Abstract
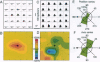
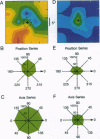
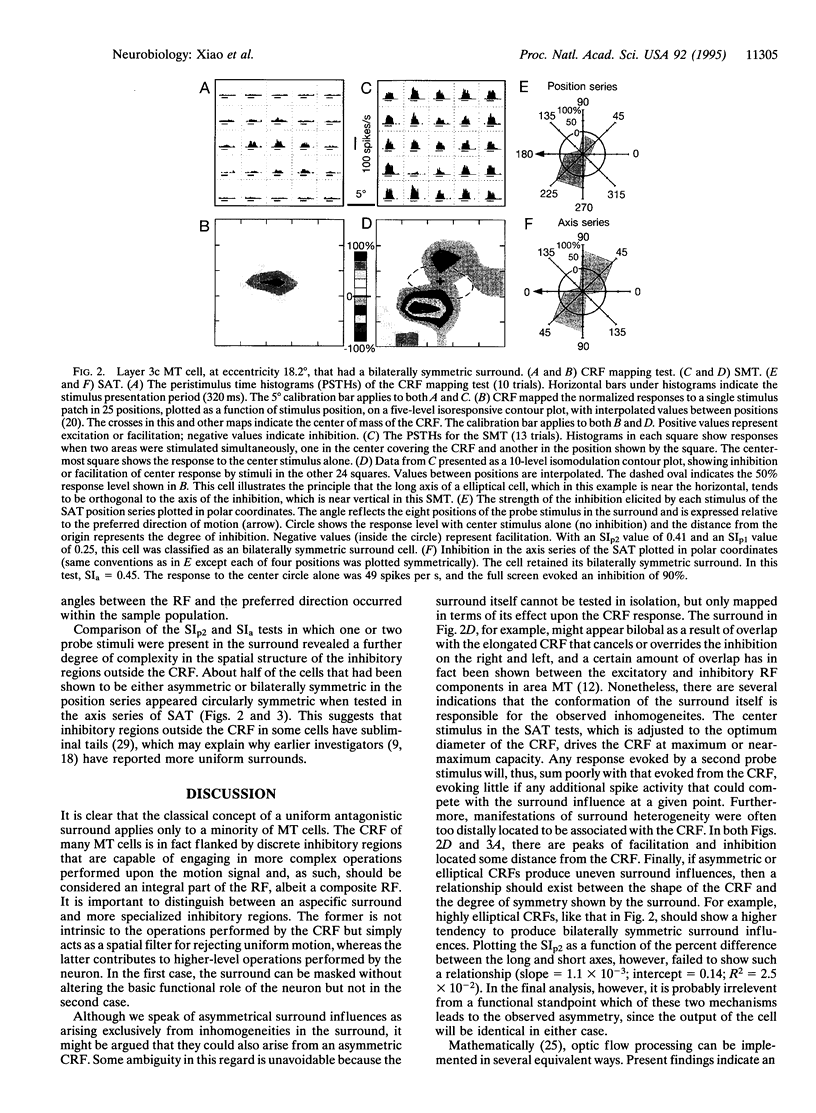
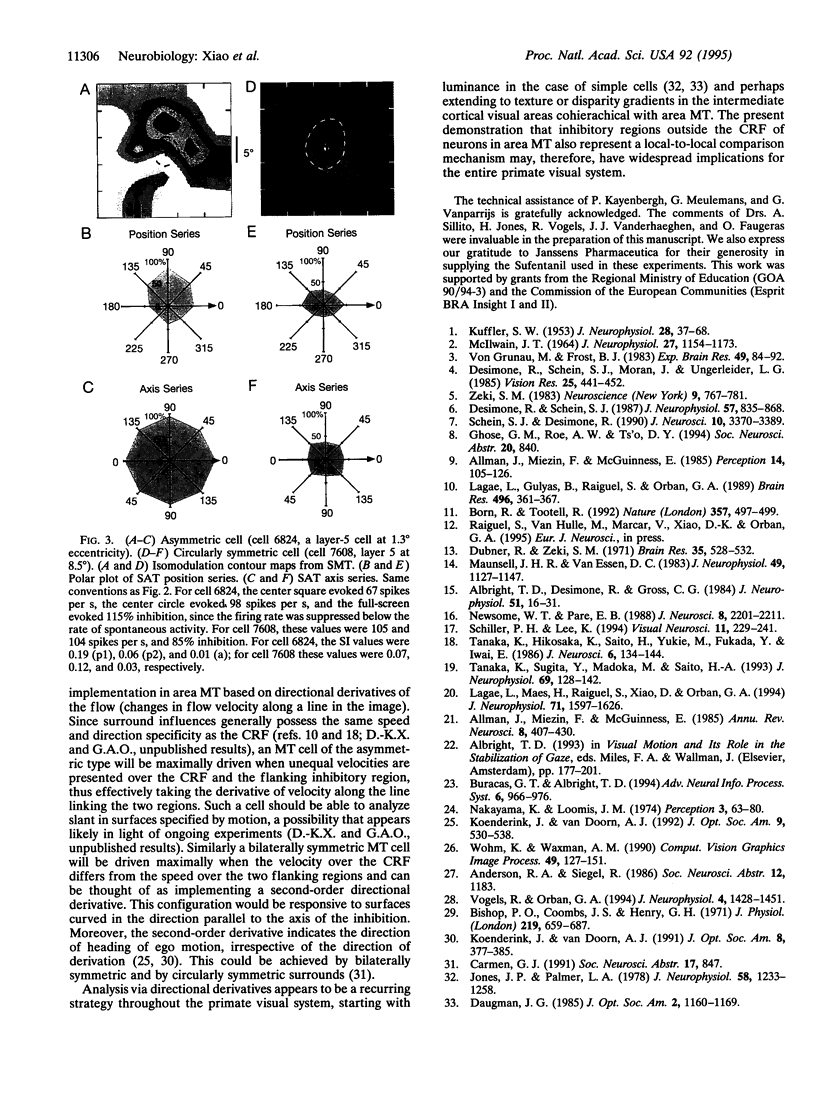
A recurrent theme in the organization of vertebrate visual cortex is that of receptive fields with an associated "silent" opponency component. In the middle temporal area (area MT), a cortical visual area involved in the analysis of retinal motion in primates, this opponency appears in the form of a region outside the classical receptive field (CRF) that in itself gives no response but suppresses responses to motion evoked within the CRF. This antagonistic motion surround has been described as very large and symmetrically arrayed around the CRF. On the basis of this view, the primary function of the surround has long been thought to consist of simple figure-ground segregation based on movement. We have made use of small stimulus patches to map the form and extent of the surround and find evidence that the surround inhibition of many MT cells is in fact confined to restricted regions on one side or on opposite sides of the CRF. Such regions endow MT cells with the ability to make local-to-local motion comparisons, capable of extracting more complex features from the visual environment, and as such, may be better viewed as intrinsic parts of the receptive field, rather than as separate entities responsible for local-to-global comparisons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright T. D., Desimone R., Gross C. G. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984 Jan;51(1):16–31. doi: 10.1152/jn.1984.51.1.16. [DOI] [PubMed] [Google Scholar]
- Allman J., Miezin F., McGuinness E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT). Perception. 1985;14(2):105–126. doi: 10.1068/p140105. [DOI] [PubMed] [Google Scholar]
- Allman J., Miezin F., McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born R. T., Tootell R. B. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992 Jun 11;357(6378):497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- Daugman J. G. Uncertainty relation for resolution in space, spatial frequency, and orientation optimized by two-dimensional visual cortical filters. J Opt Soc Am A. 1985 Jul;2(7):1160–1169. doi: 10.1364/josaa.2.001160. [DOI] [PubMed] [Google Scholar]
- Desimone R., Schein S. J., Moran J., Ungerleider L. G. Contour, color and shape analysis beyond the striate cortex. Vision Res. 1985;25(3):441–452. doi: 10.1016/0042-6989(85)90069-0. [DOI] [PubMed] [Google Scholar]
- Desimone R., Schein S. J. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J Neurophysiol. 1987 Mar;57(3):835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Dubner R., Zeki S. M. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971 Dec 24;35(2):528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- Jones J. P., Palmer L. A. An evaluation of the two-dimensional Gabor filter model of simple receptive fields in cat striate cortex. J Neurophysiol. 1987 Dec;58(6):1233–1258. doi: 10.1152/jn.1987.58.6.1233. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Koenderink J. J., van Doorn A. J. Affine structure from motion. J Opt Soc Am A. 1991 Feb;8(2):377–385. doi: 10.1364/josaa.8.000377. [DOI] [PubMed] [Google Scholar]
- Lagae L., Gulyas B., Raiguel S., Orban G. A. Laminar analysis of motion information processing in macaque V5. Brain Res. 1989 Sep 4;496(1-2):361–367. doi: 10.1016/0006-8993(89)91089-5. [DOI] [PubMed] [Google Scholar]
- Lagae L., Maes H., Raiguel S., Xiao D. K., Orban G. A. Responses of macaque STS neurons to optic flow components: a comparison of areas MT and MST. J Neurophysiol. 1994 May;71(5):1597–1626. doi: 10.1152/jn.1994.71.5.1597. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., Van Essen D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983 May;49(5):1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Loomis J. M. Optical velocity patterns, velocity-sensitive neurons, and space perception: a hypothesis. Perception. 1974;3(1):63–80. doi: 10.1068/p030063. [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Paré E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 1988 Jun;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Desimone R. Spectral properties of V4 neurons in the macaque. J Neurosci. 1990 Oct;10(10):3369–3389. doi: 10.1523/JNEUROSCI.10-10-03369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. H., Lee K. The effects of lateral geniculate nucleus, area V4, and middle temporal (MT) lesions on visually guided eye movements. Vis Neurosci. 1994 Mar-Apr;11(2):229–241. doi: 10.1017/s0952523800001590. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hikosaka K., Saito H., Yukie M., Fukada Y., Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986 Jan;6(1):134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sugita Y., Moriya M., Saito H. Analysis of object motion in the ventral part of the medial superior temporal area of the macaque visual cortex. J Neurophysiol. 1993 Jan;69(1):128–142. doi: 10.1152/jn.1993.69.1.128. [DOI] [PubMed] [Google Scholar]
- Vogels R., Orban G. A. Activity of inferior temporal neurons during orientation discrimination with successively presented gratings. J Neurophysiol. 1994 Apr;71(4):1428–1451. doi: 10.1152/jn.1994.71.4.1428. [DOI] [PubMed] [Google Scholar]
- Zeki S. Colour coding in the cerebral cortex: the responses of wavelength-selective and colour-coded cells in monkey visual cortex to changes in wavelength composition. Neuroscience. 1983 Aug;9(4):767–781. doi: 10.1016/0306-4522(83)90266-x. [DOI] [PubMed] [Google Scholar]
- von Grünau M., Frost B. J. Double-opponent-process mechanism underlying RF-structure of directionally specific cells of cat lateral suprasylvian visual area. Exp Brain Res. 1983;49(1):84–92. doi: 10.1007/BF00235544. [DOI] [PubMed] [Google Scholar]