Abstract
Objectives. We examined trends and organizational-level correlates of the availability of HCV testing in opioid treatment programs.
Methods. We used generalized ordered logit models to examine associations between organizational characteristics of 383 opioid treatment programs from the 2005 and 2011 National Drug Abuse Treatment System Survey and HCV testing availability.
Results. Between 2005 and 2011, the proportion of opioid treatment programs offering HCV testing increased but largely because of increases in off-site referrals rather than on-site testing. HCV testing availability was higher in opioid treatment programs affiliated with a hospital and those receiving federal funds. Opioid treatment programs providing both methadone and buprenorphine were more likely to offer any HCV testing, whereas opioid treatment programs providing only buprenorphine treatment were less likely to offer on-site testing. HCV testing availability was associated with more favorable staff-to-client ratios.
Conclusions. The increasing use of off-site referrals for HCV testing in opioid treatment programs likely limits opportunities for case finding, prevention, and treatment. Declines in federal funding for opioid treatment programs may be a key determinant of the availability of HCV testing in opioid treatment programs.
HCV is the most common blood-borne infection in the United States. An estimated 3.2 million people in the United States are chronically infected with HCV,1 making it 3 to 5 times more frequent than HIV.2 Results from a recent study showed that HCV has surpassed HIV as a cause of death in the United States.3 New HCV treatment regimens that are more effective and have fewer side effects have recently become available.4 Unfortunately, fewer than half of the patients living with HCV are aware of their infection.5 This is because infected persons tend to be asymptomatic: in some cases, signs of the disease do not manifest for decades.6 It is thus important to encourage and offer extensive opportunities for HCV testing, especially to the most at-risk populations.6
Advancements in testing technologies (HCV rapid testing)7 and recommendations for the identification of HCV in the general population (i.e., individuals born between 1945 and 1965) present opportunities for increasing the availability of HCV testing.8 Testing could foster increased case finding, as well as earlier linkages to HCV care and treatment services. Ensuring access to HCV testing and increasing awareness of HCV status also could help promote the adoption of preventive behaviors: for example, engaging in safer injection practices or other protective behaviors. Ultimately, this may also have a positive influence on compliance with substance abuse treatment and abstinence.9
The Centers for Disease Control and Prevention (CDC) recommends routine HCV antibody (anti-HCV) testing for injection drug users (IDUs).3,10 IDUs are particularly at risk for HCV infection as a result of sharing and reusing of needles or other injection paraphernalia.6 The estimated anti-HCV prevalence among IDUs ranges from 35% to 65%, depending on factors such as geography and rate of injection drug use.11 Unfortunately, despite the CDC recommendation, IDUs have very low rates of uptake for HCV testing and treatment.12
One factor that may account for such low testing rates is that IDUs less frequently use preventive health care services than do other population groups.13 Outpatient substance abuse treatment programs are one exception: the number of IDUs entering treatment programs has increased in recent years.14 Because injection drug use is strongly associated with opioid use (e.g., heroin), opioid treatment programs are an especially important setting for HCV testing, counseling, prevention, and links to medical care. In the case of HIV testing and case management, on-site services in substance abuse treatment programs have been associated with high-quality prevention, increased service use, earlier initiation of treatment, declines in disease transmission, improved treatment outcomes for substance use disorders, and links to ancillary services.15–17 Hence integrating HCV testing with substance abuse treatment services, particularly in opioid treatment programs, may have similar beneficial effects and is crucial for addressing the HCV epidemic in the United States.18
Unfortunately, trends in the availability of HCV testing services in opioid treatment programs across the nation are not well understood.19,20 Large gaps exist in the availability of on-site HCV testing in opioid treatment programs,21–24 with programs that have the largest proportion of IDUs among their clients being less likely to offer on-site HCV testing, even when phlebotomists were on staff.24 Opioid treatment programs often prefer to refer their clients to off-site facilities for HCV testing. Yet off-site referrals for testing and treatment of HCV are associated with significant reductions in the uptake of these services.25
There is also an urgent need to examine the organizational-level characteristics of treatment programs that may serve as facilitators or barriers to the availability of HCV testing services, either on-site or off-site, in the nation’s opioid treatment programs.26 HCV testing services may not be offered in opioid treatment programs in the United States for several potential reasons. First, opioid treatment programs may lack the required financial resources, including reimbursement and funding, to implement testing services.23,27–29 Second, treatment programs may not have the human resource capacity (e.g., low staff-to-client ratio) to effectively offer both substance abuse treatment services and ancillary services. Similarly, the ownership and affiliations of opioid treatment programs may influence the extent to which they can offer HCV testing services. For example, publicly owned opioid treatment programs might be more likely to have a prevention-driven mission, whereas hospital-affiliated opioid treatment programs may have access to networks that enable them to provide HCV testing services. Organizational-level predictors for HIV testing services in drug abuse treatment programs have been examined extensively, but similar national studies for the provision of HCV testing services are scarce.19,30–32
We first describe trends in HCV testing availability in the nation’s opioid treatment programs between 2005 and 2011. We then examine the role of organizational factors in promoting the availability of HCV testing services among opioid treatment programs.
METHODS
We defined an opioid treatment program as a physical facility with resources dedicated specifically to treating opiate dependence with methadone or buprenorphine (excluding primary care or physician offices). In 2007, the Substance Abuse and Mental Health Services Administration reported that there were 1108 licensed opioid treatment programs in the United States.33 By 2011, the number of opioid treatment programs increased to 1459, with about 304 000 opioid-dependent individuals receiving services on any given day.14 This study used 2 nationally representative surveys of opioid treatment programs. The first survey included data from opioid treatment programs collected as part of the 2005 National Drug Abuse Treatment System Survey (NDATSS), a telephone survey, with an 88% response rate.34 For the second survey (2011), we contacted opioid treatment programs that participated in 2005, and to ensure that the 2011 sample was nationally representative and had adequate statistical power, we contacted additional randomly selected opioid treatment programs from the Substance Abuse and Mental Health Services Administration’s 2011 list. Of all the 2005 and newly selected opioid treatment programs contacted in 2011, 200 completed surveys, for a response rate of 86.6%. The analysis included 187 and 196 opioid treatment programs from 2005 and 2011, respectively, for a total of 383 opioid treatment programs.
We followed established methods that maximize reliability and validity in telephone surveys.35 These methods include pretesting the survey with a random sample of programs and performing extensive computer reliability checks to signal interviewers of inconsistent or infeasible responses (e.g., percentage of clients with various demographic characteristics should sum to 100%). Interviewers then worked with respondents to resolve inconsistencies. Results were further scrutinized for reliability and validity. Reliability checks included comparisons of reported totals (e.g., total revenue) with the sum of reported detail (e.g., revenues by source); comparison of responses to related questions; comparison of responses between director and supervisor; and, for panel programs, comparison of responses over time. Results from several analyses provided support for NDATSS data reliability and validity.32,34
Study Variables
Availability of HCV testing services.
The outcome measure of interest was whether an opioid treatment program offered HCV testing (availability of HCV testing services in opioid treatment programs). We asked opioid treatment program directors whether their staff routinely provide on-site or off-site HCV testing services to clients. We used responses to these 2 questions (yes or no) to categorize programs as providing on-site HCV testing (i.e., they responded “yes” to providing on-site HCV testing), off-site HCV testing (i.e., they responded “no” to the question about on-site testing but responded “yes” to the off-site testing question), or no testing (i.e., they did not respond “yes” to either question).
Independent variables.
An opioid treatment program manager reported sociodemographic characteristics of clients, including the proportion of clients from the most recent complete fiscal year who were African American, non-Hispanic White, or Hispanic. The manager also reported the percentage of clients in the most recent complete fiscal year whose use of drugs involved injection with needles. We used this information to create a categorical variable describing the prevalence of injection drug use among opioid treatment program clients (< 25%, 25%–74%, or ≥ 75%). We created 2 dummy variables identifying opioid treatment programs with fewer than 10% Hispanic or African American clients.
We examined several variables describing resource availability in opioid treatment programs. This included an assessment of human resources available to conduct or support testing. We combined 2 data components: (1) the number of full-time-equivalent staff (i.e., individuals employed by the program who worked 35 hours per week or more in the most recent complete fiscal year) and (2) the number of clients served by the program during the same period. We created a staff-to-client ratio by dividing the number of full-time-equivalent staff by the number of clients. We also measured sources of revenue for each opioid treatment program by using (1) the percentage of total revenue received by the unit directly from the federal government, including Medicare and block grants but excluding courts and prisons; and (2) the percentage of total revenue received by the unit from private insurance companies, including health maintenance organizations and preferred provider organizations. We created a dummy variable for each measure (0–1) set to 1 if the program received at least some of its revenue from either source.
Measures of opioid treatment program characteristics also included program ownership (private for-profit, private not-for-profit, or public) and affiliation. Program directors were asked if their unit had an affiliation with a hospital. We used a dummy variable coded as 1 for “yes” and 0 otherwise. We used data provided by opioid treatment program directors to measure whether the treatment program currently held accreditation from CARF International (formerly known as the Commission on the Accreditation of Rehabilitation Facilities). Finally, we examined the influence of pharmacological method of treatment. We included an indicator for whether the treatment program provided methadone treatment only, buprenorphine prescription only, or both methadone and buprenorphine prescription.
Analysis
The current analysis included 187 opioid treatment programs in 2005 and 196 in 2011. We first measured changes in organizational characteristics of opioid treatment programs between 2005 and 2011. We used the Pearson χ2 test for categorical variables and the t test for continuous variables to test for differences in the organizational characteristics of opioid treatment programs between 2005 and 2011.
We then estimated ordered logit models of the availability of HCV testing services in opioid treatment programs. Specifically, the dependent variable was coded as follows: 1 = no testing services, 2 = off-site testing, 3 = on-site testing. Ordered logit models estimate 1 equation for each pair of levels of the dependent variable with the assumption that the effect of each covariate is the same across all categories of the dependent variable.36,37 This means, for example, that the odds ratio (OR) associated with hospital affiliation is the same when we compare opioid treatment programs with no testing services with opioid treatment programs with off-site testing as when we compare opioid treatment programs with no testing with opioid treatment programs with on-site testing. This is known as the proportional odds assumption.
In standard ordered logit models, the proportional odds assumption is strong, because it must be met for all coefficients in the model. We thus used a less restrictive extension of the ordered logit models, known as generalized ordered logit models.38 In generalized ordered logit models, we make only a “partial” proportional odds assumption: specific coefficients are allowed to vary across categories of the dependent variable, whereas other coefficients are constrained to be equal. To decide which coefficients to constrain, we conducted Wald tests of the proportional odds assumption for each covariate defined earlier. We estimated these models separately by year to investigate possible changes in patterns of coefficients over time. For each model, we calculated robust SEs. All analyses were performed with Stata version 12 (StataCorp LP, College Station, TX), including the GOLOGIT2 add-on.37
RESULTS
Table 1 describes changes in the characteristics of opioid treatment programs between 2005 and 2011 and shows notable differences. The proportion of opioid treatment programs receiving federal funding declined significantly: in 2005, 37.9% of opioid treatment programs received at least some of their revenue from federal government funding versus only 21.4% of opioid treatment programs in 2011 (P < .001). In 2005, the average staff-to-client ratio in opioid treatment programs was 4 staff members per 100 clients versus 5 staff members per 100 clients in 2011. Finally, method of treatment changed over time, with several opioid treatment programs diversifying their treatment approach. The proportion of opioid treatment programs that used buprenorphine as the only method of treatment increased from 20.3% in 2005 to 29.0% in 2011. On the other hand, methadone-only treatment programs declined from 66.8% in 2005 to 46.9% in 2011.
TABLE 1—
Characteristics of Opioid Treatment Programs in the United States: National Drug Abuse Treatment System Survey, 2005 and 2011
| Characteristic | 2005 (n = 187) | 2011 (n = 196) | P |
| Prevalence of injection drug users, % | .279 | ||
| < 25 | 33.6 | 36.7 | |
| 25–74 | 40.6 | 44.3 | |
| ≥ 75 | 25.6 | 18.8 | |
| African American clients, % | .101 | ||
| < 10 | 40.6 | 49.0 | |
| ≥ 10 | 59.4 | 51.0 | |
| Hispanic clients, % | .337 | ||
| < 10 | 48.7 | 53.6 | |
| ≥ 10 | 51.3 | 46.3 | |
| Revenue from federal government | < .001 | ||
| None | 62.0 | 78.5 | |
| ≥ 1% | 37.9 | 21.4 | |
| Revenue from private insurance | .526 | ||
| None | 58.8 | 55.6 | |
| ≥ 1% | 41.1 | 44.3 | |
| Staff-to-client ratio, mean (SD) | 0.04 (0.04) | 0.05 (0.03) | .014 |
| CARF accreditation | .199 | ||
| No | 54.0 | 47.4 | |
| Yes | 45.9 | 52.5 | |
| Ownership | .15 | ||
| Private non-for-profit | 45.9 | 50.0 | |
| Private for-profit | 36.3 | 39.2 | |
| Public | 17.6 | 10.7 | |
| Hospital affiliation | .105 | ||
| No | 81.2 | 87.7 | |
| Yes | 18.1 | 12.2 | |
| Method of treatment | < .001 | ||
| Methadone only | 66.8 | 46.9 | |
| Buprenorphine only | 20.3 | 29.0 | |
| Both methadone + buprenorphine | 12.8 | 23.9 |
Note. CARF = CARF International (formerly known as the Commission on the Accreditation of Rehabilitation Facilities). Percentages may not equal 100 because of rounding.
HCV Testing in Opioid Treatment Programs in 2005 and 2011
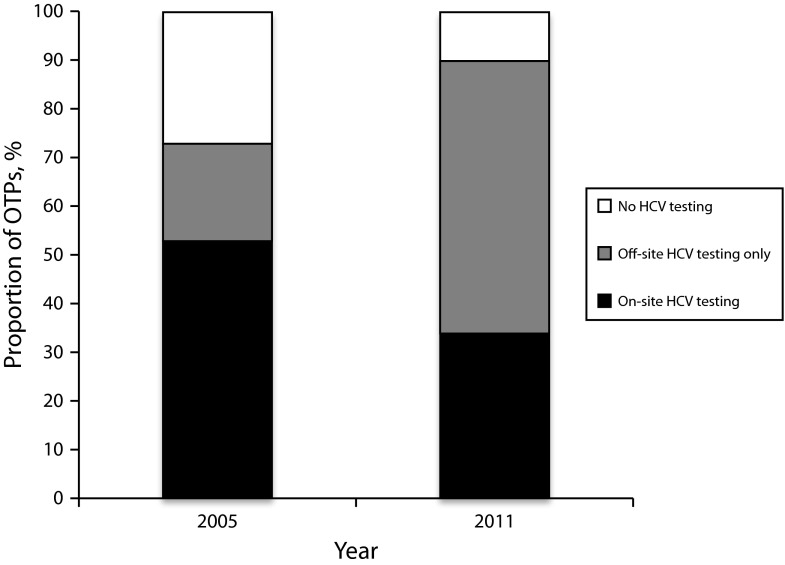
Figure 1 illustrates changes in the availability of HCV testing services in opioid treatment programs. The proportion of opioid treatment programs that reported that they did not provide any HCV testing services to their clients (i.e., no on-site or off-site testing) declined from 27% in 2005 to 10% in 2011. Concomitantly, however, the proportion of opioid treatment programs offering on-site HCV testing also declined from 53% in 2005 to only 34% in 2011. Instead, most opioid treatment programs in 2011 offered only off-site HCV testing options for their clients: 20% of opioid treatment programs had only off-site options in 2005 compared with 56% of opioid treatment programs in 2011.
FIGURE 1—
Trends in the availability of HCV testing services in opioid treatment programs (OTPs) in the United States: National Drug Abuse Treatment System Survey, 2005 and 2011.
Note. Differences in testing availability between categories of availability in 2005 and 2011 were significant at P < .001, with a χ2 test of association.
Multivariate Analysis
Table 2 shows the predictors of HCV testing availability in opioid treatment programs in 2005 and 2011, respectively. After we adjusted for other covariates in the model, the likelihood that an opioid treatment program offered any category of HCV testing was associated with several organizational characteristics.
TABLE 2—
Organizational Correlates of Availability of HCV Testing Services in Opioid Treatment Programs in the United States: National Drug Abuse Treatment System Survey, 2005 and 2011
| 2005 (n = 187) | 2011 (n = 196) | |
| Characteristic | Generalized Ordered Logit Models,a OR (95% CI) | Generalized Ordered Logit Models,a OR (95% CI) |
| Prevalence of injection drug users, % | ||
| < 25 (Ref) | 1.00 | 1.00 |
| 25–74 | 1.18 (0.51, 2.72) | 1.72 (0.79, 3.76) |
| ≥ 75 | 0.75 (0.29, 1.97) | 0.84 (0.32, 2.19) |
| African American clients, % | ||
| < 10 (Ref) | 1.00 | 1.00 |
| ≥ 10 | 0.97 (0.51, 1.85) | |
| ≥ 10 (no vs off-site and on-site testing)b | 0.30* (0.11, 0.80) | |
| ≥ 10 (no and off-site vs on-site testing)b | 1.11 (0.54, 2.29) | |
| Hispanic clients, % | ||
| < 10 (Ref) | 1.00 | 1.00 |
| ≥ 10 | 1.50 (0.82, 2.72) | 1.01 (0.53, 1.92) |
| Revenue from federal government | ||
| None (Ref) | 1.00 | 1.00 |
| ≥ 1% | 2.03* (1.01, 4.07) | 1.88 (0.91, 3.89) |
| Revenue from private insurance | ||
| None (Ref) | 1.00 | 1.00 |
| ≥ 1% | 0.58* (0.30, 1.13) | 1.01 (0.48, 2.10) |
| Staff-to-client ratio | ||
| Log | 1.69* (1.16, 2.46) | 1.50 (0.86, 2.61) |
| CARF accreditation | ||
| No (Ref) | 1.00 | 1.00 |
| Yes | 1.78 (0.85, 3.75) | 1.54 (0.75, 3.16) |
| Ownership | ||
| Private non-for-profit (Ref) | 1.00 | 1.00 |
| Private for-profit | 1.84 (0.85, 3.98) | 1.16 (0.53, 2.56) |
| Public | 1.40 (0.50, 3.92) | |
| Public (no vs off-site and on-site testing)b | 0.51 (0.15, 1.78) | |
| Public (no and off-site vs on-site testing)b | 2.33 (0.78, 6.99) | |
| Hospital affiliation | ||
| No (Ref) | 1.00 | 1.00 |
| Yes (no vs off-site and on-site testing)b | 1.46 (0.42, 5.13) | . . .c |
| Yes (no and off-site vs on-site testing)b | 7.71** (2.89, 20.53) | 3.92* (1.35, 11.38) |
| Method of treatment | ||
| Methadone only (Ref) | 1.00 | 1.00 |
| Buprenorphine only | 0.48 (0.19, 1.19) | |
| Buprenorphine only (no vs off-site and on-site testing)b | 1.94 (0.50, 7.45) | |
| Buprenorphine only (no and off-site vs on-site testing)b | 0.32* (0.11, 0.90) | |
| Both methadone + buprenorphine | 2.65 (0.85, 8.27) | 2.62* (1.26, 5.45) |
Note. CARF = CARF International (formerly known as the Commission on the Accreditation of Rehabilitation Facilities); CI = confidence interval; OR = odds ratio. The 95% CIs were estimated with robust SEs.
Dependent variable for generalized ordered logit model is coded so that higher values are equal to favorable assessment of type of HCV testing. Therefore, 1 = no HCV testing, 2 = off-site only HCV testing, 3 = on-site HCV testing.
For variables that violate the proportional odds assumption, we present 2 odds ratios: in the first, ORs are drawn from the comparison of no HCV testing (reference) vs off-site and on-site testing combined; in the second, the ORs are drawn from the comparison of no HCV testing and off-site testing (reference) vs on-site HCV testing.
The proportional odds assumption was violated, but there were too few cases to estimate this coefficient separately.
*P < .05; **P < .001.
In 2005 (Table 2), results from generalized ordered logit models showed that the availability of HCV testing services in opioid treatment programs was associated with funding from the federal government and staff-to-client ratio. Opioid treatment programs that received at least some of their revenue from federal grants were significantly more likely to offer HCV testing services to their clients than were other opioid treatment programs (OR = 2.03; 95% confidence interval [CI] = 1.01, 4.07), as were opioid treatment programs with higher staff-to-client ratios (OR = 1.69; 95% CI = 1.16, 2.46). The proportional odds assumption was rejected for only 2 variables. Opioid treatment programs that served a high proportion of African American clients were significantly less likely to offer any HCV testing, compared with no testing (OR = 0.30; 95% CI = 0.11, 0.80). Hospital affiliation was associated with significantly higher availability of on-site HCV testing (OR = 7.71; 95% CI = 2.89, 20.53).
In 2011 (Table 2), the availability of HCV testing services remained associated with receipt of funding from the federal government, although the strength of this association declined slightly (OR = 1.88; 95% CI = 0.91, 3.89). On the other hand, the availability of HCV testing was no longer significantly associated with staff-to-client ratios (OR = 1.50; 95% CI = 0.86, 2.61). The proportional odds assumption was rejected for several variables, including ownership and hospital affiliation. In particular, although there were too few cases to estimate the association of hospital affiliation with the likelihood of offering only off-site testing services, hospital affiliation was significantly associated with on-site testing (OR = 3.92; 95% CI = 1.35, 11.38).
The method of opioid dependence treatment was also associated with the availability of HCV testing services. Opioid treatment programs that prescribed methadone and buprenorphine were more likely to offer HCV testing options to their clients (OR = 2.62; 95% CI = 1.26, 5.45). However, opioid treatment programs that offered only buprenorphine treatment were significantly less likely to offer on-site HCV testing services (but not off-site testing) than were other opioid treatment programs (OR = 0.32; 95% CI = 0.11, 0.90).
DISCUSSION
We examined trends in the availability of HCV testing services and their organizational determinants in the nation’s opioid treatment programs. An increasing number of opioid treatment programs offered HCV testing services to their clients in 2011 than in 2005, but fewer opioid treatment programs offered on-site HCV testing services. Instead, they increasingly offered only referrals for HCV testing at off-site facilities. In 2005, 53% of the nation’s opioid treatment programs offered on-site HCV testing. Alarmingly, only 34% of opioid treatment programs offered on-site HCV testing to clients in 2011. Because off-site referrals are associated with lower uptake of testing and treatment services, this trend constitutes a significant threat to HCV control in the United States.
We found that structural changes in several organizational characteristics of opioid treatment programs may explain the trend toward less on-site HCV testing observed between 2005 and 2011. In particular, HCV testing availability was consistently associated with receipt of federal funding: federally funded opioid treatment programs were more likely to offer on-site testing to their clients than were other opioid treatment programs, both in 2005 and in 2011. At the same time, the proportion of federally funded opioid treatment programs among all opioid treatment programs declined sharply between 2005 and 2011. The declining availability of federal funding thus likely played a key role in explaining the observed adverse trends in on-site HCV testing availability. The importance of funding for increasing on-site availability of services was already noted in a review of the role of organization and management factors in substance abuse treatment.26 Reversing the trend in the availability of on-site HCV testing services in opioid treatment programs thus may require new funding for opioid treatment programs to facilitate the integration and sustainability of HCV testing and treatment services.
Written policies or guidelines for HCV testing services as an important factor in increasing access to funds also may be required. A study reported that substance abuse treatment programs do not adequately access available funds, particularly state funds.29 Although opioid treatment programs need better program funding and adequate material resources to increase on-site testing for HCV, mechanisms to facilitate and enhance treatment programs’ capacity to access available funds are also needed. The absence of coordination and underfunding of public health services have been associated with the health system being inadequately positioned to improve awareness, prevention, diagnosis, and treatment of HCV.39
Our results also show that affiliation with a hospital was associated with increased likelihood of on-site HCV testing. This finding is supported by previous studies that examined the effects of hospital affiliation on service delivery and quality of care in substance abuse treatment programs. For example, treatment units affiliated with hospitals have been shown to provide more physical examinations and routine primary care.40 Another study showed that hospital affiliation is associated with a greater likelihood of treatment availability and the use of medications for treating substance abuse.41 Our finding thus suggests that addressing the HCV epidemic among at-risk populations will likely require the development of collaboration between substance abuse treatment programs and testing and care sites, including hospitals and other settings.
Establishing links with a broader range of sites is particularly important because there has been a decline in the proportion of opioid treatment programs affiliated with hospitals between 2005 and 2011 (Table 1). Additionally, considering the overall increase in HCV testing services over time (driven mostly by increases in off-site relative to on-site testing) and evidence suggesting that referrals do not necessarily translate into uptake of services, future studies should test approaches to effectively establish links to off-site facilities that can increase uptake of testing and access to treatment.
Opioid treatment programs that use buprenorphine as the only approach to treatment were less likely to offer on-site HCV testing services relative to no testing. Other studies have compared hepatitis services offered at methadone maintenance treatment programs with other program types and showed that drug treatment programs were 4.5 times more likely to provide HCV services if they dispensed methadone.42 Another study found that approximately 66% of methadone programs versus only 33% of drug-free programs offered HCV screening to all patients.43 The types of clients served by buprenorphine programs may explain this variation in HCV testing availability by method of opioid dependence treatment. For example, buprenorphine-only programs may have fewer clients who inject drugs or a mix of clients considered to be less at risk for contracting HCV. This may influence the mission or focus of these programs and influence their likelihood to offer preventive services.44
Other aspects of treatment programs, particularly characteristics of providers, may offer some insight into this phenomenon. Several studies showed that deficits in providers’ knowledge of HCV infection prevalence, available tests, testing procedures, and treatment of HCV may be a barrier to the availability of HCV testing services in substance abuse treatment.45–47 Further study of these dynamics in opioid treatment programs, especially buprenorphine-only programs, is critical: the proportion of buprenorphine-only programs in the nation has increased significantly since 2005.48 Although buprenorphine is an effective approach to addiction treatment,49 it may have unanticipated and adverse effects on the delivery of more comprehensive health services, especially for individuals at high risk for contracting and transmitting HCV, that must be investigated. Finally, we found that staff-to-client ratio was associated with an increased likelihood of the availability of HCV testing (although not significantly in 2011). The availability of human resources may support new practices. Particularly, increases in staff may motivate greater focus on activities and practices related to ancillary services, including HCV testing.
Study Limitations
This study had several limitations. First, the NDATSS focused on organizational-level measures and had limited questions about clients of opioid treatment programs. We thus could not measure uptake of HCV testing among clients of opioid treatment programs that offered HCV testing. We also could not identify the set of opioid treatment programs where it would be particularly urgent to develop on-site HCV testing opportunities (e.g., because clients had limited HCV testing history or because they were more likely to be infected with HCV, thus suggesting a high yield of HCV testing). Our data also did not provide a comprehensive assessment of the availability of other HCV-related services in opioid treatment programs, including counseling, care, and treatment services, nor did they assess opportunities for links to care after HCV testing among opioid treatment program clients.
Second, the NDATSS data did not permit assessing whether the relation between key covariates and HCV testing availability was causal. For example, the observed association between HCV testing and receipt of federal funds could be a result of reverse causality: opioid treatment programs that offer HCV testing on-site may be more likely to receive federal funds because managers of these opioid treatment programs have more experience with applications for federal funding. The causal effects of federal funding (and other covariates) cannot be isolated because only a limited number of opioid treatment programs are followed longitudinally. Measuring the causal effects of these key organizational characteristics will require panel data in conjunction with sophisticated econometric techniques. Finally, the sample size available in each wave of the NDATSS may occasionally be limited. It was thus not possible to investigate possible interactions between organizational factors or between client characteristics and organizational factors. Despite these limitations, the study addressed an important health services delivery problem with considerable implications for substance-abusing patients and population health. Results from this study suggest that investments, treatment practices, and policies are needed to facilitate organizational change and promote offering of on-site HCV testing services in opioid treatment programs.
Conclusions
In an era of advancements in testing technologies (introduction of rapid HCV antibody testing) and the advent of more effective treatments for HCV, early diagnosis, facilitated by increased availability of on-site HCV testing in the nation’s opioid treatment programs, is critical. This is particularly important because providing health services on-site is more effective in reaching patients compared with providing referrals to an outside site.16 Interventions that address organizational-level barriers (i.e., availability of funding and the capacity of treatment programs to effectively access the available funds) are essential to increasing the availability of on-site testing. Recent efforts to increase awareness of HCV infection status and address the HCV burden in the nation (i.e., the recent release of recommendations for the identification of HCV among individuals born during 1945–1965) also may present opportunities (increased funding, political will) for increasing the availability of HCV testing in opioid treatment programs. Notably, initiatives that promote the adoption of rapid HCV testing in opioid treatment programs also may have an important role in accelerating the ability of opioid treatment programs to offer on-site HCV testing. Given the high prevalence of HCV infection among persons with drug use disorders, particularly IDUs, and increasing mortality from HCV, the importance of on-site HCV testing services, early detection, and treatment, as well as reductions in transmission, cannot be overstated. Future research should examine barriers to the adoption of HCV testing services, with a focus on strategies for enabling the implementation and sustainability of rapid HCV testing. These efforts also must consider organizational and patient-level factors that may influence uptake of testing services.
Acknowledgments
This publication uses data from the National Drug Abuse Treatment System Survey, funded by the National Institute on Drug Abuse (R01DA030459). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant KL2 TR000081), formerly the National Center for Research Resources (grant KL2 RR024157).
Thanks to Stéphane Helleringer for his feedback on drafts of the article.
Note. The content is solely the responsibility of the authors and does not represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Human Participant Protection
The institutional review board at Columbia University Medical Center approved all aspects of this study.
References
- 1.Centers for Disease Control and Prevention. The ABCs of Hepatitis. Atlanta, GA: Centers for Disease Control and Prevention; August 2012. Available at: http://www.cdc.gov/hepatitis/Resources/Professionals/PDFs/ABCTable.pdf. Accessed October 28, 2012. [Google Scholar]
- 2.Institute of Medicine (IOM) Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Torresi J, Johnson D, Wedemeyer H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J Hepatol. 2011;54(6):1273–1285. doi: 10.1016/j.jhep.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Drobeniuc J, Jewett A et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204(6):825–831. doi: 10.1093/infdis/jir422. [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Morgan RL, Beckett GA et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 9.Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103(1):e44–e52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 11.Amon JJ, Garfein RS, Ahdieh-Grant L et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46(12):1852–1858. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SH, Genberg BL, Astemborski J et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drobnik A, Judd C, Banach D, Egger J, Konty K, Rude E. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101(11):2151–2155. doi: 10.2105/AJPH.2011.300251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The TEDS Report: Injection Drug Abuse Admissions to Substance Abuse Treatment: 1992 and 2009. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; December 1, 2011. [Google Scholar]
- 15.Rothman J, Rudnick D, Slifer M, Agins B, Heiner K, Birkhead G. Co-located substance use treatment and HIV prevention and primary care services, New York State, 1990-2002: a model for effective service delivery to a high-risk population. J Urban Health. 2007;84(2):226–242. doi: 10.1007/s11524-006-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umbricht-Schneiter A, Ginn DH, Pabst KM, Bigelow GE. Providing medical care to methadone clinic patients: referral vs on-site care. Am J Public Health. 1994;84(2):207–210. doi: 10.2105/ajph.84.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsch LR, Feaster DJ, Gooden L et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160–1167. doi: 10.2105/AJPH.2011.300460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassilev ZP, Strauss SM, Astone JM, Friedmann PD, Des Jarlais DC. Provision of on-site medical care to patients with hepatitis C in drug treatment units. J Health Care Poor Underserved. 2004;15(4):663–671. doi: 10.1353/hpu.2004.0075. [DOI] [PubMed] [Google Scholar]
- 19.Bini EJ, Kritz S, Brown LS, Jr et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42(4):438–445. doi: 10.1016/j.jsat.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LS, Jr, Kritz SA, Goldsmith RJ et al. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2006;30(4):315–321. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin AH, Soloway I, Gourevitch MN. Integrating services for injection drug users infected with hepatitis C virus with methadone maintenance treatment: challenges and opportunities. Clin Infect Dis. 2005;40(suppl 5):S339–S345. doi: 10.1086/427450. [DOI] [PubMed] [Google Scholar]
- 22.Grebely J, Genoway KA, Raffa JD et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1-2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Bini EJ, Kritz S, Brown LS, Jr, Robinson J, Alderson D, Rotrosen J. Barriers to providing health services for HIV/AIDS, hepatitis C virus infection and sexually transmitted infections in substance abuse treatment programs in the United States. J Addict Dis. 2011;30(2):98–109. doi: 10.1080/10550887.2011.554780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frimpong JA. Missed opportunities for hepatitis C testing in opioid treatment programs. Am J Public Health. 2013;103(6):1028–1030. doi: 10.2105/AJPH.2012.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37(3):1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- 26.D’Aunno T. The role of organization and management in substance abuse treatment: review and roadmap. J Subst Abuse Treat. 2006;31(3):221–233. doi: 10.1016/j.jsat.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Brown LS, Jr, Kritz S, Goldsmith RJ et al. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Rep. 2007;122(4):441–451. doi: 10.1177/003335490712200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Substance Abuse and Mental Health Services Administration. Addressing Viral Hepatitis in People With Substance Use Disorders. Treatment Improvement Protocol (TIP) Series 53. HHS publication (SMA) 11–4656. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. [PubMed] [Google Scholar]
- 29.Kritz S, Brown LS, Jr, Goldsmith RJ et al. States and substance abuse treatment programs: funding and guidelines for infection-related services. Am J Public Health. 2008;98(5):824–826. doi: 10.2105/AJPH.2007.119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss SM, Astone J, Vassilev ZP, Des Jarlais DC, Hagan H. Gaps in the drug-free and methadone treatment program response to hepatitis C. J Subst Abuse Treat. 2003;24(4):291–297. doi: 10.1016/s0740-5472(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 31.Abraham AJ, O’Brien LA, Bride BE, Roman PM. HIV/AIDS services in private substance abuse treatment programs. Drug Alcohol Depend. 2011;115(1-2):16–22. doi: 10.1016/j.drugalcdep.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Aunno T, Vaughn TE, McElroy P. An institutional analysis of HIV prevention efforts by the nation’s outpatient drug abuse treatment units. J Health Soc Behav. 1999;40(2):175–192. [PubMed] [Google Scholar]
- 33.National Survey of Substance Abuse Treatment Services (N-SSATS): 2009. Data on Substance Abuse Treatment Facilities. DASIS Series: S-54, HHS publication (SMA) 10–4579. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2010. [Google Scholar]
- 34.Pollack HA, D’Aunno T. HIV testing and counseling in the nation’s outpatient substance abuse treatment system, 1995–2005. J Subst Abuse Treat. 2010;38(4):307–316. doi: 10.1016/j.jsat.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Adams T, Heeringa S. Outpatient Substance Abuse Treatment System Survey: Technical Documentation for OSATSS-5, 1999-2000. Ann Arbor, MI: Institute for Social Research; 2001. [Google Scholar]
- 36.Long JS, Freese J. Regression Models for Categorical Dependent Variables Using Stata. 2nd ed. College Station, TX: Stata Press; 2006. [Google Scholar]
- 37.Willimas R. Generalized ordered logit/partial proportional-odds models for ordinal dependent variables. Stata J. 2006;6:58–82. [Google Scholar]
- 38.Agresti A. Categorical Data Analysis. New York, NY: Wiley; 1990. [Google Scholar]
- 39.Clark PJ, Muir AJ. Overcoming barriers to care for hepatitis C. N Engl J Med. 2012;366(26):2436–2438. doi: 10.1056/NEJMp1202608. [DOI] [PubMed] [Google Scholar]
- 40.Friedmann PD, Lemon SC, Durkin EM, D’Aunno TA. Trends in comprehensive service availability in outpatient drug abuse treatment. J Subst Abuse Treat. 2003;24(1):81–88. doi: 10.1016/s0740-5472(02)00323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knudsen HK, Ducharme LJ, Roman PM. The adoption of medications in substance abuse treatment: associations with organizational characteristics and technology clusters. Drug Alcohol Depend. 2007;87(2–3):164–174. doi: 10.1016/j.drugalcdep.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Astone J, Strauss SM, Vassilev ZP. Des Jarlais DC. Provision of hepatitis C education in a nationwide sample of drug treatment programs. J Drug Educ. 2003;33(1):107–117. doi: 10.2190/YEGL-GX4W-HGRA-EDC7. [DOI] [PubMed] [Google Scholar]
- 43.Strauss SM, Astone JM, Jarlais DD, Hagan H. A comparison of HCV antibody testing in drug-free and methadone maintenance treatment programs in the United States. Drug Alcohol Depend. 2004;73(3):227–236. doi: 10.1016/j.drugalcdep.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Wells R, Lemak CH, D’Aunno TA. Organizational survival in the outpatient substance abuse treatment sector, 1988-2000. Med Care Res Rev. 2005;62(6):697–719. doi: 10.1177/1077558705281062. [DOI] [PubMed] [Google Scholar]
- 45.Strauss SM, Munoz-Plaza C, Tiburcio NJ et al. Barriers and facilitators to undergoing hepatitis C virus (HCV) testing through drug treatment programs. J Drug Issues. 2008;38(4):25. [Google Scholar]
- 46.Birkhead GS, Klein SJ, Candelas AR et al. Integrating multiple programme and policy approaches to hepatitis C prevention and care for injection drug users: a comprehensive approach. Int J Drug Policy. 2007;18(5):417–425. doi: 10.1016/j.drugpo.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Strauss SM, Astone-Twerell JM, Munoz-Plaza C et al. Hepatitis C knowledge among staff in U.S. drug treatment programs. J Drug Educ. 2006;36(2):141–158. doi: 10.2190/3EMQ-N350-W4XN-WT1X. [DOI] [PubMed] [Google Scholar]
- 48.Knudsen HK, Abraham AJ, Johnson JA, Roman PM. Buprenorphine adoption in the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2009;37(3):307–312. doi: 10.1016/j.jsat.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]