Abstract
Objectives. We described hepatitis C virus antibody (anti-HCV) prevalence in a state prison system and retrospectively evaluated the case-finding performance of targeted testing of the 1945 to 1965 birth cohort in this population.
Methods. We used observational data from universal testing of Pennsylvania state prison entrants (June 2004–December 2012) to determine anti-HCV prevalence by birth cohort. We compared anti-HCV prevalence and the burden of anti-HCV in the 1945 to 1965 birth cohort with that in all other birth years.
Results. Anti-HCV prevalence among 101 727 adults entering prison was 18.1%. Prevalence was highest among those born from 1945 to 1965, but most anti-HCV cases were in people born after 1965. Targeted testing of the 1945 to 1965 birth cohort would have identified a decreasing proportion of cases with time.
Conclusions. HCV is endemic in correctional populations. Targeted testing of the 1945 to 1965 birth cohort would produce a high yield of positive test results but would identify only a minority of cases. We recommend universal anti-HCV screening in correctional settings to allow for maximum case identification, secondary prevention, and treatment of affected prisoners.
HCV is the most common blood-borne viral infection in the United States, with an estimated 4.1 million persons having been exposed to the virus, and 3.2 million people, or about 1.3% of the population, having chronic HCV infection.1 Although overall HCV prevalence in the United States is declining,2 recently there have been multiple reports of outbreaks among young people, predominantly in suburban and rural areas.3–5 The primary mode of HCV transmission is injection drug use,6 and as a result, HCV disproportionately affects people in contact with the criminal justice system.7 An estimated 17.4% of US state prisoners were HCV antibody positive (anti-HCV positive) in 2006, and perhaps 28.5% to 32.8% of the US case burden was in contact with the criminal justice system in that year.8
People may be infected with HCV for several decades without symptoms. At least half of the affected individuals in the United States are unaware of their infection9 and thus are unable to receive treatment. Without treatment, HCV infection can lead to cirrhosis, chronic liver disease, and hepatocellular carcinoma.10–12 At current treatment rates, HCV will kill nearly 380 000 people in the United States by 2030 and more than 1 million by 2060.13
Until recently, the Centers for Disease Control and Prevention (CDC) recommended HCV testing only for people with known or at high risk for past or current HCV exposure, including people who had ever injected drugs, who had certain medical conditions, or who had received blood transfusions or blood products before HCV screening of such products became routine.14 In recognition of the urgent need to diagnose and treat extant infections and reduce HCV-related mortality, in 2012 the CDC also recommended 1-time HCV testing of all people born between 1945 and 1965.14 This birth cohort was selected on the basis of findings from the National Health and Nutrition Examination Survey (NHANES). NHANES is an ongoing nationally representative survey of the civilian, noninstitutionalized population. NHANES data from 1999 to 2008 indicated that 81.6% of anti-HCV–positive people in the United States were born between 1945 and 1965.15 However, an acknowledged limitation of the NHANES data in assessing the epidemiology of HCV is the exclusion of incarcerated people from the sample.1 As such, it is unclear how applicable the 1945 to 1965 birth cohort screening recommendation may be for prisoner populations.
The Federal Bureau of Prisons now recommends HCV antibody testing for all inmates who request a test or report risk factors for infection.16 This approach assumes that inmates will reliably report a history of injection drug use, but concerns about self-incrimination and confidentiality may prevent this disclosure. Although 1 study has reported success in using risk-based testing to identify acute HCV in an incarcerated population,17 that study did not assess the proportion of all chronic HCV cases identified by risk-based testing. Analysis of data from a large representative sample of prison entrants found that testing only those inmates who reported injection drug use would have identified 56% of anti-HCV–positive women and just 35% of anti-HCV–positive men.18
Given the high anti-HCV prevalence and limited case-finding performance of risk-based HCV screening in correctional settings, universal screening has been suggested as an alternative approach.19 If, however, HCV infection in the correctional population is concentrated in the 1945 to 1965 birth cohort, targeting testing toward this group may be an efficient and cost-effective approach to HCV case finding.20 Limited recent epidemiological data on HCV prevalence in correctional settings hamper evaluation of these different approaches to HCV testing. We present data from universal HCV screening on entry to state prisons in Pennsylvania and consider the case-finding performance of the CDC 1945 to 1965 birth cohort recommendation in this setting.
METHODS
Since 2003, the Pennsylvania Department of Corrections has operated a comprehensive HCV testing and treatment program. All state prison entrants are tested for anti-HCV unless they explicitly opt out. All prisoners, regardless of test result, are provided with risk reduction education and counseling. All prisoners with anti-HCV–positive test results are offered hepatitis A and B vaccinations and viral load testing, and those with confirmed infection are evaluated for HCV therapy. Viral load testing is not offered to inmates who will not qualify for treatment because of sentence length (i.e., too little time remaining to complete treatment protocol) or medical contraindications.
Data for this study were provided by the Pennsylvania Department of Corrections’ contracted laboratory, Bio-Reference. The data consisted of de-identified anti-HCV test results and basic demographic information. Each record in the data set included a coded identifier that remained consistent for repeated tests of the same individual. Anti-HCV results were recorded as positive, negative, or indeterminate. Other variables available were inmate sex, year of birth, and date of anti-HCV test. Data on race and ethnicity were not available. Data were for the period June 2004 to December 2012. No major changes to relevant policies or clinical practice were made during this time.
Data Cleaning
The supplied data included 131 791 records for 101 727 participants imprisoned between June 2004 and December 2012. We deleted 1296 duplicate records that were assumed to be data entry errors. Year of birth was missing for 7783 participants. In addition, when year of birth differed across admissions for a single individual, or year of birth suggested that the individual was younger than 17 years at the time of the anti-HCV test, we assumed data entry errors and set the year of birth to missing. The data set used for analysis included 130 495 records for 101 727 participants.
We were unable to calculate a precise participation rate (the proportion of all prison entrants who were tested for anti-HCV) because of lack of data on the number of unique individuals received to prisons during the observation period. Instead, we estimated the proportion of prison receptions in which an anti-HCV test was conducted by dividing the number of tests performed by the number of prison receptions as reported separately by the Pennsylvania Department of Corrections.21
Data Analysis
We calculated sex-specific anti-HCV prevalence and 95% binomial confidence intervals (CIs) for the total time period (June 2004–December 2012), for birth cohorts (prior to 1940, then 5-year birth cohorts from 1940 to 1995), and for several 3-year testing periods (2004–2006, 2007–2009, and 2010–2012). We then calculated the proportion of male and female anti-HCV–positive cases in each birth cohort and time period. Finally, we compared anti-HCV prevalence and the burden of anti-HCV–positive cases in the CDC-nominated 1945 to 1965 birth cohort with prevalence and burden in all other birth years. This analysis was conducted for the total observation time and the 3 periods specified earlier. In all analyses, participants with multiple entries to prison during the time period under analysis were counted once to obtain the denominator. Participants with at least 1 positive anti-HCV test result during the period under analysis were counted as case participants. Participants with missing year of birth were excluded from birth cohort analyses.
RESULTS
A blood sample was provided for anti-HCV testing in 93% of prison receptions. Test coverage increased from 76% of prison receptions in 2004 to 2006 to 97% in 2010 to 2012. Of 101 727 unique participants, 9.4% (n = 9534) were women. Year of birth was missing for 13% (n = 13 179) of the participants. Of the participants with complete data (n = 88 548), the majority (55.9%; n = 49 480) were born since 1975 (range = 1911–1995). The median age at first test during the observation period was 32 years (range = 17–95 years).
Overall anti-HCV prevalence was 18.1% (95% CI = 17.9%, 18.4%; Table 1). Anti-HCV prevalence was nearly twice as high among women (31.3%; 95% CI = 30.4%, 32.3%) as among men (16.8%; 95% CI = 16.5%, 17.0%; relative risk = 1.87; 95% CI = 1.81, 1.93). Overall, the highest anti-HCV prevalence was observed among those born from 1950 to 1954 (44.7%; 95% CI = 42.7%, 46.8%), although among women, prevalence peaked in the 1955 to 1959 birth cohort (44.7%; 95% CI = 40.6%, 48.9%). Anti-HCV prevalence was less than 10% in men born since 1985 but exceeded 20% in women born in these years.
TABLE 1—
Total and Sex-Specific HCV Antibody (Anti-HCV) Prevalence Among Entrants to Pennsylvania State Prisons in 2004–2012, by Birth Cohort and Test Period
| All Persons |
Women |
Men |
|||||||
| Variable | No. Tested | No. Anti-HCV+ | % Anti-HCV+ (95% CI) | No. Tested | No. Anti-HCV+ | % Anti-HCV+ (95% CI) | No. Tested | No. Anti-HCV+ | % Anti-HCV+ (95% CI) |
| Total | 101 727 | 18 454 | 18.1 (17.9, 18.4) | 9534 | 2986 | 31.3 (30.4, 32.3) | 92 193 | 15 468 | 16.8 (16.5, 17.0) |
| Birth cohorta | |||||||||
| < 1940 | 258 | 29 | 11.2 (7.7, 15.7) | 21 | 3 | 14.3 (3.0, 36.3) | 237 | 26 | 11.0 (7.3, 15.7) |
| 1940–1944 | 394 | 73 | 18.5 (14.8, 22.7) | 21 | 5 | 23.8 (8.2, 47.2) | 373 | 68 | 18.2 (14.4, 22.5) |
| 1945–1949 | 1044 | 361 | 34.6 (31.7, 37.6) | 83 | 28 | 33.7 (23.7, 44.9) | 961 | 333 | 34.7 (31.6, 37.8) |
| 1950–1954 | 2289 | 1024 | 44.7 (42.7, 46.8) | 211 | 75 | 35.5 (29.1, 42.4) | 2078 | 949 | 45.7 (43.5, 47.8) |
| 1955–1959 | 5030 | 2097 | 41.7 (40.3, 43.1) | 573 | 256 | 44.7 (40.6, 48.9) | 4457 | 1841 | 41.3 (40.0, 42.8) |
| 1960–1964 | 8406 | 2645 | 31.5 (30.5, 32.5) | 1106 | 409 | 37.0 (34.1, 39.9) | 7300 | 2236 | 30.6 (29.6, 31.7) |
| 1965–1969 | 10 100 | 2225 | 22.0 (21.2, 22.9) | 1484 | 445 | 30.0 (27.7, 32.4) | 8616 | 1780 | 20.7 (19.8, 21.5) |
| 1970–1974 | 11 547 | 1662 | 14.4 (13.8, 15.0) | 1328 | 354 | 26.7 (24.3, 29.1) | 10 219 | 1308 | 12.8 (12.2, 13.5) |
| 1975–1979 | 14 802 | 2101 | 14.2 (13.6, 14.8) | 1395 | 384 | 27.5 (25.2, 30.0) | 13 407 | 1717 | 12.8 (12.2, 13.4) |
| 1980–1984 | 17 307 | 2466 | 14.2 (13.7, 14.8) | 1799 | 608 | 33.8 (31.6, 36.0) | 15 508 | 1858 | 12.0 (11.5, 12.5) |
| 1985–1989 | 13 917 | 1194 | 8.6 (8.1, 9.1) | 1131 | 320 | 28.3 (25.7, 31.0) | 12 786 | 874 | 6.8 (6.4, 7.3) |
| 1990–1995 | 3454 | 140 | 4.1 (3.4, 4.8) | 198 | 40 | 20.2 (14.8, 26.5) | 3256 | 100 | 3.1 (2.5, 3.7) |
| Test period | |||||||||
| 2004–2006 | 17 604 | 3483 | 19.8 (19.2, 20.4) | 2350 | 761 | 32.4 (30.5, 34.3) | 15 254 | 2722 | 17.8 (17.2, 18.5) |
| 2007–2009 | 40 178 | 7170 | 17.8 (17.5, 18.2) | 3873 | 1143 | 29.5 (28.1, 31.0) | 36 305 | 6027 | 16.6 (16.2, 17.0) |
| 2010–2012 | 42 477 | 7058 | 16.6 (16.3, 17.0) | 4268 | 1415 | 33.2 (31.7, 34.6) | 38 209 | 5643 | 14.8 (14.4, 15.1) |
Note. CI = confidence interval.
Year of birth missing for 13 179 participants.
Sex disparities were also apparent in anti-HCV prevalence across the testing periods. Among men, anti-HCV prevalence was 17.8% (95% CI = 17.2%, 18.5%) in 2004 to 2006, decreasing to 14.8% (95% CI = 14.4%, 15.1%) in 2010 to 2012. Among women, however, anti-HCV prevalence was relatively uniform across time, at 32.4% (95% CI = 30.5%, 34.3%) in 2004 to 2006 and 33.2% (95% CI = 31.7%, 34.6%) in 2010 to 2012.
The distribution of anti-HCV prevalence is illustrated in Figure 1. Among men, the greatest proportion of anti-HCV cases (17.1%) was identified in the 1960 to 1964 birth cohort and the adjacent 5-year birth cohorts (1955–1959: 14.1%; 1965–1969: 13.6%; Figure 1a). A second peak was observed in the 1980 to 1984 birth cohort (14.2% of cases). Among women, the largest proportion of anti-HCV cases was seen in the 1980 to 1984 birth cohort (20.8%); birth years 1960 to 1979 collectively accounted for more than half (54.4%) of the female anti-HCV cases. Panels b, c, and d of Figure 1 show that among men and women, a greater proportion of anti-HCV–positive cases were seen in more recent birth cohorts in each successive time period.
FIGURE 1—
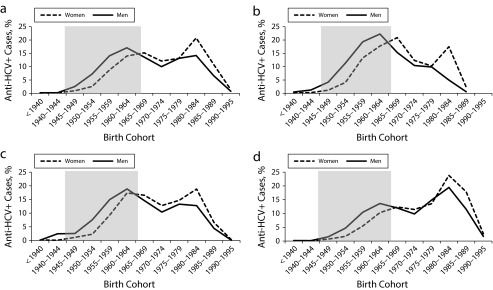
Proportion of HCV antibody (anti-HCV)–positive entrants to Pennsylvania state prisons (2004–2012) in each birth cohort, by sex, in (a) 2004–2012 (n = 101 727), (b) 2004–2006 (n = 17 604), (c) 2007–2009 (n = 40 178), and (d) 2010–2012 (n = 42 477).
Note. Shaded area denotes the 1945–1965 birth cohort recommended by the Centers for Disease Control and Prevention for 1-time anti-HCV testing.
Among both men and women, anti-HCV prevalence was higher in the 1945 to 1965 birth cohort than in all other birth years, a finding consistent across time (Table 2). Testing only this birth cohort would have identified 44% of male and 29% of female anti-HCV–positive inmates. The proportion of positive cases that would be identified from testing just this birth cohort is decreasing with time; by 2010 to 2012, targeted testing of the 1945 to 1965 birth cohort would have identified 33% of male anti-HCV–positive inmates and 20% of female anti-HCV–positive inmates (Table 2).
TABLE 2—
HCV Antibody (Anti-HCV) Prevalence and Percentage of Anti-HCV Cases in the 1945–1965 Birth Cohort and All Other Birth Years (n = 88 548) Among Entrants to Pennsylvania State Prisons in 2004–2012
| 1945–1965 Birth Cohort |
All Other Birth Years |
|||||||
| Sex and Time Period | No. Tested | No. Anti-HCV+ | % Anti-HCV+ (95% CI) | % of Anti-HCV+ Cases | No. Tested | No. Anti-HCV+ | % Anti-HCV+ (95% CI) | % of Anti-HCV+ Cases |
| Male | ||||||||
| 2004–2006 | 4163 | 1653 | 39.7 (38.2, 41.2) | 61 | 11 091 | 1069 | 9.6 (9.1, 10.2) | 39 |
| 2007–2009 | 7850 | 2834 | 36.1 (35.0, 37.2) | 47 | 28 455 | 3193 | 11.2 (10.9, 11.6) | 53 |
| 2010–2012 | 6271 | 1851 | 29.5 (28.4, 30.7) | 33 | 31 938 | 3792 | 11.9 (11.5, 12.2) | 67 |
| 2004–2012 | 16 444 | 5750 | 35.0 (34.2, 35.7) | 44 | 62 754 | 7340 | 11.7 (11.4, 12.0) | 56 |
| Female | ||||||||
| 2004–2006 | 751 | 311 | 41.4 (37.9, 45.0) | 41 | 1599 | 450 | 28.1 (25.9, 30.4) | 59 |
| 2007–2009 | 1016 | 377 | 37.1 (34.1, 40.2) | 33 | 2857 | 766 | 26.8 (25.2, 28.5) | 67 |
| 2010–2012 | 771 | 288 | 37.4 (33.9, 40.9) | 20 | 3497 | 1127 | 32.2 (30.7, 33.8) | 80 |
| 2004–2012 | 2261 | 851 | 37.6 (35.6, 40.0) | 29 | 7089 | 2076 | 29.3 (28.2, 30.4) | 71 |
Note. CI = confidence interval.
DISCUSSION
Anti-HCV prevalence was 18.1% in this large sample of state prison entrants in Pennsylvania. Few recent data on anti-HCV prevalence exist in US correctional populations, but this result is similar to an estimate of national anti-HCV prevalence in prisons (17.4%)8 and substantially lower than that reported in some states (e.g., 40% in New Mexico).22 Variation among states likely reflects variation in the prevalence of injection drug use outside prisons as well as sentencing policies for drug offenses. Women entering prison were almost twice as likely as men entering prison to be anti-HCV positive, a pattern that has been observed elsewhere in the United States23 and internationally7 and can also be attributed to background prevalence of injection drug use.7
Anti-HCV prevalence was highest among those born in 1945 to 1965, as in the US general population.14 In the general population, it is estimated that this birth cohort accounts for more than 80% of prevalent anti-HCV15; in this prisoner sample, however, the 1945 to 1965 birth cohort accounted for fewer than half of male anti-HCV cases and fewer than one third of female anti-HCV cases. Thus, although targeted screening of this birth cohort in correctional settings would produce a high yield of positive results, it would identify only a minority of the total HCV caseload. Furthermore, the proportion of anti-HCV cases that would be identified with targeted testing of the 1945 to 1965 birth cohort is decreasing with time. Our findings suggest that female prisoners, especially those in more recent birth cohorts, would be particularly poorly served by targeted testing of the 1945 to 1965 birth cohort.
The CDC recommends screening of the 1945 to 1965 birth cohort in conjunction with risk-based screening.14 Because we did not have data on HCV risk factors in our sample of prison entrants, we were unable to assess the case-finding performance of risk-based and birth cohort screening combined. A combination of these approaches may successfully identify most anti-HCV cases in correctional settings; however, we do not consider this to be likely. Most prisoners nationally are younger than 40 years,24 outside the 1945 to 1965 birth cohort, and therefore would be tested only on self-report of injection drug use or other HCV risk factors. As the 1945 to 1965 birth cohort ages, they will constitute a diminishing proportion of the correctional population. In practice, a combined birth cohort and risk-based testing strategy in a correctional setting would closely resemble a risk-based testing strategy, becoming more so with time, and the poor case-finding performance of risk-based testing has already been shown.18
Public Health Implications
In light of endemic anti-HCV in the prisoner population and demonstrated limitations of targeted testing strategies (both risk based and birth cohort), we recommend universal opt-out screening as the most appropriate strategy for HCV testing in correctional settings. Universal HIV screening is already recommended in correctional settings,25 and the data presented here indicate the feasibility of this approach for HCV screening. Universal screening ensures that the largest possible number of prevalent infections is identified and allows for confirmatory testing, secondary prevention, and treatment of infected individuals. Screening can be undertaken on reception to a correctional setting, with repeat testing available on request or as medically indicated during incarceration.
Given the concentration of the total HCV caseload in correctional settings,19 universal opt-out screening of incarcerated people with follow-up testing and treatment has the potential to reduce general population prevalence of this infection, analogous to HIV “treatment as prevention” approaches.26 Furthermore, unlike HIV therapy, HCV therapy can be curative and is increasingly so with the advent of new antiviral therapies.27 Direct-acting antiviral agents have increased sustained viral response rates and decreased the length of therapeutic regimens in the treatment of some genotypes.27 Interferon-free therapies with very high sustained viral response rates and of 12 weeks’ duration are rapidly moving through the development pipeline.28 Sentence length is often a criterion for HCV treatment in prisons to allow for treatment completion before release; shorter therapeutic regimens will therefore increase the pool of treatment-eligible prisoners.19 Although this has cost implications for correctional authorities, screening and treatment may ultimately be less costly than providing care for inmates with chronic liver disease or in need of a liver transplant.29 Further work assessing the cost-effectiveness of universal screening and treatment in correctional settings is needed. Given the potential public health benefits of widespread HCV treatment in prison, the feasibility of cost-sharing arrangements between correctional authorities and public health departments also should be explored.
Limitations
As noted earlier, we did not have data on HCV risk factors, which would have allowed for evaluation of a combined risk-based and birth cohort testing strategy in a correctional population. We also lacked data on racial/ethnic backgrounds of the participants. Recent data suggest important racial/ethnic disparities in incident HCV infections,17 and data on race/ethnicity in our cohort would have allowed further examination of these trends. Finally, year of birth was missing for 13% of the participants, potentially introducing bias to our birth cohort analyses. However, there was no association between missing year of birth and anti-HCV status (year of birth missing in 12.9% of anti-HCV–negative or equivocal cases and 13.2% of anti-HCV–positive cases; χ21 = 1.4; P = .2).
Conclusions
We observed extremely high anti-HCV prevalence in a state prison population and showed the limitations of applying a birth cohort recommendation that is suitable in the general community to a correctional population. Given the high prevalence of HCV exposure and limitations of birth cohort and risk-based testing in correctional populations, we recommend universal anti-HCV screening of people entering correctional facilities, with follow-up testing and treatment of HCV infection.
Acknowledgments
Sarah Larney is supported by a National Health and Medical Research Council Early Career Fellowship (APP1035149). The National Drug and Alcohol Research Centre at the University of New South Wales is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grants Fund. This research was facilitated by the infrastructure and resources provided by the Lifespan/Tufts/Brown Center for AIDS Research (P300AI042853).
We thank the Pennsylvania Department of Corrections and Bio-Reference Laboratory for data access and supply.
Human Participant Protection
As a secondary analysis of de-identified data, the Brown University Research Protections Office did not require us to seek institutional review board approval for this study. The research protocol was approved by the Research Review Committee of the Pennsylvania Department of Corrections.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Razavi H, El Khoury AC, Elbasha E et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christian WJ, Hopenhayn C, Christian AMY, McIntosh D, Koch A. Viral hepatitis and injection drug use in Appalachian Kentucky: a survey of rural health department clients. Public Health Rep. 2010;125:121–128. doi: 10.1177/003335491012500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002-2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):537–541. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Notes from the field: hepatitis C virus infections among young adults - rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- 6.Allison RD, Conry-Cantilena C, Koziol D et al. A 25-year study of the clinical and histologic outcomes of hepatitis C virus infection and its modes of transmission in a cohort of initially asymptomatic blood donors. J Infect Dis. 2012;206:654–661. doi: 10.1093/infdis/jis410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larney S, Kopinski H, Beckwith CG et al. The incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58:1215–1224. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2):187–195. doi: 10.1177/003335491412900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spradling PR, Rupp L, Moorman AC et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047–1055. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1–21. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Dore GJ, Freeman AJ, Law MG, Kaldor JM. Is severe liver disease a common outcome for people with chronic hepatitis C? J Gastroenterol Hepatol. 2002;17:423–430. doi: 10.1046/j.1440-1746.2002.02730.x. [DOI] [PubMed] [Google Scholar]
- 12.Freeman AJ, Dore GJ, Law MG et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 13.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Smith BD, Morgan RL, Beckett GA et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 15.Smith BD, Patel N, Beckett GA, Jewett A, Ward JW. Hepatitis C virus antibody prevalence, correlates and predictors among persons born from 1945 through 1965, United States, 1999-2008 [conference abstract] Hepatology. 2011;54(suppl 1):554A–555A. [Google Scholar]
- 16.Federal Bureau of Prisons. Clinical Practice Guidelines: Evaluation and Treatment of Hepatitis C and Cirrhosis. Washington, DC: Federal Bureau of Prisons; 2012. [Google Scholar]
- 17.Kim AY, Nagami EH, Birch CE, Bowen MJ, Lauer GM, McGovern BH. A simple strategy to identify acute hepatitis C virus infection among newly incarcerated injection drug users. Hepatology. 2013;57:944–952. doi: 10.1002/hep.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95(10):1739–1740. doi: 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaulding AS, Kim AY, Harzke AJ et al. Impact of new therapeutics for hepatitis C virus in incarcerated populations. Top Antivir Med. 2013;21(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- 20.Spaulding AC, Thomas DL. Screening for HCV infection in jails. JAMA. 2012;307(12):1259–1260. doi: 10.1001/jama.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pennsylvania Department of Corrections. Annual Statistical Report. 2012. Available at: http://www.cor.state.pa.us/portal/server.pt/community/research___statistics/10669/reports/1069947. Accessed October 22, 2013.
- 22.Arora S, Thornton K, Murata G et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baillargeon J, Wu H, Kelley MJ, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117(1):43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 24.Carson EA, Sabol WJ. Prisoners in 2011. December 2012. Available at: http://www.bjs.gov/content/pub/pdf/p11.pdf. Accessed October 22, 2013.
- 25. Centers for Disease Control and Prevention. HIV testing implementation guidance for correctional settings. 2009. Available at: http://www.cdc.gov/hiv/pdf/risk_Correctional_Settings_Guidelines.pdf. Accessed October 15, 2013.
- 26.Granich R, Williams B, Montaner J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Curr Opin HIV AIDS. 2013;8(1):41–49. doi: 10.1097/COH.0b013e32835b80dd. [DOI] [PubMed] [Google Scholar]
- 27.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poordad F, Lawitz E, Kowdley KV et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 29.Baillargeon J, Soloway RD, Paar DP et al. End-stage liver disease in a state prison population. Ann Epidemiol. 2007;17:808–813. doi: 10.1016/j.annepidem.2007.04.005. [DOI] [PubMed] [Google Scholar]


