monoclonal antibody (MabTech).
PBMCs from functional assays were stained with the common antibody panel P2, fixed in 2% paraformaldehyde, permeabilized with FACS Perm II (BD Biosciences), and stained with ECD-conjugated anti-CD3 (Beckman Coulter) and APC-conjugated anti–IFN-γ (BD Biosciences). Anti–mouse immunoglobulin compensation particles (BD CompBeads; BD Biosciences) were used as compensation controls for software-based compensation. Cells were analyzed by flow cytometry using a 4-laser LSR-II instrument (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (TreeStar). The gating strategy used is shown in figure 1C–1J. Briefly, we identified NK cells by gating on single cells within a lymphocyte gate, excluding CD3+ cells, followed by gating on CD56+ and/or CD16+ cells. For analyses of CD56−CD16+ NK cells, we excluded a subset of CD3−CD14−CD19−CD56−CD4dimCD11cbright cells that also express CD16. Statistical analysis was performed using GraphPad Prism statistical software (version 4.0; GraphPad Software). Unless otherwise noted, frequencies and absolute cell counts were compared over time in the same treatment group. Statistical significance was tested using a Wilcoxon signed rank test, unless otherwise noted.
RESULTS
Expansion of NK cells by combined ART and IL-2 therapy but not by ART alone in HIV-1–infected individuals
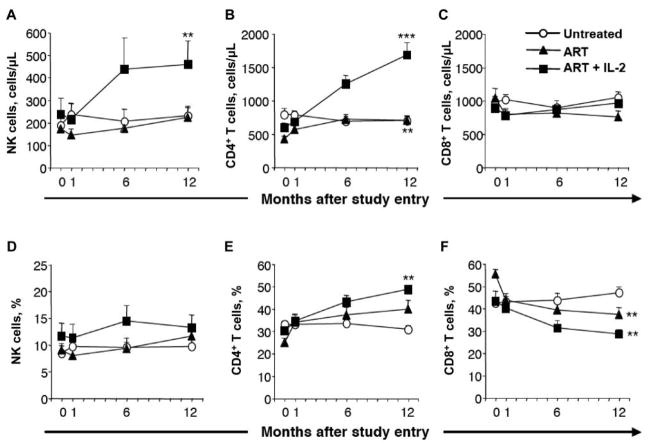
At study entry, there were no statistically significant differences in the frequency or absolute number of NK cells between the subjects randomized to receive ART alone or combined ART and IL-2 therapy (figure 2A and 2D). Moreover, there was no correlation between the overall absolute number or frequency of NK cells and HIV load or CD4+ T cell count at study entry (data not shown). However, the frequency of NK cells in the HIV-infected subjects was significantly lower than that in the healthy control subjects (P < .05, Mann-Whitney U test; data not shown). The absolute number and frequency of NK cells did not change significantly over 1 year in the subjects randomized to receive ART alone (figure 2A and 2D). In contrast, there was a 2-fold increase in the absolute number of NK cells in subjects randomized to receive combined ART and IL-2 therapy (figure 2A; P < .01). In addition, this group had a significantly higher number of NK cells at week 48 than did the subjects receiving ART alone (figure 2A; P < .05 by Student’s unpaired t test). The increase in NK cells was evident at 6 months after study entry and was sustained thereafter (figure 2A). The time from the last dose of IL-2 to sampling, as well as the time from the first dose to sampling, varied between the subjects receiving combined ART and IL-2 therapy. However, there was no correlation between the fold increase in NK cell numbers and the time from the last or first dose to sampling (data not shown). It should be noted that, despite the large increase in the absolute number of NK cells, there was only a moderate increase in NK cell frequency in response to combined ART and IL-2 therapy (figure 2D; P > .05), reflecting a parallel increase in CD4+ T cells (figure 2B). Interestingly, the fold increase in NK cell numbers correlated with the fold increase in CD4+ T cells (P < .01 and r = 0.77, linear regression analysis; data not shown). As a reference, the nonrandomized subjects who chose to remain untreated throughout the study had similar frequencies and absolute numbers of NK cells at study entry as the randomized, treated groups. The absolute number and frequency of NK cells and CD4+ T cells did not change significantly over 1 year in these subjects (figure 2).
Figure 2.
Expansion of NK cells after combined antiretroviral therapy (ART) and interleukin (IL)–2 therapy. White circles depict subjects who remained untreated throughout the study (untreated). Black triangles depict subjects who initiated ART at study entry (ART) and continued receiving ART alone throughout the study. Black squares depict subjects who initiated ART at study entry, followed by IL-2 therapy once their viral load had decreased to <500 copies/mL (ART + IL-2). A, Mean no. of NK cells (defined as CD3−CD56+) per microliter of blood over time. B, Mean no. of CD4+ T cells per microliter of blood over time. C, Mean no. of CD8+ T cells per microliter of blood over time. D, Mean percentage of NK cells over time. E, Mean percentage of CD4+ T cells over time. F, Mean percentage of CD8+ T cells over time. Bars indicate SEs. **P < .01 and ***P < .001 (Wilcoxon signed rank test), indicating statistically significant differences between study entry and month 12 within the respective groups (n = 11, 11, and 10 for the untreated, ART, and ART + IL-2 groups, respectively).
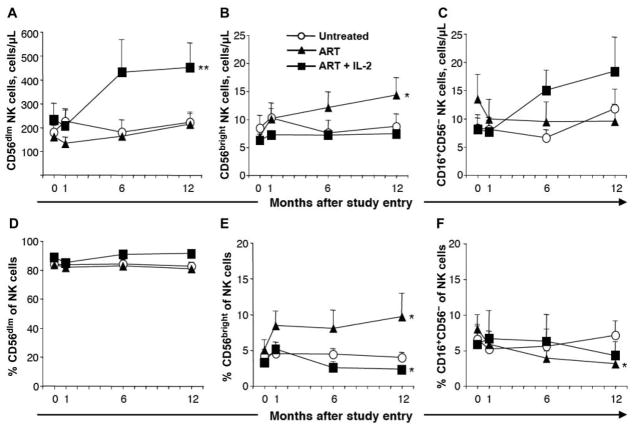
Increase in CD56dim, but not CD56bright, NK cells after combined ART and IL-2 therapy in early HIV-1 infection
To investigate whether NK cells expanded uniformly as a consequence of IL-2 therapy, we analyzed changes in each of the major NK cell subsets separately. The number of CD56dim NK cells increased ~2-fold in subjects receiving combined ART and IL-2 therapy (figure 3A; P < .01), making up the majority of the increase in NK cells. Surprisingly, there was no increase in the number or frequency of CD56bright NK cells in peripheral blood as a consequence of IL-2 therapy (figure 3B and 3E). In fact, the frequency of these cells was reduced after the initiation of IL-2 therapy (figure 3E; P < .05), reflecting the expansion of CD56dim NK cells (figure 3A). In contrast, there was a significant increase in the number and frequency of CD56bright NK cells apparent by 4 weeks after the initiation of ART (figure 3B and 3E; P < .05), whereas no significant increase in CD56dim NK cells was seen at that time (figure 3A). The increase in CD56bright NK cells continued over time in the group receiving ART alone, but not in the group receiving combined ART and IL-2 therapy (figure 3B and 3E). The number and frequency of CD56dim and CD56bright NK cells did not change significantly over time in the nonrandomized subjects who chose to remain untreated throughout the study (figure 3A, 3B, 3D, and 3E).
Figure 3.
Expansion of CD56dim, but not CD56bright, NK cells after interleukin (IL)–2 therapy. A, Mean no. of CD56dim NK cells per microliter of blood over time. B, Mean no. of CD56bright NK cells per microliter of blood over time. C, Mean no. of CD3−CD16+CD56− lymphocytes per microliter of blood over time. D, Mean percentage of CD56dim NK cells of total NK cells over time. E, Mean percentage of CD56bright NK cells of total NK cells over time. F, Mean percentage of CD3−CD16+CD56− cells of total NK cells over time. Bars indicate SEs. *P < .05 and **P < .01 (Wilcoxon signed rank test), indicating statistically significant differences between study entry and month 12 within the respective groups (n = 11, 11, and 10 for the untreated, antiretroviral therapy [ART], and ART + IL-2 groups, respectively in panels A, B, D, and E; n = 5 for each group in panels C and F).
In addition to the CD56dim and CD56bright NK cell subsets, we analyzed the size of the CD16+CD56− NK cell subset. This subset constituted, on average, 7% of the NK cells at study entry, when all subjects were untreated, and correlated with the viral load at this time (P < .01 and r = 0.7, linear regression analysis; data not shown). The frequency of these cells in HIV-1–infected individuals was elevated compared with that in healthy control subjects (P < .05, Mann-Whitney U test; data not shown), and a statistically significant decrease in the frequency (figure 3F), but not absolute number (figure 3C), was noted over time in subjects receiving ART alone, in agreement with previous reports [27]. In contrast, the number of CD16+CD56− NK cells was elevated 2-fold after IL-2 therapy (figure 3C), although this did not reach statistical significance (P = .16). No significant changes in the frequency or absolute number of CD16+CD56− NK cells were found over time in subjects who chose to remain untreated (figure 3C and 3F).
Effect of combined ART and IL-2 therapy on NKR and perforin expression
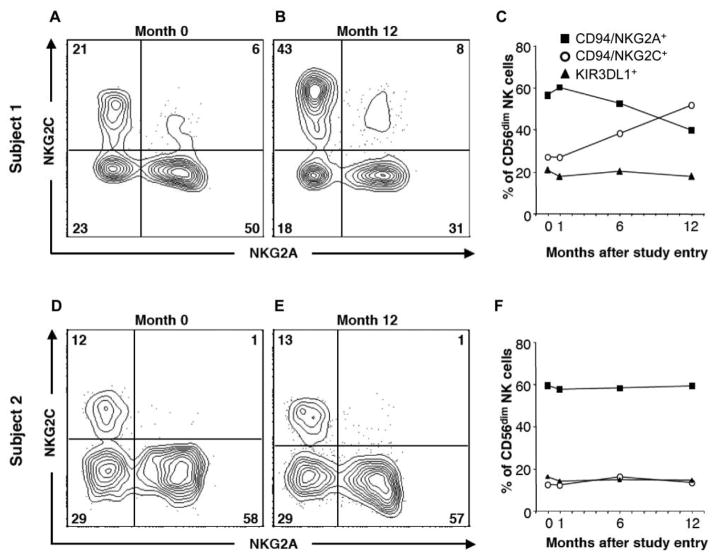
Overall, NKR expression was stable over time in all groups, with some individual exceptions. Subjects receiving combined IL-2 therapy and ART maintained the composition of NKR expression on their expanding CD56dim NK cells, and no changes in expression of NKG2C (figure 4A), NKG2A (figure 4B), KIR3DL1 (figure 4C), KIR2D (figure 4D), NKp30 (figure 4E), NKp46 (figure 4F), CD161 (figure 4G), or perforin (figure 4H) could be attributed to the combined ART and IL-2 therapy (figure 4A–4H). However, a small decrease in KIR3DL1-expressing NK cells was detected by 4 weeks after the initiation of ART (P < .05), but no further decrease was noted over time in subjects receiving ART alone or combined ART and IL-2 therapy (figure 4C). In addition, there were small but significant increases in the frequency of CD94/NKG2C+ cells in all 3 groups (figure 4A; P < .05). In some individuals, the increased frequency of NKG2C+ NK cells was dramatic and correlated with a decrease in the frequency of NKG2A+ NK cells (figure 5A–5C), whereas it remained stable in others (figure 5D–5F).
Figure 4.
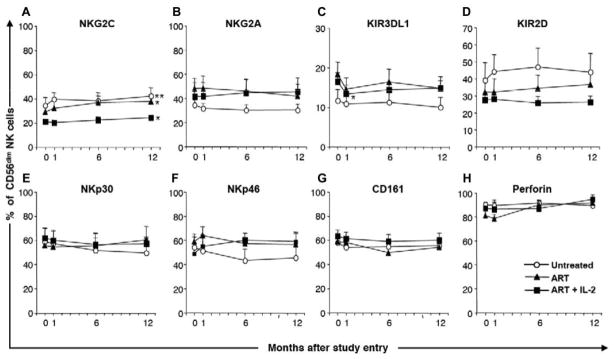
Conserved NK cell receptor expression over time in HIV-1–infected individuals. Graphs show the percentages of CD56dim NK cells expressing NKG2C (A), NKG2A (B), KIR3DL1 (C), KIR2D (D), NKp30 (E), NKp46 (F), CD161 (G), and perforin (H). Bars indicate SEs. *P < .05 and **P < .01 (Wilcoxon signed rank test), indicating statistically significant differences between study entry and month 12 within the respective groups (n = 11, 11, and 10 for the untreated, antiretroviral therapy [ART], and ART + interleukin (IL)–2 groups, respectively, in panels A, C, G, and H; n = 5 for each group in panels B, D, E, and F).
Figure 5.
Expansion of NKG2C+ NK cells is paralleled by a decrease in NKG2A+ NK cells in some, but not all, HIV-1-infected subjects over time. Shown are flow cytometry plots of NKG2A and NKG2C expression by CD56dim NK cells in 2 subjects receiving antiretroviral therapy (ART) at study entry (month 0) (A and D) and after 12 months of ART (B and E). C and F, Percentage of CD56dim NK cells expressing NKG2A, NKG2C, and KIR3DL1 over time. Note how the expression of NKG2A and NKG2C was stable in one of the subjects, but not the other, whereas KIR3DL1 expression remained stable over time in both subjects.
Maintenance of NK cell production of IFN-γ after combined IL-2 therapy and ART
The frequency of NK cells producing IFN-γ after culture of PBMCs in medium alone did not increase as a consequence of IL-2 therapy (data not shown). IL-12 stimulation of PBMCs in vitro induced a strong response by NK cells in all 3 groups, with no significant changes over time (figure 6A–6C). The absolute number of IL-12–induced IFN-γ–producing NK cells increased over time in the group undergoing combined ART and IL-2 therapy; however, this increase was secondary to the overall expansion of NK cells (data not shown).
Figure 6.
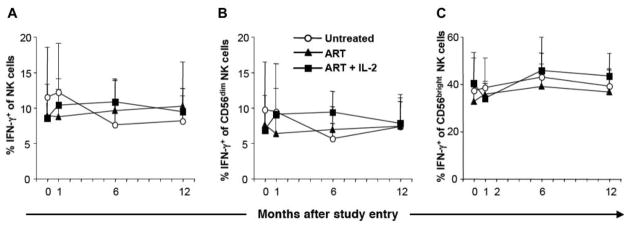
Conserved NK cell interferon (IFN)– γ production after interleukin (IL)–2 therapy in HIV-1–infected subjects. A, Percentage of NK cells expressing IFN-γ after stimulation with IL-12. B, Percentage of CD56dim NK cells expressing IFN-γ after stimulation with IL-12. C, Percentage of CD56bright NK cells expressing IFN-γ after stimulation with IL-12. Bars indicate SEs. IFN-γ production by NK cells (defined by gating on CD3−CD14−CD19−CD56+ lymphocytes) was measured by intracellular cytokine flow cytometry (n = 5 for all groups).
DISCUSSION
Here we demonstrate that a sustained reconstitution of NK cells in HIV-1–infected subjects, in parallel with CD4+ T cells and NKT cells, can be achieved by combining ART with intermittent subcutaneous administration of IL-2. The expansion of NK cells was sustained for at least 6 weeks after IL-2 treatment cycles and was mainly restricted to CD56dim NK cells.
Several lines of evidence point to an important role for NK cells in the immune defense against HIV. Epidemiological data indicate that NK cells are important both in the protection against de novo infection and in slowing progression to AIDS [17, 33, 34]. Highly exposed intravenous drug users who remain HIV seronegative have increased NK cell activity compared with that in intravenous drug users who seroconvert [35], and NK cells can inhibit HIV infection of CD4+ T cells in vitro through production of chemokines [14, 15, 36]. In addition, NK cells are important in the control of infections with other pathogens, including viruses, bacteria, and parasites [37–41], in particular in immunocompromised individuals. Reconstituting or increasing NK cell numbers in HIV-1–infected individuals, where the number of NK cells is decreased, is thus potentially clinically important not only for control of HIV but also for the defense against other pathogens and tumors.
From previous studies of IL-2 therapy, both in subjects with chronic HIV-1 infection [5, 10–13] and in HIV-1–seronegative subjects with malignancies [42–44], it is known that IL-2 therapy can induce an expansion of NK cells. However, it has not been well characterized which NK cells expand in response to IL-2 therapy nor how NKR expression and function is affected. Virtually all NK cells express the intermediate-affinity IL-2 receptor (CD122), and, in addition, the CD56bright subset expresses the high-affinity IL-2 receptor (CD25). Previous studies of IL-2 therapy in subjects with cancer have demonstrated that the CD56bright subset expands more than the CD56dim NK cell subset [42–44]. In contrast, the expansion of NK cells observed in the present study is seen virtually only within the CD56dim NK cell subset, and no increase attributable to IL-2 therapy could be noted in the CD56bright subset. There are several potential explanations for this discrepancy, including the time between IL-2 therapy and sampling and the dosage and duration of IL-2 therapy. First, previous studies of IL-2 therapy in cancer patients have seen that the expansion of CD56bright NK cells is transient, with decreases evident within days to weeks after the initiation of therapy [45]. Our study aimed at investigating the longer-term effects of IL-2 therapy. The samples were analyzed on average 6 weeks after the last dose of IL-2, when it is possible that NK cell levels already have decreased from peak levels. It thus remains possible that an initial, transient increase in CD56bright NK cells might have been missed because of the lag between therapy and sampling. A second potential explanation for the differences in NK cell expansion is differences in the dose and the duration of IL-2 therapy. It is possible that the higher dose (~5–10-fold higher) and shorter duration in this study, compared with the longer, lower-dose treatment in previous studies, resulted in an advantage for the CD56dim NK cells. Finally, it should be noted that the present and previous studies have sampled only peripheral blood. CD56bright cells are the major NK cell subset in lymph nodes, whereas CD56dim NK cells make up the major NK cell subset found in peripheral blood. It is thus possible that there is an expansion of CD56bright NK cells in the lymph nodes, which is not reflected by the expansion of NK cells in the blood. Because of the discomfort and risk for complications of sequential biopsies of secondary lymphoid tissue (e.g., lymph nodes and spleen), we collected samples only from peripheral blood and could not address the question of the effects of IL-2 on NK cells in lymphoid tissue directly.
The balance between activating and inhibitory receptors governs the function of NK cells, allowing maintenance of NK cell self-tolerance and recognition of aberrant cells. IL-2 therapy could potentially distort the balance between these receptors and result in NK cell autoreactivity. Here we show that the NKR expression and spontaneous IFN-γ production was conserved during IL-2 therapy, despite the large increase in NK cell numbers. Together, these findings indicate that combined ART and IL-2 therapy expands the CD56dim NK cells without excessive, potentially harmful, activation or skewing of the NKR repertoire.
In addition to investigating the effects of combined ART and IL-2 therapy, our study enabled us to investigate the dynamics of NKR expression over time in HIV infection. Previous cross-sectional studies have noted large differences in NKR expression between treated (aviremic) and untreated (viremic) groups of patients [29, 31]. This has been interpreted as a causal link between viral load and changes in NKR expression. However, comparing subjects who choose to initiate ART and subjects who chose to remain untreated is inherently biased—subjects who chose to remain untreated are more likely to have some degree of viral control and hence have lower viral loads than do subjects who subsequently choose to initiate ART (and have higher viral loads before ART). This is evident also in our study, where the subjects who remained untreated had 1–2 log lower viral load at study entry than did the subjects who subsequently initiated ART. Here we show that the expression of a number of NKRs is remarkably stable over 1 year in the same patients, irrespective of treatment or reductions in viral loads. There are, however, large variations in the pattern of NKR expression between patients, even though the pattern is conserved within each patient over the year. These findings support the notion that NKR expression could be a determinant of viral load rather than being determined by the viral load. Although the expression of NKRs remained stable over time in most individuals, we noted a marked expansion of CD94/NKG2C+NK cells in some individuals, irrespective of treatment (figure 5). Increased frequencies of NKG2C+NK cells in HIV-1–infected individuals was recently shown to be associated with seropositive reactions to cytomegalovirus (CMV) rather than HIV-1 infection [46], indicating that the marked increase in NKG2C+NK cells over time in some individuals in our study may reflect a change in CMV serostatus or reactivation of CMV during early HIV-1 infection.
In conclusion, we show that CD56dim NK cells are expanded in subjects with early HIV infection undergoing combined ART and IL-2 therapy, with conserved receptor expression and IFN-γ production over time. The impact of IL-2 treatment on increasing the frequency of innate immune cells, including NKT cells [9] and NK cells, in primary HIV-1 infection may be critical in restoring immune responses to other pathogens and tumors, as well as in helping contain HIV viremia if patients choose to discontinue therapy or develop drug resistance.
Acknowledgments
Financial support: National Institute of Health (grants U01 AI41531, P01 AI064520, and U01 AI41531); Swedish Foundation for Strategic Research; Swedish Research Council (grant to J.M.); Karolinska Institute (grant to J.M.); Åke Wiberg Foundation (grant to J.M.). L.L.L. is an American Cancer Society Research Professor.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Dybul M, Hidalgo B, Chun TW, et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)–specific immune responses in patients treated during recently acquired HIV infection. J Infect Dis. 2002;185:61–8. doi: 10.1086/338123. [DOI] [PubMed] [Google Scholar]
- 2.Farel CE, Chaitt DG, Hahn BK, et al. Induction and maintenance therapy with intermittent interleukin-2 in HIV-1 infection. Blood. 2004;103:3282–6. doi: 10.1182/blood-2003-09-3283. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–6. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 4.Kuekrek H, Schlingmann T, Valdez H, et al. Differential effect of interleukin-2 treatment on primary and secondary immunizations in HIV infected individuals. AIDS. 2005;19:1967–74. doi: 10.1097/01.aids.0000189859.59559.9b. [DOI] [PubMed] [Google Scholar]
- 5.Levy Y, Durier C, Krzysiek R, et al. Effects of interleukin-2 therapy combined with highly active antiretroviral therapy on immune restoration in HIV-1 infection: a randomized controlled trial. AIDS. 2003;17:343–51. doi: 10.1097/00002030-200302140-00008. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Marino B, Shiboski S, Hecht FM, Kahn JO, Levy JA. Interleukin-2 therapy restores CD8 cell non-cytotoxic anti-HIV responses in primary infection subjects receiving HAART. AIDS. 2004;18:1991–9. doi: 10.1097/00002030-200410210-00003. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan V, Lempicki RA, Sereti I, et al. Increased peripheral expansion of naive CD4+ T cells in vivo after IL-2 treatment of patients with HIV infection. Proc Natl Acad Sci USA. 2002;99:10712–7. doi: 10.1073/pnas.162352399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsuyasou RT. The potential role of interleukin-2 in HIV. AIDS. 2001;15:S22–7. doi: 10.1097/00002030-200102002-00005. [DOI] [PubMed] [Google Scholar]
- 9.Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, Nixon DF. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006;107:3081–3. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aladdin H, Larsen CS, Moller BK, et al. Effects of subcutaneous interleukin-2 therapy on phenotype and function of peripheral blood mononuclear cells in human immunodeficiency virus infected patients. Scand J Immunol. 2000;51:168–75. doi: 10.1046/j.1365-3083.2000.00673.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci USA. 1996;93:10405–10. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatri VP, Fehniger TA, Baiocchi RA, et al. Ultra low dose interleukin-2 therapy promotes a type 1 cytokine profile in vivo in patients with AIDS and AIDS-associated malignancies. J Clin Invest. 1998;101:1373–8. doi: 10.1172/JCI2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CS, Ostergard L, Moller BK, Buhl MR. Subcutaneous interleukin-2 in combination with anti-retroviral therapy for treatment of HIV-1-infected subjects. Scand J Infect Dis. 2000;32:153–60. doi: 10.1080/003655400750045259. [DOI] [PubMed] [Google Scholar]
- 14.Fehniger TA, Herbein G, Yu H, et al. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol. 1998;161:6433–8. [PubMed] [Google Scholar]
- 15.Kottilil S, Chun TW, Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 16.Oliva A, Kinter AL, Vaccarezza M, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–31. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 18.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 19.Azzoni L, Rutstein RM, Chehimi J, Farabaugh MA, Nowmos A, Montaner LJ. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1–infected children. J Infect Dis. 2005;191:1451–9. doi: 10.1086/429300. [DOI] [PubMed] [Google Scholar]
- 20.Eger KA, Unutmaz D. Perturbation of natural killer cell function and receptors during HIV infection. Trends Microbiol. 2004;12:301–3. doi: 10.1016/j.tim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Alter G, Malenfant JM, Delabre RM, et al. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol. 2004;173:5305–11. doi: 10.4049/jimmunol.173.8.5305. [DOI] [PubMed] [Google Scholar]
- 22.Bruunsgaard H, Pedersen C, Skinhoj P, Pedersen BK. Clinical progression of HIV infection: role of NK cells. Scand J Immunol. 1997;46:91–5. doi: 10.1046/j.1365-3083.1997.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 23.Hu PF, Hultin LE, Hultin P, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56-cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–40. [PubMed] [Google Scholar]
- 24.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarazona R, Casado JG, Delarosa O, et al. Selective depletion of CD56dim NK cell subsets and maintenance of CD56bright NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–83. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 26.Andre P, Brunet C, Guia S, et al. Differential regulation of killer cell Ig-like receptors and CD94 lectin-like dimers on NK and T lymphocytes from HIV-1-infected individuals. Eur J Immunol. 1999;29:1076–85. doi: 10.1002/(SICI)1521-4141(199904)29:04<1076::AID-IMMU1076>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 28.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavilio D, Benjamin J, Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100:15011–6. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Maria A, Fogli M, Costa P, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 31.Kottilil S, Shin K, Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–8. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 32.Roederer M, Herzenberg LA, Herzenberg LA. Changes in antigen densities on leukocyte subsets correlate with progression of HIV disease. Int Immunol. 1996;8:1–11. doi: 10.1093/intimm/8.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Jennes W, Verheyden S, Demanet C, et al. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006;177:6588–92. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y, Martin MP, Gao X, et al. KIR/HLA pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathog. 2006;2:e79. doi: 10.1371/journal.ppat.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott-Algara D, Truong LX, Versmisse P, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–7. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein HB, Kinter AL, Jackson R, Fauci AS. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res Hum Retroviruses. 2004;20:1189–95. doi: 10.1089/aid.2004.20.1189. [DOI] [PubMed] [Google Scholar]
- 37.Baratin M, Roetynck S, Lepolard C, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci USA. 2005;102:14747–52. doi: 10.1073/pnas.0507355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004;34:2579–88. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 39.Khan IA, Thomas SY, Moretto MM, et al. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le-Barillec K, Magalhaes JG, Corcuff E, et al. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–40. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 41.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–8. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–26. doi: 10.1007/s002620050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meropol NJ, Porter M, Blumenson LE, et al. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res. 1996;2:669–77. [PubMed] [Google Scholar]
- 44.Vlasveld LT, Hekman A, Vyth-Dreese FA, et al. A phase I study of prolonged continuous infusion of low dose recombinant interleukin-2 in melanoma and renal cell cancer. II. Immunological aspects. Br J Cancer. 1993;68:559–67. doi: 10.1038/bjc.1993.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–32. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guma M, Cabrera C, Erkizia I, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1–positive patients. J Infect Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]