Abstract
Objectives. We assessed the relationship between individual characteristics and receipt of oseltamivir (Tamiflu) in the United States during the H1N1 pandemic and other flu seasons.
Methods. In a cohort of individuals enrolled in pharmacy benefit plans, we used a multivariate logistic regression model to measure associations between subscriber characteristics and filling a prescription for oseltamivir during 3 flu seasons (October 2006–May 2007, October 2007–May 2008, and October 2008–May 2010). In 19 states with county-level influenza rates reported, we controlled for disease burden.
Results. Approximately 56 million subscribers throughout the United States were included in 1 or more study periods. During pandemic flu, beneficiaries in the highest income category had 97% greater odds of receiving oseltamivir than those in the lowest category (P < .001). After we controlled for disease burden, subscribers in the 2 highest income categories had 2.18 and 1.72 times the odds of receiving oseltamivir compared with those in the lowest category (P < .001 for both).
Conclusions. Income was a stronger predictor of oseltamivir receipt than prevalence of influenza. These findings corroborate concerns about equity of treatment in pandemics, and they call for improved approaches to distributing potentially life-saving treatments.
Influenza outbreaks are common and cause substantial morbidity and mortality.1 Although vaccination represents the primary strategy for the prevention of influenza, antiviral therapies including oseltamivir (Tamiflu) can be used to treat the disease and serve as prophylaxis when initiated shortly after exposure.2–4 In a pandemic, it takes up to 6 months to create a novel vaccine; thus, at the beginning of a major outbreak, antiviral medications are likely to be the only countermeasure available.5,6 In the 2009 H1N1 pandemic, early oseltamivir treatment of hospital patients successfully reduced deaths and admissions to critical care units.7 Therefore, the effective and equitable allocation of antivirals, particularly if they are scarce, is a central public health concern and an important measure of the ability of the public health system to launch a robust response.
Low-income populations are at greater risk in an influenza pandemic. Because of crowded living conditions, they may be more likely to be exposed to the disease. They also are less likely to receive timely and effective treatment after disease has developed.5,8 Evidence from the 1918 influenza pandemic demonstrates that low-income populations experienced greater morbidity and mortality.9 Evidence emerging from the 2009 H1N1 pandemic also indicates that poverty was a risk factor for severe disease.10 Too little is known about the ability of low-income populations to access timely treatment during outbreaks of influenza. Although an article from the United Kingdom showed that socioeconomic deprivation was associated with decreased likelihood of accessing antivirals during H1N1,11 no studies in the United States have explored the relationship between individual characteristics and receipt of oseltamivir.
The 2009 H1N1 pandemic turned out to be a relatively mild event that did not push the response system to its limits.12 However, when H1N1 first emerged, there was substantial media attention and public health concern about its potential risks to human health and society.10 In anticipation of shortages of antiviral medications, the Centers for Disease Control and Prevention (CDC) released guidance to ensure that antivirals were reserved for influenza patients with, or at risk for, severe disease.4 Even though demand for antiviral medications was ultimately weaker than many predicted, the majority of communities still witnessed shortages, especially of the pediatric formulation.13 The large volume of flu cases in the winter of 2012–2013 highlights the ongoing need for careful evaluation of how influenza is managed across the country.
We aimed to assess individual-level predictors of oseltamivir receipt, with a specific focus on the equitability of care. This analysis is key to understanding whether CDC recommendations (e.g., treat children, those with severe disease, and those with comorbidities) were followed by clinicians and whether communication and distribution strategies achieved equitable allocation.
METHODS
Data included selected pharmacy claims and enrollment data from CVS Caremark (Woonsocket, RI), a large national pharmacy benefits manager. The pharmacy data included all claims for oseltamivir either requested by the pharmacy or reimbursed by Caremark from all pharmacies that a subscriber visited. Subscribers’ health insurance coverage included a mix of commercial insurance, Medicare, Medicaid, and others, but it was not available on an individual level. We drew income information from 2000 census data and retrieved data on flu diagnosis rates from states’ public health Web sites.
Cohort
To evaluate overall trends in antiviral prescribing across the study period, we extracted the count of all claims for oseltamivir (Tamiflu; Genentech, South San Francisco, CA) during each month from October 2006 to June 2010, separately by prophylactic (once-a-day) versus therapeutic (twice-daily) dose. Oseltamivir, which represents more than 95% of the antivirals prescribed for pandemic influenza, was the most effective therapy for H1N114; accordingly, we followed the example of past studies3 and focused on oseltamivir prescribing in this study.
From these data, we identified 3 flu seasons: (1) October 1, 2006, to May 31, 2007; (2) October 1, 2007, to May 31, 2008; and (3) October 1, 2008, to May 31, 2010. We established these time periods empirically, on the basis of patterns of antiviral purchasing; the precise start dates of flu diagnoses were deemed less relevant than the patterns of medication purchasing. As a result, these periods included time prior to increases in influenza diagnoses and, in the case of the H1N1 pandemic, included another mild flu season that overlapped with the H1N1 pandemic onset (April 2009).
We identified all subscribers with continuous enrollment in a pharmacy benefit insurance plan from CVS Caremark during each of the 3 flu seasons. These flu seasons defined 3 separate study periods, and subscribers could be included in 1 or more periods, depending on their enrollment. In each study period, we used pharmacy claims to determine whether subscribers filled a prescription for oseltamivir. The primary outcome was a binary indicator of any oseltamivir receipt during each flu season. A secondary outcome indicated receipt of treatment doses only.
We used enrollment data to identify a subscriber’s gender, age, and geographic region of residence (defined as New England, South, West, and Midwest). We assessed the number of unique medications for each subscriber during the first 4 months of each study period, which represented a proxy for level of comorbidity.15 We linked subscribers’ enrollment and prescription data to 2000 census zip code–level data in order to assign the median income in the zip code of residence on the basis of subscribers’ residential address. We used census thresholds to determine whether each subscriber lived in a rural or urban area, on the basis of the population density of each zip code; we defined a rural neighborhood as a population density of fewer than 1000 persons per square mile.16 We assessed all of these variables at the beginning of each study period.
We also searched each state’s public health Web sites for reports of influenza diagnosis rates. These data were available for only a portion of the final study period, August 2009 through April 2010, which coincided with the second wave of the 2009 H1N1 pandemic. We therefore created an additional cohort of subscribers with continuous enrollment during this period who resided in any of the 19 states with diagnosis data available. In this cohort, we recorded receipt of oseltamivir during each month of the study period and assessed covariates as described earlier in this section. We then assigned local influenza diagnosis rates to each subscriber on the basis of the rates reported in the current and prior month in the county of home residence.
Statistical Analysis
We categorized age into 5 groups: younger than 18, 18 to 34, 35 to 49, 50 to 64, and 65 years and older. We also created 5 categories of median income in zip code of home residence. We then examined the characteristics of each cohort. For categorical variables, we calculated the proportion of subscribers in each category separately by study period. For continuous variables, we calculated the mean and standard deviation separately by study period.
To assess characteristics associated with oseltamivir receipt, we estimated a multivariate logistic regression model with the indicator of oseltamivir receipt in each study period as the dependent variable. Independent variables included indicators of study period and demographic factors (age group, gender, geographic region, number of unique medications, income category, and nonrural residence). We also included interactions between study period and demographic factors, so that the odds ratio effect of individual characteristics could vary across study periods.
In the cohort with influenza diagnosis data available, we fit 2 logistic regression models that estimated the association between oseltamivir receipt and the subscriber characteristics listed in the previous paragraph. Both models adjusted for temporal trends by including indicators for each month as independent variables in the model. The second model additionally adjusted for influenza diagnosis rates in the county of residence in the current and prior month (1-month lag).
Each of these models contained several potential levels of clustering, including repeated measurements of the outcome within each individual and multiple subscribers clustered in each zip code. We found that accounting for this correlation was impossible because of the extremely large study size, so the confidence intervals reported might underestimate the true variability in odds ratio estimates. However, the observed correlations were negligible and study conclusions are unlikely to be affected. We performed all analyses with SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Figure 1 displays the trends in oseltamivir prescribing across all study periods. The lines represent the count of prophylactic doses claimed each month, the count of therapeutic doses, and the total number of claims for oseltamivir. During both of the first 2 flu seasons, claims for oseltamivir peaked in February, as expected during a normal flu season. In the 2009–2010 season, there was an initial peak in oseltamivir claims in February 2009 (prior to the start of the H1N1 pandemic), followed by a second peak in June 2009 (which coincided with the first wave of the pandemic) and a much larger peak in October 2009 during the pandemic’s second wave. Claims for oseltamivir throughout this period remained high and did not return to the low levels usually seen during the summer. In general, variation in claims for oseltamivir was driven by prophylactic doses, and prophylactic doses comprised the majority of doses during periods of peak prescribing.
FIGURE 1—
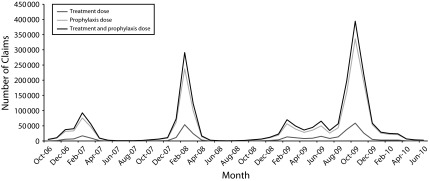
Monthly claims for oseltamivir in the CVS Caremark database from October 2006 through June 2010: United States.
We identified approximately 56 million subscribers with continuous enrollment in a CVS Caremark plan during at least 1 of the 3 study periods, including 41.6 million in the first period, 37.9 million in the second, and 26.7 million in the third. Overall, 0.5%, 1.1%, and 2.8% of subscribers filled a prescription for oseltamivir during the first, second, and third study periods, respectively. Of the 15.8 million subscribers that had continuous eligibility from August 2009 to April 2010 and had local influenza diagnosis rates available, 2.3% received at least 1 dose of oseltamivir during that 9-month period.
Table 1 presents cohort characteristics in each study period. In general, the cohort did not vary across periods. Subscribers were on average 41 years old and filled prescriptions for 1.9 unique medications during the first 4 months of each study period. In all cohorts, the largest share of subscribers lived in the South, followed by New England and the Midwest. Approximately 51% of subscribers lived in rural areas and 52% were female.
TABLE 1—
Cohort Characteristics in Each Study Period: CVS Caremark Database, United States, October 2006–May 2010
| Variable | October 2006–May 2007, No., %, or Mean ±SD | October 2007–May 2008, No., %, or Mean ±SD | October 2008–May 2010, No., %, or Mean ±SD |
| Subscribers continuously enrolled | 41.6 million | 37.9 million | 26.7 million |
| Receipt of oseltamivir | 0.5 | 1.1 | 2.8 |
| Age, y | |||
| < 18 | 22.4 | 22.8 | 22.4 |
| 18–34 | 14.8 | 15.6 | 13.7 |
| 35–49 | 20.4 | 20.2 | 19.8 |
| 50–64 | 21.3 | 20.7 | 22.4 |
| ≥ 65 | 15.7 | 15.4 | 16.5 |
| Unknown | 5.4 | 5.3 | 5.1 |
| Gender | |||
| Male | 47.4 | 47.5 | 47.4 |
| Female | 52.3 | 52.3 | 52.5 |
| Unknown | 0.3 | 0.2 | 0.1 |
| Urban area | 49.1 | 49.3 | 48.3 |
| Income, $ | |||
| 2499–19 999 | 0.7 | 0.8 | 0.7 |
| 20 000–39 999 | 35.7 | 36.2 | 36.2 |
| 40 000–59 999 | 38.2 | 38.2 | 38.4 |
| 60 000–79 999 | 17.1 | 16.7 | 16.7 |
| 80 000–200 000 | 5.9 | 5.7 | 5.6 |
| Unknown | 2.4 | 2.4 | 2.5 |
| Region | |||
| New England | 25.8 | 25.1 | 22.4 |
| South | 37.6 | 36.4 | 39.7 |
| West | 15.7 | 17.5 | 18.8 |
| Midwest | 20.9 | 21.0 | 19.2 |
| Patient’s age, y | 40.6 ±23.4 | 40.1 ±23.4 | 41.3 ±23.6 |
| Unique medications taken in 4 mo | 1.89 ±3.06 | 1.85 ±3.02 | 1.88 ±3.02 |
Table 2 shows the results from the multivariate logistic regression model in the full cohort. This model shows that in all study periods, age was inversely related to the likelihood of receiving oseltamivir. For example, during the pandemic period (period 3), children aged birth to 17 years had 96% higher odds (odds ratio [OR] = 1.96; 95% confidence interval = 1.94, 1.97) of receiving oseltamivir compared with young adults aged 18 to 34 years. In contrast, beneficiaries aged 35 to 49, 50 to 64, and 65 years or older had 13% (OR = 0.87; 95% CI = 0.86, 0.88), 54% (OR = 0.46; 95% CI = 0.46, 0.47), and 79% (OR = 0.21; 95% CI = 0.21, 0.21) lower odds, respectively, than young adults aged 18 to 34 years. Gender was also significantly associated with receipt of oseltamivir, but the associations were generally small and inconsistent across study periods.
TABLE 2—
Associations Between Subscriber Characteristics and Likelihood of Receiving Oseltamivir: CVS Caremark Database, United States, October 2006–May 2010
| Independent Variable | October 2006–May 2007, OR (95% CI) | October 2007–May 2008, OR (95% CI) | October 2008–May 2010, OR (95% CI) |
| Age | |||
| Birth–17 y | 2.16 (2.13, 2.19) | 1.16 (1.15, 1.17) | 1.96 (1.94, 1.97) |
| 18–34 y (Ref) | 1.00 | 1.00 | 1.00 |
| 35–49 y | 0.95 (0.93, 0.96) | 0.92 (0.91, 0.93) | 0.87 (0.86, 0.88) |
| 50–64 y | 0.37 (0.36, 0.38) | 0.51 (0.50, 0.51) | 0.46 (0.46, 0.47) |
| ≥ 65 y | 0.15 (0.15, 0.15) | 0.28 (0.28, 0.29) | 0.21 (0.21, 0.21) |
| Male gender | 1.03 (1.02, 1.04) | 0.99 (0.99, 1.00) | 0.96 (0.95, 0.96) |
| Urban area | 0.59 (0.59, 0.60) | 0.61 (0.61, 0.62) | 0.84 (0.83, 0.84) |
| Income, $ | |||
| 2499–19 999 (Ref) | 1.00 | 1.00 | 1.00 |
| 20 000–39 999 | 1.69 (1.57, 1.82) | 1.48 (1.42, 1.55) | 1.21 (1.17, 1.25) |
| 40 000–59 999 | 1.62 (1.50, 1.74) | 1.49 (1.42, 1.56) | 1.26 (1.22, 1.30) |
| 60 000–79 999 | 1.85 (1.72, 1.98) | 1.68 (1.60, 1.76) | 1.48 (1.43, 1.53) |
| 80 000–200 000 | 2.47 (2.30, 2.66) | 2.14 (2.20, 2.25) | 1.97 (1.90, 2.04) |
| Region | |||
| New England (Ref) | 1.00 | 1.00 | 1.00 |
| South | 3.48 (3.43, 3.54) | 3.31 (3.27, 3.34) | 2.05 (2.03, 2.06) |
| West | 0.80 (0.78, 0.82) | 0.74 (0.72, 0.75) | 0.87 (0.86, 0.88) |
| Midwest | 1.54 (1.51, 1.57) | 1.87 (1.85, 1.89) | 1.01 (1.00, 1.01) |
| Unique medications taken | 1.18 (1.17, 1.18) | 1.12 (1.11, 1.12) | 1.13 (1.12, 1.13) |
Note. CI = confidence interval; OR = odds ratio. We estimated associations from a multivariate logistic regression model using data from all study periods. Indicators for study period, subscriber characteristics, and interactions between study period and subscriber characteristics were included as independent variables. All effects were significantly different across study periods (P < .005 for all).
Subscribers living in zip codes with median income greater than $80 000 per year had consistently higher rates of filling prescriptions for oseltamivir than those in zip codes with the lowest median income (< $20 000). During the first study period, subscribers in the highest income category had 2.47 (95% CI = 2.30, 2.66) times the odds of receiving oseltamivir than those in the lowest income category. During the second and third study periods, the association between income and the likelihood of receiving oseltamivir decreased significantly; individuals in the highest income category had 2.14 (95% CI = 2.20, 2.25) and 1.97 (95% CI = 1.90, 2.04) times the odds of individuals in the lowest income category during the second and third study periods, respectively (P < .001 for both compared with the first study period).
The effects of all other characteristics were qualitatively consistent across study periods. Specifically, urban residence was associated with lower odds of receiving oseltamivir, and patients taking a higher number of unique medications had higher odds of oseltamivir receipt, consistent with CDC recommendations. Subscribers in the South had the highest odds of receiving oseltamivir, followed by those in the Midwest and New England, although the effects of region and urban residence were smaller in magnitude during the pandemic flu than during the other study periods.
Observed associations between income and oseltamivir receipt were even more extreme when we evaluated the secondary outcome, receipt of a treatment dose of oseltamivir (Table 3). In particular, during the pandemic flu, subscribers in the highest income category had nearly 3 times the odds of filling an oseltamivir prescription for a treatment dose compared with subscribers in the lowest category (OR = 2.83; 95% CI = 2.60, 3.09).
TABLE 3—
Associations Between Subscriber Characteristics and Likelihood of Receiving a Treatment Dose of Oseltamivir: CVS Caremark Database, United States, October 2006–May 2010
| Independent Variable | October 2006–May 2007, OR (95% CI) | October 2007–May 2008, OR (95% CI) | October 2008–May 2010, OR (95% CI) |
| Age | |||
| Birth–17 y | 2.27 (2.19, 2.35) | 1.87 (1.82, 1.91) | 1.60 (1.57, 1.63) |
| 18–34 y (Ref) | 1.00 | 1.00 | 1.00 |
| 35–49 y | 1.38 (1.33, 1.43) | 1.29 (1.26, 1.33) | 1.25 (1.23, 1.27) |
| 50–64 y | 0.37 (0.35, 0.39) | 0.59 (0.57, 0.61) | 0.54 (0.53, 0.55) |
| ≥ 65 y | 0.24 (0.23, 0.26) | 0.58 (0.56, 0.60) | 0.31 (0.30, 0.21) |
| Male gender | 1.01 (0.98, 1.03) | 0.92 (0.90, 0.93) | 0.93 (0.92, 0.94) |
| Urban area | 0.48 (0.47, 0.50) | 0.48 (0.47, 0.49) | 0.70 (0.69, 0.71) |
| Income, $ | |||
| 2499–19 999 (Ref) | 1.00 | 1.00 | 1.00 |
| 20 000–39 999 | 2.74 (2.19, 3.43) | 2.42 (2.10, 2.79) | 1.37 (1.26, 1.49) |
| 40 000–59 999 | 2.53 (2.02, 3.17) | 2.42 (2.10, 2.79) | 1.40 (1.29, 1.53) |
| 60 000–79 999 | 3.18 (2.54, 3.99) | 2.97 (2.57, 3.43) | 1.80 (1.65, 1.96) |
| 80 000–200 000 | 5.36 (4.27, 6.73) | 4.40 (3.81, 5.09) | 2.83 (2.60, 3.09) |
| Region | |||
| New England (Ref) | 1.00 | 1.00 | 1.00 |
| South | 4.76 (4.56, 4.96) | 4.20 (4.09, 4.32) | 2.29 (2.25, 2.33) |
| West | 0.93 (0.87, 0.99) | 0.85 (0.81, 0.88) | 0.68 (0.67, 0.70) |
| Midwest | 2.32 (2.21, 2.43) | 2.77 (2.69, 2.85) | 1.07 (1.05, 1.10) |
| Unique medications taken | 1.15 (1.15, 1.15) | 1.11 (1.10, 1.11) | 1.11 (1.10, 1.11) |
Note. CI = confidence interval; OR = odds ratio. We estimated associations from a multivariate logistic regression model using data from all study periods. Indicators for study period, subscriber characteristics, and interactions between study period and subscriber characteristics were included as independent variables. All effects were significantly different across study periods (P < .005 for all).
Table 4 presents the results of the logistic regression models estimated for those subscribers with data on local influenza diagnosis rates available during the second wave of the H1N1 pandemic. We included monthly influenza diagnoses in the model as cases per 1000 persons in the county, which had a median rate of 0.014 (interquartile range = 0.001–0.083). We found no association between contemporaneous local influenza diagnosis rates and oseltamivir receipt (OR = 1.00; 95% CI = 1.00, 1.01) and a weak negative association between prior month influenza rates and oseltamivir receipt (OR = 0.98; 95% CI = 0.98, 0.99). Adjustment for diagnosis rates changed the estimated time trends in oseltamivir receipt but did not affect other odds ratio estimates. In a separate model, we included quartiles of influenza diagnosis rates and found no association between diagnosis rate quartiles and receipt of oseltamivir (results not shown).
TABLE 4—
Associations Between Subscriber Characteristics and Likelihood of Receiving Oseltamivir: CVS Caremark Database, United States, August 2009–April 2010
| OR (95% CI) |
||
| Independent Variable | Unadjusted for Diagnosis Rates | Adjusted for Diagnosis Rates |
| Influenza diagnosis rate | ||
| Current month | 1.00 (1.00, 1.01) | |
| Prior month | 0.98 (0.98, 0.99) | |
| Month | ||
| August 2009 (Ref) | 1.00 | 1.00 |
| September 2009 | 3.64 (3.56, 3.71) | 2.21 (2.14, 2.29) |
| October 2009 | 5.71 (5.60, 5.83) | 4.04 (3.92, 4.17) |
| November 2009 | 3.47 (3.39, 3.54) | 2.55 (2.47, 2.64) |
| December 2009 | 1.03 (1.00, 1.06) | 0.74 (0.71, 0.76) |
| January 2010 | 0.53 (0.51, 0.54) | 0.38 (0.36, 0.39) |
| February 2010 | 0.45 (0.44, 0.47) | 0.32 (0.31, 0.33) |
| March 2010 | 0.40 (0.39, 0.42) | 0.29 (0.28, 0.30) |
| April 2010 | 0.13 (0.12, 0.14) | 0.09 (0.08, 0.09) |
| Age | ||
| Birth–17 y | 1.87 (1.84, 1.90) | 1.68 (1.65, 1.72) |
| 18–34 y (Ref) | 1.00 | 1.00 |
| 35–49 y | 0.90 (0.88, 0.91) | 0.85 (0.84, 0.87) |
| 50–64 y | 0.53 (0.52, 0.54) | 0.55 (0.54, 0.57) |
| ≥ 65 y | 0.22 (0.22, 0.23) | 0.23 (0.22, 0.24) |
| Male gender | 0.95 (0.94, 0.96) | 0.94 (0.93, 0.95) |
| Urban area | 0.82 (0.81, 0.82) | 0.89 (0.88, 0.90) |
| Income, $ | ||
| 2499–19 999 (Ref) | 1.00 | 1.00 |
| 20 000—39 999 | 1.19 (1.11, 1.28) | 1.21 (1.10, 1.34) |
| 40 000—59 999 | 1.39 (1.30, 1.50) | 1.47 (1.33, 1.62) |
| 60 000—79 999 | 1.69 (1.57, 1.81) | 1.72 (1.56, 1.90) |
| 80 000—200 000 | 2.23 (2.07, 2.4) | 2.18 (1.97, 2.41) |
| Region | ||
| New England (Ref) | 1.00 | 1.00 |
| South | 2.11 (2.08, 2.14) | 1.44 (1.41, 1.48) |
| West | 1.02 (1.00, 1.04) | 0.81 (0.79, 0.83) |
| Midwest | 1.07 (1.05, 1.10) | 0.98 (0.95, 1.01) |
| Unique medications taken | 1.13 (1.12, 1.13) | 1.12 (1.12, 1.13) |
Note. CI = confidence interval; OR = odds ratio. We estimated the associations from multivariate logistic regression models with and without adjustment for influenza diagnosis rates using data from 19 states. Indicators for each month during the study period and subscriber characteristics are included as independent variables.
Controlling for influenza diagnosis rates in the county of residence did not substantially affect the odds ratio estimates for age group, gender, number of unique medications, or urban residence. Individuals in the South still had the highest likelihood of receiving oseltamivir after we controlled for local influenza rates, but the estimated odds ratio compared with individuals in New England was reduced from 2.11 (95% CI = 2.08, 2.14) without adjustment to 1.44 (95% CI = 1.41, 1.48) after adjustment. Estimates of the effects of income were strengthened after adjustment for influenza diagnosis rates. For example, after adjustment, subscribers living in a zip code with a median income in the highest category had 2.18 (95% CI = 1.97, 2.41) times the odds of receiving oseltamivir than the lowest income category. In addition, compared with subscribers in the lowest income category, subscribers in the second highest, third highest, and fourth highest income categories had odds ratios for oseltamivir receipt of 1.72 (95% CI = 1.56, 1.90), 1.47 (95% CI = 1.33, 1.62), and 1.21 (95% CI = 1.10, 1.34).
DISCUSSION
In this large study of more than 50 million individuals, we identified several correlates of receipt of medications to treat or prevent influenza. We found that children, patients with more comorbidities, and those living in rural areas and in higher-income zip codes had significantly greater likelihood of oseltamivir receipt. Higher rates of oseltamivir receipt in children and those with more comorbidities are consistent with CDC guidelines. Subscribers who lived in higher-income zip codes were more likely to receive oseltamivir during both seasonal and pandemic influenza periods, but the association was weaker during the pandemic. These differences could not be explained by confounding by indication. Analyses that adjusted for county-level rates of influenza diagnoses during the pandemic did not eliminate the income–oseltamivir relationship. In fact, after we controlled for H1N1 diagnosis rates, the association grew even stronger.
We also observed a large increase in the overall rate of oseltamivir filling across the 3 flu seasons. The proportion of subscribers receiving oseltamivir increased from 0.5% in the 2006–2007 season to 1.1% in the 2007–2008 season and 2.8% during the pandemic flu season, which was much longer, overlapped with 2 winter flu seasons, and included a higher proportion of subscribers from the South and of older age. However, even with these increases, fewer than 3% of subscribers filled a prescription for oseltamivir during the pandemic flu season. It is likely that even fewer actually used oseltamivir, as data from the United Kingdom indicated that 50% of the oseltamivir dispensed during the pandemic went unused.17
The low demand for antivirals reflects the fact that the H1N1 pandemic was less severe than many had feared.12 Despite concerns about widespread oseltamivir shortages and the need to limit distribution, the only major shortages of oseltamivir were for the pediatric formulation. Thus, the observed differences in access to oseltamivir probably did not result in large differences in patients’ outcomes across income levels. However, problems observed in the response to the H1N1 pandemic would likely be magnified if a future pandemic were worse than what occurred in 2009. Indeed, the 2012–2013 influenza season caused significant morbidity and mortality in many locations, with mortality levels similar to what was seen during the 2009 pandemic.18 Hospital overcrowding and patients’ difficulty in obtaining treatment were also reported.19 In this context, the lessons of the 2009 pandemic are critical for planning improved responses for the immediate future.
Our study did not aim to assess why those who live in higher-income zip codes would be more likely to receive treatment, but a number of possibilities could explain this finding. Considering that the pandemic was far less virulent than was feared, the increased likelihood of oseltamivir receipt in high-income areas may represent overprescribing to those in wealthier neighborhoods rather than an access problem for those in lower-income areas. Access to health care, more generous health care and pharmacy benefits, and the ability to exert pressure on providers to prescribe oseltamivir may also have been a factor. Media outlets reported that some companies provided oseltamivir for their employees, and treatment was not necessarily dispensed in accordance with CDC recommendations.20 Poor health literacy and lack of understanding of the importance of therapy can limit access for patients with lower socioeconomic status.21 During the H1N1 pandemic, this problem may have been exacerbated by the lack of a targeted communications strategy to promote appropriate and equitable use. Concerns about the cost of the medication22 or difficulties with transportation to providers or pharmacies may also have contributed to this trend.
Our study is limited by the granularity of available data. Although we had information on prescription claims for oseltamivir on an individual level, we assessed both income and flu diagnosis rates on the basis of the area of an individual’s residence. Therefore, the association measures for those variables could be subject to ecological bias, implying that income and influenza diagnosis status measured at the individual level could have alternate associations with oseltamivir receipt. In addition, the prescription claims in our data may not capture all doses of oseltamivir or other antivirals that were distributed to the study population. For example, the CDC released 11 million antiviral treatment courses, primarily of oseltamivir, from the federal Strategic National Stockpile (SNS) to state health departments.23 There is little documentation available on how states distributed their SNS doses, and the distribution system varied from state to state. Some state and local health authorities used publicly purchased antivirals for uninsured or underinsured patients, although the subscribers in our database would be unlikely to receive these doses, since oseltamivir was covered by their medication insurance. Other factors that may have contributed to the likelihood of oseltamivir receipt, such as the risk of adverse events or antiviral resistance, could not be assessed in our data. The differing prevalence of resistant influenza strains across flu seasons may partly explain the increasing use of oseltamivir across flu seasons; however, there is no evidence that these risks vary with income level.
The response of the health care system to the H1N1 pandemic provides a window into our emergency response system, and should be used to highlight opportunities for improvement. Our study demonstrates an association between income and receipt of both treatment and prophylactic doses of oseltamivir, but it cannot elucidate the mechanism of this association. Therefore, additional studies with greater clinical detail would be needed to inform future policy actions. Such policies may rely on mathematical optimization models that have been developed for distribution of the antiviral SNS during the early stages of a pandemic.24 Front-line clinicians may need to take a more active role in implementing a distribution strategy and ensuring that antiviral prescriptions are directed to patients with valid indications. Retail pharmacies may also play a more proactive role in counseling patients by using data at their disposal to target guidance to those at greatest risk. Strategies for educational outreach to target low-income neighborhoods, to reduce financial barriers to treatment, and to stress the importance of equitable distribution and prevent stockpiling may also be considered.
Acknowledgments
This research was funded by an unrestricted grant from CVS Caremark to the Brigham and Women’s Hospital.
We thank Elaine Kilabuk and Kellie Swanton for their assistance with the article.
Human Participant Protection
The institutional review board of Brigham and Women’s Hospital approved this study.
References
- 1.Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 3.Greene SK, Shay DK, Yin R et al. Patterns in influenza antiviral medication use before and during the 2009 H1N1 pandemic, Vaccine Safety Datalink Project, 2000–2010. Influenza Other Respir Viruses. 2012;6(6):e143–e151. doi: 10.1111/j.1750-2659.2012.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. 2009 Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed November 10, 2012. [Google Scholar]
- 5.Blumenshine P, Reingold A, Egerter S, Mockenhaupt R, Braveman P, Marks J. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis. 2008;14(5):709–715. doi: 10.3201/eid1405.071301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Pandemic influenza vaccine manufacturing process and timeline. 2009 Available at: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090806/en/index.html. Accessed November 10, 2012. [Google Scholar]
- 7.Muthuri SG, Myles PR, Venkatesan S, Leonardi-Bee J, Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A (H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207(4):553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uscher-Pines L, Duggan P, Garoon J, Karron R, Faden R. Social justice and disadvantaged groups in an influenza pandemic. Hastings Cent Rep. 2007;37(4):32–39. doi: 10.1353/hcr.2007.0064. [DOI] [PubMed] [Google Scholar]
- 9.Sydenstricker E. The incidence of influenza among persons of different economic status during the epidemic of 1918. Public Health Rep. 2006;121(suppl 1):191–204. [PubMed] [Google Scholar]
- 10.LaRussa P. Pandemic novel 2009 H1N1 influenza: what have we learned? Semin Respir Crit Care Med. 2011;34(2):393–399. doi: 10.1055/s-0031-1283279. [DOI] [PubMed] [Google Scholar]
- 11.Haroon S, Barbosa G, Saunders P. The determinants of health-seeking behaviour during the A/H1N1 influenza pandemic: an ecological study. J Public Health. 2011;33(4):503–510. doi: 10.1093/pubmed/fdr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An HHS Retrospective on the 2009 H1N1 Influenza Pandemic to Advance All Hazards Preparedness. Washington, DC: US Dept of Health and Human Services; 2012. [Google Scholar]
- 13.Hunter J, Rodríguez D, Aragón T. Public health management of antiviral drugs during the 2009 H1N1 influenza pandemic: a survey of local health departments in California. BMC Public Health. 2012;12:82–88. doi: 10.1186/1471-2458-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borders-Hemphill V, Mosholder A. US utilization patterns of influenza antiviral medications during the 2009 H1N1 influenza pandemic. Influenza Other Respir Viruses. 2012;6(6):e129–e133. doi: 10.1111/j.1750-2659.2012.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 16.Census Bureau. Urban area criteria for the 2010 census. Federal Register. 2011;76 Available at: https://www.federalregister.gov/articles/2011/08/24/2011-21647/urban-area-criteria-for-the-2010-census. Accessed March 7, 2014. [Google Scholar]
- 17.Singer AC, Järhult JD, Grabic R et al. Compliance to oseltamivir among two populations in Oxfordshire, United Kingdom affected by influenza A(H1N1)pdm09, November 2009—a waste water epidemiology study. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060221. e60221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Pneumonia and influenza (P&I) mortality surveillance. Weekly US Influenza Surveillance Report, 2013. Available at: http://www.cdc.gov/flu/weekly. Accessed February 13, 2013.
- 19.Kotz D, Conaboy C. Boston declares a flu emergency. Boston Globe. January 9, 2013 [Google Scholar]
- 20.McKay B. Uproar as firms get swine-flu vaccine. Wall Street Journal. November 6, 2009 A8. [Google Scholar]
- 21.Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. Am J Health Behav. 2007;31(suppl 1):S19–S26. doi: 10.5555/ajhb.2007.31.supp.S19. [DOI] [PubMed] [Google Scholar]
- 22.Shrank WH, Hoang T, Ettner SL et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166(3):332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 23.US Dept of Health and Human Services. Secretary Sebelius takes two key actions on Strategic National Stockpile. 2009 Available at: http://www.hhs.gov/news/press/2009pres/04/20090430a.html. Accessed December 29, 2012. [Google Scholar]
- 24.Dimitrov NB, Goll S, Hupert N, Pourbohloul B, Meyers LA. Optimizing tactics for use of the US antiviral strategic national stockpile for pandemic influenza. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0016094. e16094. [DOI] [PMC free article] [PubMed] [Google Scholar]


