Abstract
Water storage drums are often a primary breeding site for Aedes aegypti in developing countries. Habitat characteristics can impact both adult and larval fitness and survival, which may potentially influence arbovirus transmission. Our objective was to compare fundamental environmental differences in water drums based on the presence or absence of larvae in Trinidad. Drums were categorized according to the larval status, and if the drum was constructed of steel or plastic. Water samples were analyzed for ammonium, nitrate, and soluble reactive phosphorus (SRP). Continuous surface water temperatures were also recorded. Nutrient concentrations were considerably lower than those reported for other container breeding mosquitoes. No nutrient measured differed in concentration between drums positive compared to those that were negative for the presence of Aedes aegypti larvae. Levels of SRP and ammonium in steel drums were significantly lower than in plastic water drums. Both maximum and minimum surface temperatures were significantly lower in drums positive for the presence of larvae than in drums without larvae. Water temperatures in March and May were warmer than during October sampling periods. Larval presence is likely dependent upon the interaction among multiple biotic and abiotic factors. Despite appearance, not all water storage drums are equally suitable for Aedes aegypti development. Exposing water storage drums to direct sunlight or increased heat may be used in conjunction with sealing containers to reduce production of Aedes aegypti when draining and chemical treatment are impractical.
Keywords: nutrients, dengue control, mosquito control, stress
Introduction
Vector control remains the most effective method in combating the spread of the mosquito Aedes aegypti, the primary vector of dengue virus. About 50 to 100 million cases of dengue fever occur each year, resulting in more than 20,000 deaths (WHO, 2008). No vaccines or treatment are available. The Caribbean Epidemiology Centre (CAREC) recommends sustainable control strategies including elimination of non-essential water holding containers and covering water storage containers to prevent Ae. aegypti infestation. A clearer understanding of factors influencing mosquito presence and production and how environmental conditions encountered by immature mosquitoes in water storage drums impact potential disease transmission should lead to more effective control strategies.
Multiple studies have implicated water storage drums as key containers for Ae. aegypti production (Barrera et al., 2006; Chadee, 1984; 2004; Kling et al., 2007; Tun-Lin et al., 1995; Nathan and Knudsen, 1991). However, a common observation is that not all water drums at a given site are positive for larvae, and it is not clear as to what factors impact individual drum suitability as oviposition sites. Water in drums that have been colonized by Ae. aegypti is generally described as being predominantly clean with some detritus, but is otherwise unremarkable when compared to drums that have not been colonized (Christophers, 1960).
In Trinidad, storage of water in drums by individual households is common due to the lack of, or unpredictability in municipal sources. Water drums routinely provide water for a variety of purposes including drinking, bathing, washing and other household needs (Chadee and Rahaman, 2000). Water drums account for over 70% of Ae. aegypti positive containers and represent the key container for Ae. aegypti production throughout the country (Chadee and Rahaman, 2000). Nathan and Knudsen (1991) reported that 543 of 1605 (34%) containers suitable for Ae. aegypti larvae in the Caribbean were infested, and of these, water storage drums were the most important of these.
Previous studies have suggested that the presence and abundance of mosquito larvae in individual containers may be associated with various aspects of water chemistry (Kengluecha et al., 2005; Petersen and Chapman, 1969; Pitcarin et al., 1987; Udevitz et al., 1987; Vrtiska and Pappas, 1984). Containers supporting Ae. aegypti larvae frequently had lower average concentrations of sulfate, nitrate, soluble reactive phosphate (SRP) and lower pH than containers with the related species Aedes albopictus and Aedes vittatus, while total alkalinity and chloride concentrations were generally higher in containers with Ae. aegypti (Sehgal and Pillai, 1970). Laboratory and field studies of temperate tree holes used as breeding sites for Ochlerotatus triseriatus (formerly Aedes triseriatus) have shown that stemflow that adds nutrients and simultaneously flushes toxic metabolites can significantly increase production and speed larval development rates (Kaufman et al., 1999; 2002; 2006; Walker et al., 1991).
Water hardness, water temperature, carbonates, calcium, magnesium, phosphate, pH, and chloride were good predictors for anopheline species in tropical and temperate climates (Kengluecha et al., 2005; Pitcarin et al., 1987; Udevitz et al., 1987). Culex tarsalis abundance was predicted by calcium and magnesium levels in California rice fields, while in Iowa wetlands Cx. tarsalis production was associated with nitrate and phosphate levels (Mercer et al., 2005; Pitcarin et al., 1987).
Water drums are unique among artificial and natural larval habitats. They are usually constructed from plastic or steel, and because they are used to store water for household purposes including drinking water, they are usually well maintained and occasionally drained and cleaned. Despite routine maintenance, many drums become colonized and produce large numbers of adult Ae. aegypti. Allochthonous nutrient inputs are critical for artificial container habitats, as primary production is likely nutrient limited (Carpenter, 1983; Kesavaraju et al., 2007). Rainfall, debris collected from eaves and roofs, as well as leaves and other organic material falling or blowing into drums are the most likely sources of new nutrient inputs. It is well known that nutrient availability may limit rates of algal and microbial production in aquatic ecosystems and significantly influence food webs (Hecky and Kilham, 1988; Rhee, 1978; Schindler, 1977).
Mosquito production and development is regulated through complex interactions of biotic and abiotic factors in water drum habitats. Environmental conditions in larval breeding sites, including nutrient availability, and temperature likely play a vital role in the ecology of Ae. aegypti, and subsequently dengue transmission. Mosquito body size is dependent upon intrinsic genetic factors as well as larval nutrition and temperature during development has been correlated with vector competence (Alto et al., 2008; Kierans and Fay, 1968; Kamimura et al., 2002; Reisen et al., 1997). Understanding their effects on mosquito populations is important to the development of novel control programs. Our objective was to evaluate the influence of typical water quality indicators (e.g. ammonium, nitrate, SRP) and water temperature profiles with the presence of Ae. aegypti larvae in water storage drums in Trinidad, West Indies.
Materials and Methods
Field Sampling
Trinidad has a tropical climate and is characterized by a dry season from January to May and a wet season from June to December. Average temperatures range from a low of ~22 °C to a high of ~30.5 °C year round, but are generally cooler in the rainy season. Collection of water samples were limited by logistics to March 2005 and October 2005 in Tamana, a rural community in northeast Trinidad (Fig. 1). Most households maintain several metal or plastic drums for water storage as the community has no municipal water source, and rainfall water is routinely collected as roof runoff (Fig. 2). Also, due to its isolated location, vector control efforts in the community are limited and sporadic. Water drums were inspected for the presence of Ae aegypti larvae, and larvae were identified by field technicians from the Ministry of Health’s Insect Vector Control Division. Water drums that were identified as being positive for larvae had instars 1–4 and pupae present. If possible, adjacent water drums (<2 m) were matched based on larval status (presence vs. absence) and drum type (steel vs. plastic) to minimize potential environmental differences due to location. Larval status for each drum was scored qualitatively as presence/absence.
Figure 1.
Collection sites located in Tamana, Windy Hills, Ravine Sable and Oropouche, Trinidad.
Figure 2.
Examples of typical steel (left) and plastic (right) water drums that provide habitats for Ae. aegypti larvae.
We collected approximately equal numbers of water samples from both steel and plastic drums. One water sample per drum was obtained by filling a 50 ml nalgene jar with water that had been collected in the middle of the drum and filtered using a 40 cc syringe adapted with a 0.45 μm glass fiber filter (GFF). Water samples were kept on ice during transport and thereafter stored at −20°C until laboratory analysis.
Continuous temperatures were recorded in water drums in March 2005, May 2005, October 2007, and October 2008 using Stowaway® TidbiT® (TBI32-05+37) and Hobo® Pendant Temperature (UA-002-64) loggers (Onset Computer Corporation, Pocasset, MA). Data loggers recorded temperature every 10–15 min over 2–3 days. During the 2005 sampling periods in March and May in Tamana, two probes were placed in each water drum, the first was located ~20 cm from the surface of the water and the second was placed ~20 cm from the bottom of the drum. In 2007, a single probe was placed ~20 cm from the surface of the water in neighborhoods in Ravine Sable and Oropouche in central and southwestern Trinidad, respectively. In 2008, water surface temperature was recorded in the same matter in the Windy Hills district outside of Curepe.
Water Chemistry Analyses
Ammonium (NH4+) concentrations were determined using the colorimetric phenylhypochlorite technique (Solorzano, 1969) using a 10 cm cell on a Shimadzu UV1601 spectrophotometer (Columbia, MD) at 630 nm (detection limit=1 μg N L−1; APHA, 1995). Phosphate (PO4−3) concentrations were measured as soluble reactive phosphorus (SRP) using the molybdate-antimony method (Murphy and Riley, 1962) also using a spectrophotometer. Nitrate (NO3−) was measured using ion chromatography (Dionex Model DX600, Sunnyvale, CA) equipped with a conductivity detector, AS14A analytical and guard columns, and a 500 μL injection loop (APHA 1995).
Statistical Analyses
Statistical analyses were performed using Stata 8 and Graphpad Prism version 4.00 (StataCorp, 2003). Analysis of Variance (ANOVA) was used to investigate the relationships between nutrient concentrations among water drums (steel vs. plastic), month of sample collection and larval Ae. aegypti presence. Bartlett’s test for equal variances and the Shapiro-Francia normality test revealed significant departures from the assumptions of normality and homogeneity of variances, all data were then transformed using the natural log (ln). The data were partitioned by category and examined for outliers. Two nitrate observations exceeded the 95% quantile but were not significant outliers according to Grubbs’ test (Grubbs, 1969; http://www.graphpad.com/quickcalcs/Grubbs1.cfm (accessed January 2009); Iglewicz and Hoaglin,1993). Samples were collected from some of the same barrels in March and October, but we did not treat them as repeated measures because individual barrels are periodically drained and cleaned. Total dissolved inorganic nitrogen (DIN) was calculated by ln transforming the sum of ammonium and nitrate concentrations. ANOVA was used to examine differences in DIN by category (drum material, and by the presence of larvae). Analysis of water temperature data included the calculation of the magnitude of temperature difference (maximum temperature–minimum temperature over ~48 hr in a single drum.), as well as maximum and minimum temperatures. Effects of temperature were analyzed using ANOVA, P<0.05 was considered significant while P<0.1 was considered marginally significant.
Results
Water Chemistry
In March 2005, water samples were collected from 12 drums: 2 of 5 plastic drums were positive for Ae. aegypti larvae, while 4 of 7 steel drums were positive. In October 2005, samples were collected from 29 drums: 8 of 15 plastic drums and 9 of 14 steel drums were positive for larvae. As previously reported for Ae. aegypti breeding sites (Christophers, 1960), we observed little obvious organic debris in any of the sampled drums. The effect of seasonality (i.e, March vs. October, 2005) on nutrient concentrations was not significant (data not shown).
Although inorganic nutrient concentrations were variable among individual drums, especially within drum type (plastic vs. steel), there were differences across drum type. Ammonium concentrations were significantly different between steel and plastic drums (F1,36 = 15.35, P = 0.0004, Table 1). In general, ammonium levels in plastic drums were ~3.5-fold higher than steel (Fig. 3A). There was no significant influence of ammonium concentration on mosquito larval presence (F1,36 = 0.64, P = 0.4279), and the interaction between mosquito larval presence and drum material was also not significant (F1,36 = 0.25, P = 0.6171, Table 1).
Table 1.
Results of Two-way ANOVA comparing effects on larval presence, of construction material of water drums and inorganic nutrients including ammonium, nitrate, dissolved inorganic nitrogen (DIN), and soluble reactive phosphate, (SRP)
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| Ammonium | ||||
| Larval presence | 1, 36 | 1.22 | 0.64 | 0.4279 |
| Drum material | 1, 36 | 29.16 | 15.35 | 0.0004 |
| Larvae x Drum | 1, 36 | 0.48 | 0.25 | 0.6171 |
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| Nitrate | ||||
| Larval presence | 1, 35 | 2.57 | 1.72 | 0.1984 |
| Drum material | 1,35 | 2.88 | 1.92 | 0.1743 |
| Larvae x Drum | 1,35 | 0.00 | 0.00 | 0.9603 |
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| DIN | ||||
| Larval presence | 1, 34 | 0.98 | 0.66 | 0.4225 |
| Drum material | 1, 34 | 0.00 | 0.00 | 0.9813 |
| Larvae x Drum | 1, 34 | 0.03 | 0.02 | 0.8887 |
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| SRP | ||||
| Larval presence | 1, 37 | 0.25 | 0.14 | 0.7122 |
| Drum material | 1, 37 | 15.13 | 8.28 | 0.0066 |
| Larvae x Drum | 1, 37 | 5.60 | 3.07 | 0.0882 |
Figure 3.
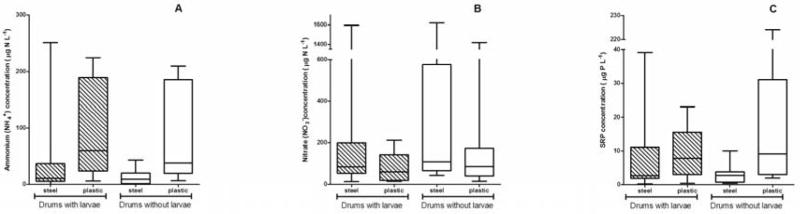
Variability in nutrient concentrations among water storage drums by larval status (present or absent) and by drum material (steel or plastic). (A) ammonium (NH4+) concentration (μg N L−1), (B) nitrate (NO3−) concentration (μg N L−1), (C) soluble reactive phosphorous (SRP) concentration (μg P L−1). Box plots identify medians and quartiles, with whiskers representing 10th and 90th percentiles.
Unlike ammonium, there were no significant differences in nitrate concentrations between steel and plastic drums (F1,35 = 1.92, P = 0.1743). Nitrate concentrations in drums with larva did not differ significantly from those drums without larvae (F1,35 = 1.72, P = 0.1984, Table 1), although concentrations were generally higher in steel drums without larvae (Fig. 3B). There was no significant interaction between drum type and larval status (F1,35 = 0.00, P = 0.9603).
Total dissolved inorganic nitrogen (DIN) did not differ between steel and plastic drums (F1,34 = 0.66, P = 0.4225, Table 1). No significant differences in DIN were found between water drums with or without larvae (F1,34 = 0.00, P = 0.9813, Table 1), and the interaction term was not significant (F1,34 = 0.02, P = 0.8887, Table 1).
Concentrations of SRP were low in all drums but did not significantly differ between drums with larvae and without larvae (F1,37 = 0.14, P = 0.7122, Table 1). As for ammonium, SRP concentrations varied significantly between steel and plastic drums (F1,37 = 8.28, P = 0.0066), with steel drums containing ~5.5-fold greater concentrations than plastic drums (Fig. 3C). Further, the interaction between larval status and drum material was suggestive (F1,37 = 3.07, P = 0.0882), wherein drums positive for larvae contained ~1.4-fold lower SRP concentrations than drums without larvae in both metal and plastic drums, respectively (Fig. 3C).
Water Temperature
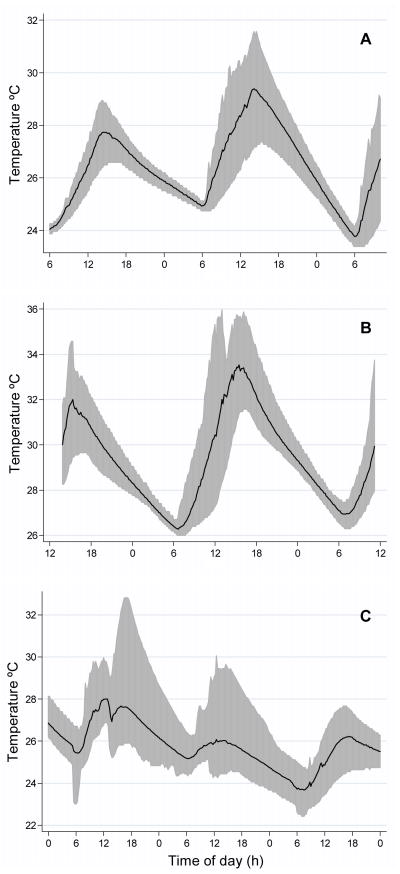
Water temperature data was recorded from 39 drums during March and May 2005 as well as October 2007 and 2008. In March 2005, we monitored temperature in 5 drums; 4 were steel drums of which 1 was positive for the presence of larvae and the remaining drum was plastic and was negative for larvae. Surface temperatures in water drums from March ranged from 23.4–31.6 °C (Fig. 4A). Probes placed in the bottom of drums in March did not reach as high of temperatures compared to surface probes, and ranged from 23.2–29.1 °C (data not shown). In May 2005, 7 drums were examined, 2 steel and 5 plastic. Among those, 1 steel drum and 1 plastic drum were positive for Ae. aegypti. Overall temperatures in May were higher than in March, ranging from 26.0 °C to 36.0 °C near the surface (Fig. 4B). Similar to March, probes placed near the bottom were buffered and did not reach as high of temperature as surface probes but were warmer than bottom probes in March (data not shown). The 4 larvae-positive, ½-filled drums from May ranged from 27.1–34.5°C (data not shown).
Figure 4.
Range of surface water temperatures (~20 cm from surface) recorded in (A) March 2005, (B) May 2005, and (C) October 2007 and 2008 regardless of larval status or drum type. The black line indicates the average water temperature and the gray shading indicates minimum and maximum temperatures recorded.
In October 2007, 9 drums were monitored for temperature fluctuations, 5 steel and 4 plastic and all were positive for larvae. In October 2008, 14 drums were monitored: of these, 8 were plastic with one negative for larvae while all 6 steel drums were positive for larvae. Overall, October water temperatures from 2007 and 2008 ranged from 22.4 °C to 32.8 °C (Fig. 4C). Water temperature oscillations were more variable in October than in March or May (Fig. 4A–4C).
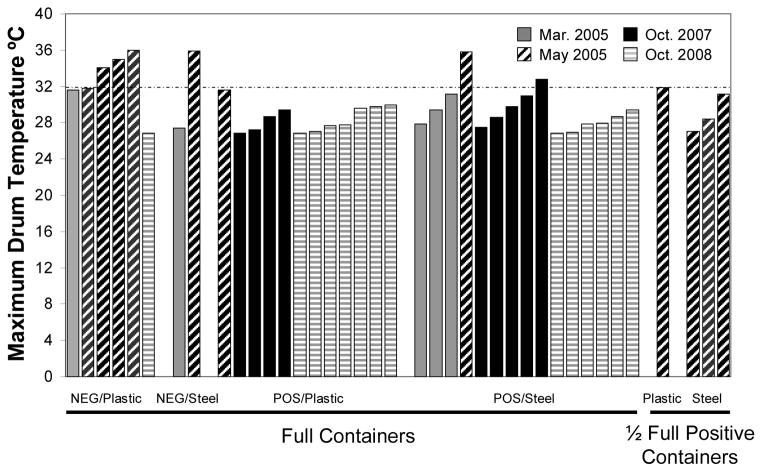
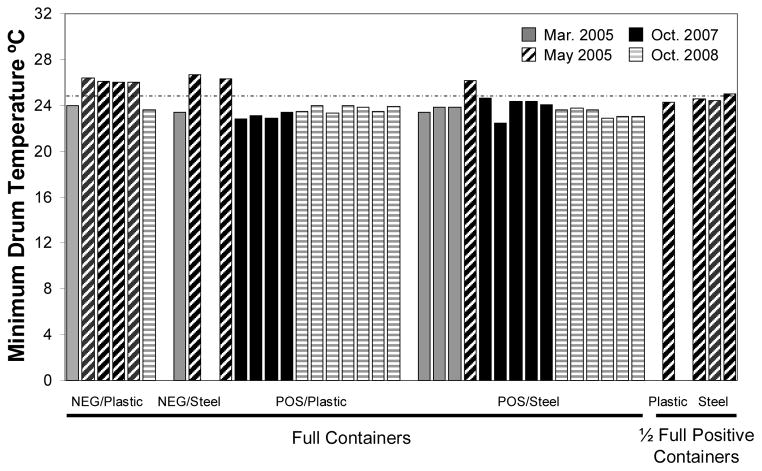
Maximum water temperatures at the surface of water drums by drum category and by sampling date are displayed in Fig. 5. All drums positive for the presence of larvae, with the exception of two steel drums sampled in May 2005 and October 2007, did not reach a maximum temperature over 32°C. However, 4 of the 8 (50%) of the negative drums exceeded 32°C at least once during the sampling period. Similar observations were made for minimum surface water temperature. Five of the 8 (62.5%) negative drums’ minimum water temperature was warmer than 25°C, while only 3 of the 31 (0.1%) larvae positive drums had minimum water temperatures exceed 25°C (Fig. 6). The removal or inclusion of 4 water drums that were filled ~1/2 from May 2005 (Fig. 5 and 6) did not influence the results of the analyses.
Figure 5.
Maximum recorded temperatures in water drums by larval status, material and date recorded. Solid gray = March 2005, black stripes = May 2005, Solid black = October 2007, Gray stripes = October 2008.
Figure 6.
Minimum recorded temperatures in water drums by larval status, material and date recorded. Solid gray = March 2005, black stripes = May 2005, Solid black = October 2007, Gray stripes = October 2008.
The magnitude of the temperature change in drums with larvae compared to those without was suggestive for significance (F1,35 = 3.78, P = 0.0599, Table 2). No significant differences were observed between steel and plastic drums (F1,35 = 0.03, P = 0.8752, Table 2), nor was the interaction term between larvae status and drum material significant (F1,24 = 0.32, P = 0.5736, Table 2). Maximum water temperature in drums was significantly different in those with larvae and without (F1,35 = 7.35, P = 0.0103, Table 2). No significant difference in maximum temperature was detected between steel and plastic water drums (F F1,35 = 0.03, P = 0.8697, Table 2). The interaction between drum type and larval presence was also not significant (F1,35 = 0.44, P = 0.5129, Table 2). Minimum surface water temperature was significantly lower in drums that were positive for the presence of larvae (F1,35 = 8.86, P = 0.0053, Table 2) than in those without. Minimum temperature did not differ by drum material (F1,35 = 0.01, P = 0.9153, Table 2), and no significant interaction between larval presence and drum material was observed (F1,35 = 0.30, P = 0.5880, Table 2).
Table 2.
Results of Two-way ANOVA comparing effects of temperature (maximum, minimum, and magnitude) and drum material on larval presence.
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| Magnitude of Temperature Swing | ||||
| Larval presence | 1, 35 | 14.01 | 3.78 | 0.0599 |
| Drum material | 1, 35 | 0.09 | 0.03 | 0.8752 |
| Larvae x Drum | 1, 35 | 1.19 | 0.32 | 0.5736 |
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| Maximum Temperature | ||||
| Larval presence | 1, 35 | 45.60 | 7.35 | 0.0103 |
| Drum material | 1, 35 | 0.17 | 0.03 | 0.8697 |
| Larvae x Drum | 1, 35 | 2.71 | 0.44 | 0.5129 |
| SOURCE OF VARIATION | DF | MS | F-value | P-value |
|---|---|---|---|---|
| Minimum Temperature | ||||
| Larval presence | 1, 35 | 9.06 | 8.86 | 0.0053 |
| Drum material | 1, 35 | 0.01 | 0.01 | 0.9153 |
| Larvae x Drum | 1, 35 | 0.31 | 0.30 | 0.5880 |
Discussion
The variable yet surprisingly low concentrations of inorganic nutrients, especially SRP, found in water drums and the general lack of association with Ae. aegypti larval presence in Trinidad supports evidence for the complex and dynamic relationship of container habitats and their suitability for mosquito development. Our observed nutrient concentrations for larvae positive containers were markedly lower (i.e. 35, 66, and 1989-fold lower for nitrate, ammonium and SRP, respectively) compared to what has been observed in tree hole larval habitats commonly used by Oc. triseriatus (Walker et al., 1991). The differences in nutrient concentrations between drums and natural tree holes is interesting, but not surprising. Tree holes are replenished by nutrient rich stemflow and liquids from the vascular system of the tree, whereas drums are replenished solely by rainfall, runoff, random windblown materials, or physical replacement by humans (Copeland, 1984; 1987; Kaufman et al., 1999; 2002; 2006; Walker et al., 1991). Water drums might not be as sensitive to new rainfall inputs due to their larger volumes; if there is an influence, each new rainfall event likely serves to dilute nutrient concentrations rather than increase them. There is a paucity of studies examining nutrient availability in Ae. aegypti larval habitats; samples collected from temporary rain pools in Delhi, India used as breeding sites by Ae. aegypti had an average nitrate concentration of 10 mg N L−1, and 0.95 mg P L−1 of phosphate, which were respectively, ~50-fold and ~100-fold higher in concentration than we detected in water drums in rural Trinidad. This difference may be explained by the type of larval habitat and nutrient rich runoff collecting in the pools in India (Sehgal and Pillai, 1970), and the likely concentration effect of seasonal drying. These contrasting results also suggest that Ae. aegypti larvae can successfully complete development across a large gradient of nutrient concentrations, and nutrient availability may have a stronger impact on mosquito production and size as opposed to the determination of the suitability of the site for oviposition.
Of the three nutrients examined, ammonium, and SRP levels were significantly lower in plastic drums than in steel drums (Table 1, Fig. 3). SRP concentrations are commonly assumed to correlate strongly with primary production in aquatic ecosystems, and in non-polluted freshwater systems phosphorous is often the major limiting nutrient of primary production (Schindler, 1977). Although not significant, drums with larvae present had lower SRP concentrations than those without larvae and while the form of inorganic nitrogen differed in plastic vs. steel drums, the total inorganic N available for bacterial or algal growth did not differ across drum type. The relative abundance of total inorganic nitrogen available may indicate that phosphorous was a limiting nutrient in drums with larvae (Fig. 3C and 3D).
Undoubtedly nutrient availability in breeding habitats is important in mosquito development. It is most likely that the impact of nutrient availability will be seen in production of adult mosquitoes, shortened developmental periods, or increased body size of adults as opposed to the simple presence or absence of larvae (Barrera et al., 2006; Kaufman et al., 2002). Other nutrients not examined in this study may also impact the presence and production of Ae. aegypti as well as the abundance and composition of bacteria and algae in the microbial community. In laboratory and field experiments, Kaufman et al. (2002; 2007) reported that labile carbon was the primary stimulus of bacterial growth, while the addition of nitrate and phosphorous stimulated bacterial production on leaf surfaces. Abiotic factors including light and pH may also indirectly impact mosquito presence and development (Barrera et al., 2006).
Larval rearing temperatures can potentially have a major impact on disease transmission by influencing body size, development time, and production (Kierans and Fay, 1968; Atkinson, 1994; Kamimura et al., 2002; Reisen et al., 1997). The relationship between elevated temperatures and shortened development duration, could increase the abundance of host seeking adults (Kamimura et al., 2002; Keirans and Fay, 1968). Increased mortality and increased development time have been reported in Ae. aegypti when larvae are reared in relatively low or high temperature conditions (Bar-Zeev, 1958; Kamimura et al., 2002; Tun-Lin et al., 2000). Shorter development periods can affect the size of adult females, which is believed to be an important aspect of vector competence, vectorial capacity, and overall vector biology including longevity, biting persistence, and blood meal frequency (Alto et al., 2008; Brust, 1967; Haramis, 1985; Maciel-de-Freitas et al., 2007; Nasci, 1986; 1991; Nasci and Mitchell, 1994; Scott et al., 2000; Schneider et al., 2007; Tun-Lin et al., 2000; Yan et al., 1997).
Significantly warmer maximum (P = 0.0103) and minimum (P=0.0053) temperatures were observed in drums without larvae, and magnitude of temperature swing was suggestive (P = 0.05999). It was surprising to see that few water drums positive for larvae exceeded 32 °C (2 of 31; 6.5%), while 4 out of 8 larvae negative drums (50%) exceeded 32 °C. Similarly, minimum temperatures were lower in drums with larvae than in those without. One explanation is the increased number of positive drums that were profiled in both October sampling periods which was markedly cooler than in March or May. However, during both October sampling periods attempts were made to find larvae negative water drums with limited success. Our observations from the field are supported by laboratory results from Tun-Lin et al. (2000) where a survival curve was constructed across a gradient of increasing temperatures. Optimal survival rates for an Ae aegypti strain were obtained between 20 °C and 30 °C and a decrease in survival was observed, starting at ~30 °C to ~35 °C (Tun-Lin et al., 2000). Although mosquitoes have been successfully reared at high temperatures in laboratory settings, it is possible that elevated temperatures or extreme fluctuations in temperature are detrimental to normal larval development in the field (Kamimura et al., 2002; Keirans and Fay, 1968; Mourya et al., 2004; Yadav et al., 2005). In Puerto Rico, pupal production was negatively associated with water temperatures, and of interest, Barrera et al. (2006) recorded water temperatures as high as 40 °C. Increased water temperatures may also be detrimental to potential food sources including bacteria, algae, and fungi. There are a limited number of studies that have examined the variation in diel temperatures of larval habitats, although this is certainly an important factor impacting vector biology and should be pursued.
Certain stresses, including temperature can modulate heat-shock proteins (hsps), revealing new phenotypes capable of becoming independent of the inducing stress after several generations (Rutherford and Lindquist, 1998; Sangster et al., 2008). Adult females from Ae. aegypti larvae exposed to extreme heat shock (45.5°C for 10 min) were more susceptible to DENV-2 infection. Translation and transcription levels of Hsp70 in larvae did not differ from controls in high- and low-susceptible mosquitoes, however, higher expression levels were observed in a DDT resistant strain (Yadav et al., 2005). In a similar study, Mourya et al. (2004) reported a positive correlation between increasing intervals of larval temperature stress and increased Chikungunya virus (CHIK) dissemination among adult females. Data from the same study suggested that the expression of immune responsive genes, including cercropin and nitric oxide synthetase, were affected by heat shock, possibly impacting susceptibility (Mourya et al., 2004). Currently, it is unknown how ambient heat shock inducing temperatures experienced by larvae may affect DENV susceptibility in field populations, or even whether temperatures reached in field conditions are high enough to induce heat shock protein expression. Further investigation is needed to elucidate what role naturally occurring environmental stresses and heat shock proteins play in arbovirus transmission.
In summary, this study expands the current understanding on the interaction between water column nutrient concentrations and Ae. aegypti production in water drum habitats. No significant differences in nutrient concentrations were found in drums with larvae and those without, however, ammonium and SRP did differ by drum material. Maximum and minimum surface water temperatures were different in drums with larvae as compared to those without larvae. It was not uncommon for water temperatures in drums to vary ~6–8°C. This was more common in March and May when water temperatures were warmer than during October sampling periods. There is surprisingly limited literature on environmental conditions of larval breeding habitats for Ae. aegypti. Further research is needed, considering the potential influence of the larval environment on disease transmission. Our results indicate that increased water temperatures in water storage drums are detrimental to larval presence. It is possible that water temperatures in drums that are exposed to direct sunlight exceed optimal larval development temperatures and limit the larval production. Placing water storage drums in areas of intense sunlight exposure may enhance current practices of sealing or covering drums, especially when draining or treating with chemical larvicides is not possible.
Acknowledgments
We thank Sarah Epstein in the Severson laboratory, in addition to Tim Hoellein, Mia Stephen, Laura Taylor, Natalie Grifiths, and Sarah Roley in the Tank Laboratory at the University of Notre Dame for their assistance and support. In addition, we thank Stephen “Billy” Deonarine, Lester James and Larry Smith with the Insect Vector Control Division, Ministry of Health in Trinidad, West Indies. This research was funded by grant RO1-AI059342 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
References
- Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus and dissemination. American Journal of Tropical Medicine and Hygiene. 2008;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- Easton AD, Clesceri LS, Greenberg AE, editors. APHA. Standard methods for the examination of water and wastewater. American Public Health Association; Washington D.C: 1995. [Google Scholar]
- Atkinson D. Temperature and organism size—a biological law for ectotherms. Advances in Ecological Research. 1994;25:1–58. [Google Scholar]
- Bar-Zeev M. The temperature on the growth rate and survival of the immature stages of Aedes aegypti (L.) Bulletin of Entomological Research. 1958;49:157–163. [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. Journal of Medical Entomology. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brust RA. Weight and development time of different stadia of mosquitoes reared at various constant temperatures. Canadian Entomologist. 1967;99:986–993. [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Chadee DD. An evaluation of temephos in water drums in Trinidad, W.I. Mosquito News. 1984;44:51–53. [Google Scholar]
- Chadee DD. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. Bulletin of Entomological Research. 2004;94:201–207. doi: 10.1079/ber2004297. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Rahaman A. Use of water drums by humans and Aedes aegypti in Trinidad. Journal of Vector Ecology. 2000;25:28–35. [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L) the yellow fever mosquito. Cambridge University Press; Cambridge, United Kingdom: 1960. [Google Scholar]
- Copeland RS. Treehole-breeding mosquitoes in northern Indiana: species composition of three types of larval habitat. Proceedings of the 8th Annual Meeting of the Indian Vector Control Association. 1984;8:20–28. [Google Scholar]
- Copeland RS. Dissertation. University of Notre Dame; Notre Dame, Indiana, USA: 1987. Habitat segregation and life history patterns of the Culicidae of treeholes in northern Indiana. [Google Scholar]
- Dionex. Determination of Nitrite and Nitrate in drinking water using chemically suppressed ion chromatography AU132. Dionex Corporation; Sunnyvale, CA: Application Note 132. [Google Scholar]
- GraphPad Prism version 5.02 for Windows. GraphPad Software; San Diego California: [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Haramis LD. Larval nutrition, adult body size, and the biology of Aedes triseriatus. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Vero Beach, FL: Florida Medical Entomology Laboratory; 1985. pp. 431–437. [Google Scholar]
- Hecky RE, Kilham P. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography. 1988;33:796–822. [Google Scholar]
- Iglewicz B, Hoaglin DC. How to Detect and Handle Outliers. American Society for Quality Control; Milwaukee, WI: 1993. [Google Scholar]
- Kamimura K, Matsuse IT, Takahashi H, Komukai J, Fukuda T, Suzuki K, Aratani M, Shira Y, Mogi M. Effect of temperature on the development of Aedes aegypti and Aedes albopictus. Medical Entomology and Zoology. 2002;53:53–58. [Google Scholar]
- Kaufman MG, Walker ED, Smith TW, Merritt RW, Klug MJ. Effects of larval mosquitoes (Aedes triseriatus) and stemflow on microbial community dynamics in container habitats. Applied and Environmental Microbiology. 1999;65:2661–2673. doi: 10.1128/aem.65.6.2661-2673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on the microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquatic Microbial Ecology. 2002;29:73–88. [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM, Walker ED. Importance of algal biomass to growth and development of Anopheles gambiae larvae. Journal of Medical Entomology. 2006;43:669–676. doi: 10.1603/0022-2585(2006)43[669:ioabtg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kengluecha A, Singhasivanon P, Tiensuwan M, Jones JW, Sithprasasna R. Water quality and breeding habitats of Anopheline mosquito in northwestern Thailand. Southeast Asian Journal of Tropical Medicine and Hygiene. 2005;36:46–53. [PubMed] [Google Scholar]
- Kesavaraju B, Yee DA, Juliano SA. Interspecific and intraspecific in foraging preferences of container-dwelling mosquitoes. Journal of Medical Entomology. 2007;44:215–221. doi: 10.1603/0022-2585(2007)44[215:iaidif]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Fay RW. Effect of food and temperature on Aedes aegypti (L.) and Aedes triseriatus (SAY) larval development. Mosquito News. 1968;28:338–341. [Google Scholar]
- Kling LJ, Juliano SA, Yee DA. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: detritus type, amount, and water nutrient differences. Journal of Vector Ecology. 2007;32:207–217. doi: 10.3376/1081-1710(2007)32[207:lmcidv]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annual Review of Genetics. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Codeco CT, Lourenco-de-Oliveira R. Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Medical and Veterinary Entomology. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Mercer DR, Sheeley SL, Brown EJ. Mosquito (Diptera: Culicidae) development within microhabitats of Iowa wetland. Journal of Medical Entomology. 2005;42:685–693. doi: 10.1603/0022-2585(2005)042[0685:MDCDWM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mourya DT, Yadav P, Mishra AC. Effect of temperature stress on immature stages and susceptibility of Aedes aegypti mosquitoes Chikungunya virus. American Journal of Tropical Medicine and Hygiene. 2004;70:346–350. [PubMed] [Google Scholar]
- Murphy J, Riley J. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta. 1962;27:31–36. [Google Scholar]
- Nasci RS. The size of emerging and host-seeking Aedes aegypti and the relationship of size to blood-feeding success in the field. Journal of the American Mosquito Control Association. 1986;2:61–62. [PubMed] [Google Scholar]
- Nasci RS. Influence of larval and adult nutrition on biting persistence in Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1991;28:522–526. doi: 10.1093/jmedent/28.4.522. [DOI] [PubMed] [Google Scholar]
- Nasci RS, Mitchell CJ. Larval diet, adult size and susceptibility of Aedes aegypti (Diptera: Culicidae) to infection with Ross River virus. Journal of Medical Entomology. 1994;31:123–126. doi: 10.1093/jmedent/31.1.123. [DOI] [PubMed] [Google Scholar]
- Nathan MB, Knudsen AB. Aedes aegypti infestation characteristics in several Caribbean countries and implications for integrated community-based control. Journal of the American Mosquito Control Association. 1991;7:400–404. [PubMed] [Google Scholar]
- Petersen JJ, Chapman HC. Chemical factors of water in tree holes and related breeding of mosquitoes. Mosquito News. 1969;29:29–36. [Google Scholar]
- Pitcairn MJ, Washino RK, Palchick S. Factors affecting larval mosquito abundance in northern California ricefields. California Mosquito and Vector Control Association. 1987;55:102–107. [Google Scholar]
- Rhee GY. Effects of n:p atomic ratios and nitrate limitation on algal growth, cell composition and nitrate uptake. Limnology and Oceanography. 1978;23:10–25. [Google Scholar]
- Reisen WK, Hardy JL, Presser SB. Effects of water quality on the vector competence of Culex tarsalis (Diptera: Culicidae) for western equine encephalomyelitis (Togoviridae) and St. Louis encephalitis (Flaviviridae) viruses. Journal of Medical Entomology. 1997;34:631–643. doi: 10.1093/jmedent/34.6.631. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor to phenotypic variation. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Sangster TA, Salathia N, Undurraga S, Milo R, Schellenberg K, Lindquist S, Queitsch C. Hsp90 affects the expression of genetic variation and developmental stability in quantitative traits. Nature. 2008;105:2963–2968. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal SS, Pillai MKK. Preliminary studies on the chemical nature of mosquito-breeding waters in Delhi. World Health Organization Bulletin. 1970;42:647–650. [PMC free article] [PubMed] [Google Scholar]
- Schneider JR, Mori A, Romero-Severson J, Chadee DD, Severson DW. Investigations of dengue-2 susceptibility and body size among Aedes aegypti populations. Medical and Veterinary Entomology. 2007;21:370–376. doi: 10.1111/j.1365-2915.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- Schindler DW. Evolution of phosphorous limitation in lakes. Science. 1977;195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. Journal of Medical Entomology. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Solorzano L. Determination of ammonium in natural waters by the phenolhypochlorite method. Limnology and Oceanography. 1969;14:799–801. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 8. College Station, TX: StataCorp LP; 2003. [Google Scholar]
- Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Medical and Veterinary Entomology. 2000;14:31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W, Kay BH, Barnes A. Understanding productivity, a key to Aedes aegypti surveillance. American Journal of Tropical Medicine and Hygiene. 1995;53:595–601. doi: 10.4269/ajtmh.1995.53.595. [DOI] [PubMed] [Google Scholar]
- Udevitz MS, Bloomfield P, Apperson CS. Prediction of the occurrence of four species of mosquito larvae with logistic regression on water-chemistry variables. Environmental Entomology. 1987;6:281–285. [Google Scholar]
- Vrtiska LA, Pappas LG. Chemical analysis of mosquito larval habitats southeastern Nebraska. Mosquito News. 1984;44:506–509. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- WHO. Dengue and dengue heamorrhagic fever. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- Yadav P, Barde PV, Gokhale MD, Vipat V, Mishra AC, Pal JK, Mourya DT. Effect of temperature and insecticide stresses on Aedes aegypti larvae and their influence on the susceptibility of mosquitoes to dengue-2 virus. Southeast Asian Journal of Tropical Medicine and Hygiene. 2005;5:1139–1144. [PubMed] [Google Scholar]
- Yan G, Severson DW, Christensen BM. Costs and benefits of mosquito refractoriness to malaria parasites: implications for genetic variability of mosquitoes and genetic control of malaria. Evolution. 1997;51:441–450. doi: 10.1111/j.1558-5646.1997.tb02431.x. [DOI] [PubMed] [Google Scholar]