Abstract
The Affordable Care Act (ACA) mandates that both Medicaid and insurance plans cover life-saving preventive services recommended by the US Preventive Services Task Force, including colorectal cancer (CRC) screening and choice between colonoscopy, flexible sigmoidoscopy, and fecal occult blood testing (FOBT).
People who choose FOBT or sigmoidoscopy as their initial test could face high, unexpected, out-of-pocket costs because the mandate does not cover needed follow-up colonoscopies after positive tests. Some people will have no coverage for any CRC screening because of lack of state participation in the ACA or because they do not qualify (e.g., immigrant workers).
Existing disparities in CRC screening and mortality will worsen if policies are not corrected to fully cover both initial and follow-up testing.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States,1 but many of these deaths could be averted by screening, which decreases both CRC incidence and mortality by 30% to 60%.2 The US Preventive Services Task Force strongly recommends CRC screening for adults aged 50 to 75 years by 3 evidence-based methods: annual fecal occult blood testing (FOBT) with either high-sensitivity guaiac or fecal immunochemical tests, flexible sigmoidoscopy every 5 years with interval FOBT, or colonoscopy every 10 years.3 In large randomized trials, FOBT and sigmoidoscopy reduced CRC incidence and mortality in 2-part screening programs in which initial positive FOBT or sigmoidoscopy was followed by a colonoscopy. Colonoscopy as an initial screening test is supported by observational studies.2 CRC screening by any of the recommended options is cost-effective,4,5 and potentially cost saving, because it reduces the number of patients needing advanced CRC treatment.6 However, to reduce CRC morbidity, mortality, and associated costs, screening must be increased beyond its current rates.
SUBOPTIMAL SCREENING RATES AND HEALTH DISPARITIES
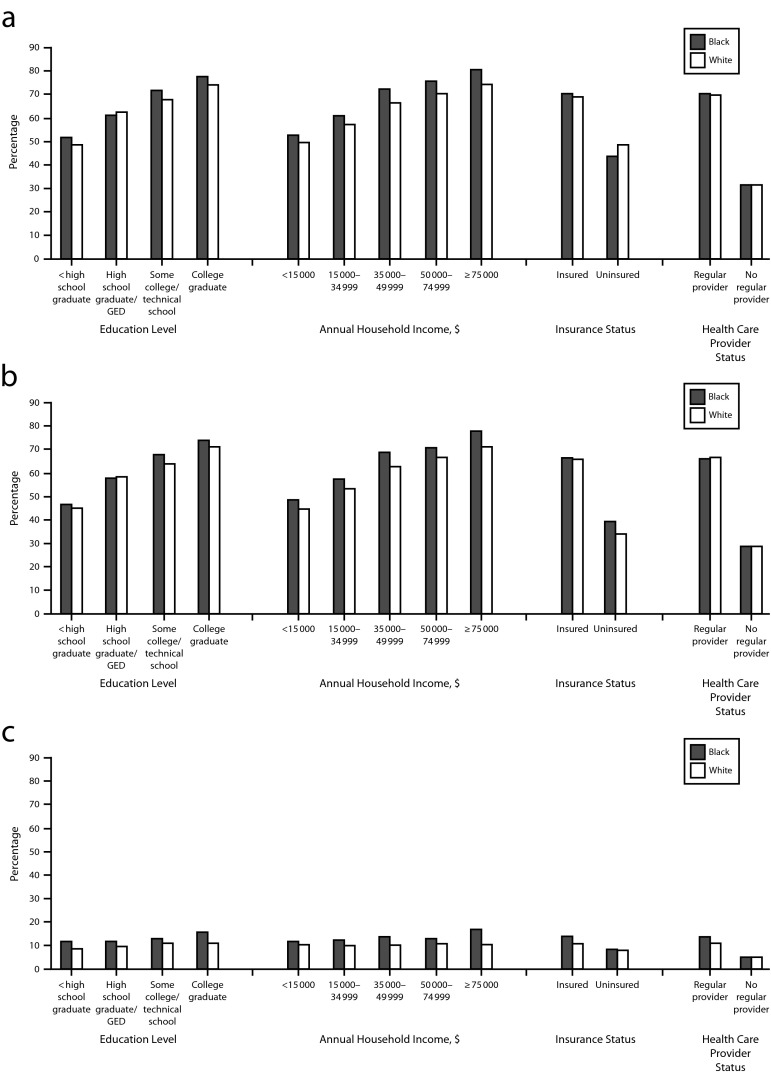
Although CRC screening rates have risen in recent years, with 65% of Americans aged 50 to 75 years reporting being current for CRC screening,7 these rates remain lower than screening rates for breast (72%) and cervical (83%) cancers. More concerning are the substantially lower CRC screening rates for certain racial and ethnic subpopulations, people living in poverty, and the uninsured. For example, only 53% of Hispanics and 37% of individuals without health insurance are up-to-date for CRC screening (Figure 1).7 Lower rates of screening directly contribute to disparities in CRC morbidity and mortality.8,9
FIGURE 1—
Percentage of respondents aged 50–75 years, by test type and selected characteristics, who reported (a) being up-to-date with colorectal cancer screening, (b) having a colonoscopy within 10 years, and (c) having FOBT within 1 year: Behavioral Risk Factor Surveillance System, United States, 2012.
Note. FOBT = fecal occult blood testing; GED = graduate equivalency diploma. Data were weighted to the age, gender, and racial/ethnic distribution of each state’s adult population with intercensal estimates and were age standardized to the 2012 Behavioral Risk Factor Surveillance System population. Up-to-date was defined as FOBT within 1 year, or sigmoidoscopy within 5 years with FOBT within 3 years, or colonoscopy within 10 years.
Source. Centers for Disease Control and Prevention.7
These disparities may be attributable in part to the fact that some professional societies recommend colonoscopy as the preferred screening method.10,11 However, increasing evidence shows that patients who are offered only colonoscopy for initial CRC screening might not screen at all.12 Inadomi et al. found that patients offered FOBT or a choice of FOBT or colonoscopy for initial screening were almost twice as likely as those offered colonoscopy only to complete CRC screening, with Latinos and Asians significantly more likely to choose FOBT.13 A randomized trial by Green et al. offered almost 5000 patients CRC screening choices of FOBT, sigmoidoscopy, or colonoscopy, with FOBT kits mailed to those who did not choose.14 The default FOBT program resulted in almost twice as many people being current for CRC screening and was less expensive than usual care because it reduced the number of expensive colonoscopies. Kaiser Permanente Northern California reported that mailed fecal immunochemical tests substantially increased CRC screening rates, with proportional increases in CRC detection, primarily early-stage disease.15
THE AFFORDABLE CARE ACT AND COLORECTAL CANCER SCREENING
The Affordable Care Act (ACA) mandates that preventive services recommended by the US Preventive Services Task Force, including CRC screening, be covered in full with no patient costs.16 This policy is supported by studies demonstrating that even when individuals have health insurance, out-of-pocket costs are a barrier to seeking preventive care.17–19 This policy could reduce health care disparities, because low-income patients are less likely to be able to afford screening and other preventive care. However, a close analysis of how the ACA prevention mandate is being implemented reveals a paradox for CRC screening: current ACA policies could lead to increased costs for patients and greater disparities in screening rates because mandated coverage applies only to initial screening FOBT, sigmoidoscopy, or colonoscopy, not to the necessary diagnostic testing after a positive FOBT or sigmoidoscopy finding.
The current ACA preventive care mandate penalizes people who choose FOBT or sigmoidoscopy because the required colonoscopy after a positive test is not covered and is subject to out-of-pocket deductibles or copays. Newer tests, such as fecal immunochemical tests, can be done in the privacy of one’s home and require only a single sample, with no dietary or medication changes. FOBT is inexpensive, at $2 to $22 per test. If an FOBT procedure is positive, the recommended next step is colonoscopy. However, people choosing FOBT or sigmoidoscopy might be unpleasantly surprised to learn, depending on their health plan, that colonoscopy after a positive test could cost between $200 and $3000.20 In an example of the dangerous effects of this policy, a physician colleague described a patient with a positive FOBT result who could not afford a follow-up colonoscopy because of her $10 000 health plan deductible. The patient and physician were left worrying about her possible CRC or advanced adenoma, and the physician had doubts about recommending FOBT to other patients.
On the other hand, policies that promote colonoscopy as the initial screening test may have advantages, because these tests find more advanced adenomas in a single screen than do the other screening tests.3,10 However, this line of reasoning ignores the fact that FOBT is a program of repeated screens with multiple opportunities to discover advanced adenomas and early CRC while they are still easy to treat. Disadvantages of colonoscopy are preparation that involves cramping and diarrhea, time away from work, and risk of adverse events. About 1 in 500 colonoscopies results in bleeding or perforation that requires hospitalization, transfusion, or surgery, and about 1 in 30 000 colonoscopies results in death.21 Studies comparing the relative efficacy of colonoscopy and FOBT in decreasing CRC morbidity and mortality are only now being conducted; final results will not be available for at least 10 years.
Screening colonoscopies could be contributing to the high costs of health care. The New York Times recently reported that the average cost of a colonoscopy in the United States is $1185.20 In 2002, more than 14 million colonoscopies were performed.22 More recent hospital claims data indicate that at least 17 million colonoscopies are performed annually, at an estimated cost of more than $20 billion.23 The risk and expense of colonoscopies might be worth the benefits for people at higher risk or with important findings. However, in average-risk adults, 10 to 20 screening colonoscopies are needed to find 1 case of CRC or advanced adenoma. Initial FOBT screening reduces the number of colonoscopies needed to benefit a single person to 3 to 4.24,25 In light of the high cost of colonoscopy and the current uncertainty of its benefits over less invasive procedures, the message to the public should not be “get a colonoscopy” but simply “get screened for CRC.” The bottom line is that at this time, the best CRC screening test is the one that will get done.
MEDICARE POLICIES AND SCREENING DISPARITIES
Medicare patients have a unique set of barriers to CRC screening. Like the ACA, original Medicare (Part B alone) covers initial CRC screening tests in full. However, if a biopsy or polypectomy is performed during a screening colonoscopy, the procedure is considered diagnostic, with a $175 deductible and 20% copay for total procedure costs. Patients go to sleep thinking they are having a screening colonoscopy that is covered in full, only to wake up and find out that cost sharing applies. Recently, Ronald J. Vender, MD, FACG, president of the American College of Gastroenterology, addressed this issue:
For Medicare patients, the unintended consequence of polyps being removed during colonoscopy is that the beneficiary is obligated to pay the coinsurance. . . . This is an unexpected and unwelcome “sticker shock” that deters others from being screened, and undermines the intended purpose of the procedure—to prevent cancer from developing in the first place.26
Initially, ACA coverage was identical to the Medicare policy, but was amended so that colonoscopies with removal of polyps are included in the no-cost-sharing provision.27 However, the policy remains unchanged for Medicare, leading—understandably—to many complaints to the Center for Medicare Services. In an attempt to fix this problematic Medicare policy, on March 19, 2013, Senator Ben Cardin (D-MD) and Representative Richard Neal (D-MA) introduced the Supporting ColoRectal Examination and Education Now (SCREEN) Act.28 The act would waive Medicare beneficiary cost sharing for colonoscopy with biopsy or polyp removal. However, neither the SCREEN Act nor current Health and Human Services regulations include language to cover costs of a colonoscopy after a positive FOBT result.
Why is there no bill to support FOBT? The explanation might be disturbing: profits from billable services would be less. Some insurance plans, such as Kaiser Permanente and Group Health Cooperative, which reimburse per patient rather than by procedure, have solved this issue for their own health plans, offering both choice and coverage for the continuum of CRC screening tests and follow-up, including colonoscopy after a positive FOBT result. However, neither the SCREEN Act nor current ACA regulations include language to cover the cost of a colonoscopy after a positive FOBT result.
SCREENING FOR THE UNINSURED
What about people without health insurance who live in states choosing not to participate in Medicaid expansion and people who do not qualify for health insurance, such as immigrant workers without required documentation? Uninsured individuals can pay for health care on a sliding scale at federally qualified health centers or safety net clinics, but even the charge for FOBT, the least expensive type of CRC screening, is often higher than what Medicaid and Medicare pay for the same service.
The Colorectal Cancer Detection and Control Program provides free CRC screening and diagnostic tests for age-eligible adults living below 250% of the US poverty level.29,30 However, this program is available in only 26 states and has limited funding that does not cover all people who might qualify. Safety net clinic providers thus face an ethical dilemma: offer no CRC screening or offer screening knowing that many patients cannot afford sigmoidoscopy or colonoscopy and that patients with a positive FOBT result cannot afford follow-up colonoscopy, let alone cancer treatment.
CONCLUSIONS
The ACA was a landmark in our national movement toward equitable, effective, affordable care. Now comes the detailed work of improving the ACA to ensure that it achieves its aims, including the substantial net benefits to patients and potential cost savings from CRC screening. Screening benefits accrue only if early CRC and advanced adenomas are found and removed before they become advanced cancers. To achieve this for all patients, policy changes—to the ACA and Medicare—are needed to fully cover FOBT and sigmoidoscopy for initial screening with no out-of-pocket costs for follow-up colonoscopy, along with mechanisms for screening and follow-up for the uninsured.
It is unfair to create policies that increase health disparities. Action taken now to improve CRC screening policies will provide a template for improving coverage of other cancer screenings, vaccinations, and beneficial preventive services.
Acknowledgments
We thank Chris Tachibana for her contributions in editing this article.
Human Participant Protection
No protocol approval was required because no human participants were involved.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 4.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating Test Strategies for Colorectal Cancer Screening—Age to Begin, Age to Stop, and Timing of Screening Intervals: A Decision Analysis of Colorectal Cancer Screening for the U.S. Preventive Services Task Force from the Cancer Intervention and Surveillance Modeling Network (CISNET) Rockville, MD: US Preventive Services Task Force; 2009. [PubMed] [Google Scholar]
- 6.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101(20):1412–1422. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 8.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, van Ballegooijen M, Zauber AG, Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev. 2012;21(5):728–736. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin B, Lieberman DA, McFarland B et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Anderson JC American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] [published correction appears in Am J Gastroenterol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739–750. [DOI] [PubMed]
- 12.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23(2):169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inadomi JM, Vijan S, Janz NK et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green BB, Wang CY, Anderson ML et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158(5 pt 1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33(1):101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 16. Patient Protection and Affordable Care Act. Pub L No. 111–148 (2010)
- 17.Rezayatmand R, Pavlova M, Groot W. The impact of out-of-pocket payments on prevention and health-related lifestyle: a systematic literature review. Eur J Public Health. 2013;23(1):74–79. doi: 10.1093/eurpub/cks034. [DOI] [PubMed] [Google Scholar]
- 18.Wharam JF, Graves AJ, Landon BE, Zhang F, Soumerai SB, Ross-Degnan D. Two-year trends in colorectal cancer screening after switch to a high-deductible health plan. Med Care. 2011;49(9):865–871. doi: 10.1097/MLR.0b013e31821b35d8. [DOI] [PubMed] [Google Scholar]
- 19.Sabatino SA, Lawrence B, Elder R et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal E. The $2.7 trillion medical bill colonoscopies explain why US leads the world in health expenditures. New York Times. June 1, 2013. Available at: http://www.nytimes.com/2013/06/02/health/colonoscopies-explain-why-us-leads-the-world-in-health-expenditures.html?partner=rss&emc=rss&smid=tw-nytimes&_r=2&. Accessed March 19, 2014.
- 21.Ko CW, Riffle S, Michaels L et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2010;8(2):166–173. doi: 10.1016/j.cgh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeff LC. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroentology. 2004;127(6):1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003–2009. JAMA. 2012;307(11):1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 24.Quintero E, Castells A, Bujanda L et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 25.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(12):1272–1278. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Cardin B, Neal R. SCREEN Act introduced in House and Senate for colorectal cancer awareness month: legislation proposed to ensure access to colorectal cancer screening under Medicare. 2013. Available at: http://www.prnewswire.com/news-releases/screen-act-introduced-in-house-and-senate-for-colorectal-cancer-awareness-month-199517591.html. Accessed March 19, 2014.
- 27.Centers for Medicare and Medicaid Services. Waiver of coinsurance and deductible for preventive services, section 4104 of the Patient Protection and Affordable Health Care Act (the Affordable Care Act), removal of barriers to preventive services in Medicare. 2011. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r864otn.pdf. Accessed March 19, 2014.
- 28. SCREEN Act of 2013, S 608, 113th Cong, 1st Sess (2013)
- 29.Seeff LC, Major A, Townsend JS et al. Comprehensive cancer control programs and coalitions: partnering to launch successful colorectal cancer screening initiatives. Cancer Causes Control. 2010;21(12):2023–2031. doi: 10.1007/s10552-010-9664-9. [DOI] [PubMed] [Google Scholar]
- 30.Colorectal Cancer Control Program. Colorectal cancer screening saves lives. 2013. Available at: http://www.cdc.gov/cancer/crccp. Accessed March 19, 2014.