Abstract
Mammals live in a co-evolutionary association with the plethora of microorganisms that reside at a variety of tissue microenvironments. The microbiome represents the collective genomes of these co-existing microorganisms, which is shaped by host factors such as genetics and nutrients but in turn is able to influence host biology in health and disease. Niche-specific microbiome, prominently the gut microbiome, has the capacity to effect both local and distal sites within the host. The gut microbiome has played a crucial role in the bidirectional gut-brain axis that integrates the gut and central nervous system (CNS) activities, and thus the concept of microbiome-gut-brain axis is emerging. Studies are revealing how diverse forms of neuro-immune and neuro-psychiatric disorders are correlated with or modulated by variations of microbiome, microbiota-derived products and exogenous antibiotics and probiotics. The microbiome poises the peripheral immune homeostasis and predisposes host susceptibility to CNS autoimmune diseases such as multiple sclerosis. Neural, endocrine and metabolic mechanisms are also critical mediators of the microbiome-CNS signaling, which are more involved in neuro-psychiatric disorders such as autism, depression, anxiety, stress. Research on the role of microbiome in CNS disorders deepens our academic knowledge about host-microbiome commensalism in central regulation and in practicality, holds conceivable promise for developing novel prognostic and therapeutic avenues for CNS disorders.
1. Introduction to microbiome
Human beings, like other mammals, live in a co-evolutionary association with huge quantities of commensal microorganisms resident on the exposed and internal surfaces of our bodies. The entirety of microorganisms in a particular habitat is termed microbiota, or microflora. The collective genomes of all the microorganisms in a microbiota are termed microbiome(Cryan and Dinan, 2012; Round and Mazmanian, 2009). Commensal microbiota and microbiome outnumber human somatic cells and genome, respectively by approximately 10-100:1 (Belkaid and Naik, 2013). The microbiota composition is influenced by temporal and spatial factors. Temporally, the human fetal gut is sterile but colonization begins immediately after birth and is affected by route of delivery, maternal transfer, diet, environmental stimuli and antibiotic usage (Sekirov et al., 2010). However, the presence of bacteria has been detected in the meconium from healthy neonates, which might hint the existence of prenatal mother-to-child transfer of microbiota(Jimenez et al., 2008; Valles et al., 2012). By 1 year of age, an idiosyncratic gut microbiome with adult-like signature is stabilized in each infant(Palmer et al., 2007). While adult gut bacterial communities vary, the concept of enterotype has been raised to classify individuals by their gut microbiota composition. Three enterotypes were characterized in human adults with relative abundance of Bacteroides, Prevotella or Ruminococcus genus(Arumugam et al., 2011). Yet, discrete enterotypes are still arguable as a later study revealed gradients of key bacterial genera(Koren et al., 2013). Whether human gut microbiota profiles fall into distinct clusters or a continuum depends on sampling strategy and methods of analysis and entails further comparison between healthy and diseased individuals.
Spatially, each body habitat is differentially dominated by specific phyla of microbiota: skin by Actinobacteria, Firmicutes and Proteobacteria; oral cavity by Bacteroidetes, Firmicutes, Fusobacteria and Proteobacteria; airway tract by Bacteroidetes, Firmicutes, and Proteobacteria; GI tract by Bacteroidetes and Firmicutes; and urogenital tract by Firmicutes (species under Lactobacillus genus)(Belkaid and Naik, 2013). Adding to the complexity, there is an uneven spatial distribution of microbiota within each specific niche. In the human GI tract, the quantity and diversity of microbiota increase from stomach to small intestine and to colon(Brown et al., 2013; Sekirov et al., 2010). Interestingly, microbiota have been identified within immune-privileged sites such as the CNS. α-proteobacteria class is reported to be the major commensals persistent in the human brain regardless of immune status(Branton et al., 2013).
While the host-microbiome interaction is not a novel concept, only recently has it been revisited by a surge of studies. Co-evolution has pre-determined that microbiota form a long-term symbiosis rather than short-term parasitism with human hosts. Yet, our prior and expanding knowledge about the effects of microbiome on host biology indicates that microbiota are not commensalistic bystanders that bring no benefit or detriment to hosts. Instead, a significant proportion of microbiota can be defined as symbionts or pathobionts, depending on whether they are mutualistic health-promoters or opportunistic pathology-inducers for hosts(Round and Mazmanian, 2009). Host-microbiota mutualism is exemplary in the gut, where gut microbiome as a joint unity can be viewed as an organ of the host(O'Hara and Shanahan, 2006). Traditionally, gut microbiome is considered to have three major categories of functions. First, it defends against pathogen colonization by nutrient competition and production of anti-microbial substances. Second, it fortifies intestinal epithelial barrier and induces secretory IgA (sIgA) to limit bacteria penetration into tissues. Third, it facilitates nutrient absorption by metabolizing indigestible dietary compounds. In line with these concepts, germ-free (GF) animals have higher susceptibility to infection but reduced digestive enzyme activities and muscle wall thickness(O'Hara and Shanahan, 2006; Round and Mazmanian, 2009). Functional metatranscriptomic analysis of human fecal microbiota demonstrated a common pattern of overrepresented genes involved in carbohydrate metabolism, energy production and synthesis of cellular components (Hemarajata and Versalovic, 2013).
The recent trend of research has focused on the fourth role of gut microbiome: guiding maturation and functionality of the host immune system. Immune defects in GF mice are evident at both structural levels, such as decreased peyer's patches, lamina propria and isolated lymphoid follicles, and at cellular levels, such as decreased intestinal CD8+ T cells and CD4+ T helper 17 (Th17) cells and reduced B cell production of secretory IgA (sIgA)(Round and Mazmanian, 2009). Th17 cells are potent mediators of mucosal immunity that produce signature cytokine IL-17, and sIgA is the principal immunoglobulin at mucosal sites that maintains barrier functions(Corthesy, 2013; Dubin and Kolls, 2008). Other immune subsets, such as Foxp3+ regulatory CD4+ T cells (Tregs), invariant natural killer T (iNKT) cells and innate lymphoid cells (ILCs), are functionally affected by microbiota at pathological conditions(Ochoa-Reparaz et al., 2009; Olszak et al., 2012; Sawa et al., 2011). Re-colonization of GF mice with a model gut commensal, Bacteroides fragilis, restored immune maturation at gut associated lymphoid tissues. Further, purified B. fragilis capsular polysaccharide A (PSA) was sufficient to expand splenic total CD4+ T cells and intestinal Foxp3+CD4 Tregs, which suggested that specific commensal antigens could drive immune regulation(Mazmanian et al., 2005; Round and Mazmanian, 2010). Gut microbiome provides diverse signals for tuning host immune status toward either effector or regulator direction, and is thus critical to peripheral immune education and homeostasis.
Microbiome at a specific niche can cast local as well as systemic effects on host biology. Disruption of a balanced composition of gut microbiome (termed dysbiosis) may cause chronic low-grade intestinal inflammation as seen in the irritable bowel syndrome (IBS) or intense intestinal autoimmunity as seen in the inflammatory bowel disease (IBD)(Collins et al., 2009; Round and Mazmanian, 2009). Dietary change can bring symptomatic improvement in IBS patients. Moreover, gut microbiome alteration was observed in IBS patients, exemplified by the reduction of species under Lactobacillus genus and Clostridium class(Kassinen et al., 2007; Malinen et al., 2005). Similarly, IBD patients showed elevated antibody titers against indigenous bacteria, a drastic change of gut microbiome, and favorable response to antibiotic intervention(Frank et al., 2007; Macpherson et al., 1996). Importantly, while genetic factors such as polymorphisms in NOD2 (nucleotide-binding oligomerization domain 2) influence susceptibility to IBD, animal studies show that dysbiosis alone suffice to induce IBD. Antibiotic depletion of microbiota cured intestinal inflammation in Tbx21-/-Rag-/- (TRUC) mice that lacked adaptive immunity and developed spontaneous IBD. Further, wild-type mice co-housed with TRUC littermates developed similar colitis symptoms(Garrett et al., 2007). Thus in the case of IBD, dysbiosis can directly lead to aberrant mucosal immunity, which in turn might maintain or exacerbate dysbiosis. On the other hand, beneficial gut bacteria can ameliorate IBD in both human studies and mouse models. Bifidobacteria, Lactobacillus and Bacteroides genera are the major components of beneficial probiotics(Round and Mazmanian, 2009). Gut microbiota-derived products and metabolites, such as B. fragilis PSA and short-chain fatty acids (SCFA), also exerted potent anti-inflammatory functions in mouse IBD models(Mazmanian et al., 2008; Smith et al., 2013).
Systemically, gut microbiome contributes to the etiology of experimental disease models affecting remote organ systems. This can be caused by the trafficking of immune cells stimulated at the intestinal site, including microbe-sensing APCs and adaptive immune cells, to distal tissue sites, by systemic diffusion of commensal microbial products or metabolites, or by bacterial translocation as a result of impaired barrier integrity. At the liver sites, endotoxemia-induced inflammation is responsible for diseases such as cirrhosis(Sekirov et al., 2010). At the airway mucosal sites, antibiotic modulation of gut commensals impaired protective anti-viral immunity during intranasal infection with influenza and systemic infection with lymphocytic choriomeningitis virus (LCMV)(Abt et al., 2012; Ichinohe et al., 2011). Gut microbiome influences various extra-intestinal autoimmune conditions as illustrated in murine models. Germ-free status confers a complete protection from spontaneous experimental autoimmune encephalomyelitis (EAE) and ankylosing spondylitis, a partial protection from spontaneous rheumatoid arthritis (RA) yet an enhanced level of spontaneous type-1 diabetes (T1D). Further, both GF and antibiotics-treated mice showed altered severity in inducible models of extra-intestinal autoimmune diseases(Berer and Krishnamoorthy, 2012; Ochoa-Reparaz et al., 2009).
In this Review, we discuss the role of microbiome, especially gut microbiome, in relation to central nervous system (CNS) disorders. We analyze how microbiome liaises the bi-directional communication between gut and the critical distal site of CNS, and the mechanisms that guide each direction of function. We summarize the range of CNS disorders influenced by microbiome, which could be broadly classified into immune- and non-immune-mediated types. We further categorize the underlying microbiome-related factors implicated in CNS disorders. Our burgeoning knowledge about microbiome may provide novel avenues for therapeutics against neurological diseases.
2. Communication between gut microbiome and the CNS
The gut receives regulatory signals from the CNS and vice versa. The term gut-brain-axis thus describes an integrative physiology concept that incorporates all, including afferent and efferent neural, endocrine, nutrient, and immunological signals between the CNS and the gastrointestinal system(Romijn et al., 2008). As accumulating literatures underpin the importance of the gut microbiome to intestinal functions, a novel concept of microbiome-gut-brain axis has been evolved (Rhee et al., 2009). The core feature of this concept is bidirectional interaction, with diverse mechanisms guiding each direction of effects.
2.1. How the CNS influences microbiome
A classical CNS-gut-microbiome signaling is operational via central regulation of satiety. Changes of dietary pattern as a result of CNS control of food intake can impact nutrient availability to gut microbiota and consequently their composition. Satiation-signaling peptides are the key molecular intermediaries that enable this downward control. These peptides, for example peptide YY (PYY), are transported through blood to the brain postprandial to exert their impact on satiety (Romijn et al., 2008). Satiation-signaling peptides arise primarily from the GI tract but most of them are also synthesized within the brain (reviewed by (Cummings and Overduin, 2007)). Beyond that, CNS can influence gut microbiome through neural and endocrine pathways in both direct and indirect manners. The autonomic nervous system (ANS) and hypothalamus-pituitary-adrenal (HPA) axis that liaise the CNS and viscera can modulate gut physiology such as motility, secretion and epithelial permeability as well as systemic hormones, which in turn affects the niche environment for microbiota and also host-microbiome interaction at the mucosae(Cryan and Dinan, 2012). Santos et al. found that stress caused epithelial barrier defects and subsequent mucosal mast cell activation(Santos et al., 2001). O'Mahony et al. illustrated that an early life stress (maternal separation) increased systemic corticosterone level and immune responses and altered fecal microbiota in rats(O'Mahony et al., 2009). Bailey et al. indicated that a social disruption (SDR) initiated by co-housing with aggressive male littermates altered murine gut bacterial populations through immune-activation(Bailey et al., 2011). Further, release of signaling molecules, cytokines, and anti-microbial peptides (AMPs) into the gut lumen by neurons, enteroendocrine cells, immune cells and Paneth cells at the direct or indirect command of the CNS is likely to have an immediate impact on gut microbiota(Rhee et al., 2009). Clarke et al. discovered the QseC sensor kinase as a bacterial receptor for host-derived epinephrine and norepinephrine, which might explain the biochemical basis for host endocrine signaling to microbiota(Clarke et al., 2006).
2.2. How microbiome influences CNS functions
The influence of microbiome on CNS functions is manifested in both normal and disease conditions. There is a crucial link between gut microbiome and CNS maturation under physiological state. External cues derived from indigenous commensal microbiota affect prenatal and postnatal developmental programming of the brain(Al-Asmakh et al., 2012; Douglas-Escobar et al., 2013). On the other hand, co-morbidity with mood disorders such as depression and anxiety is common in the intestinal pathological state of IBS. Chronic low-grade inflammation or immune activation that underlies the etiology of IBS is also a driving risk factor in mood disorders(O'Malley et al., 2011). In the more intense case of IBD, co-morbidity with stress is caused by the concurrent intestinal inflammation and microbiome alteration. Change in psychological activities is perceived in patients before and after IBD diagnosis(Bonaz and Bernstein, 2013).
Upward regulation of the CNS by microbiome can be achieved through neural, endocrine, metabolic and immunological mechanisms. The neural pathway is operational through the enteric nervous system (ENS), a main division of the ANS that governs the GI functions, and vagal afferent nerves (VAN) that convey sensory information from viscera to the CNS. Probiotic modulation of gut microbiota has been shown to influence gut neuro-motor functions(Verdu, 2009). Receptors expressed on VAN sense many of the regulatory gut peptides and also information contained in dietary components, relaying the signals to the CNS afterwards(de Lartigue et al., 2011). Indeed, vagal activation is necessary for a range of effects of gut microbiome or probiotics on brain functions(Cryan and Dinan, 2012). Recent studies suggest a direct interaction between gut microbiome and enteric neurons. TLR-3, 7 (recognizing viral RNA) and TLR-2, 4 (recognizing peptidoglycan and lipopolysaccharide) are expressed by the ENS in both mice and human(Barajon et al., 2009; Brun et al., 2013). Kunze et al. observed that Lactobacillus reuteri enhanced excitability of colonic neurons in naïve rats by inhibiting calcium-dependent potassium channel(Kunze et al., 2009). Mao et al. found that ex vivo, both Lactobacillus rhamnosus (strain JB-1) and B. fragilis could activate intestinal afferent neurons, while PSA completely mimicked the neuronal effects of its parent organism B. fragilis(Mao et al., 2013). Chiu et al. indicated that Staphylococcus aureus activation of sensory neurons could transduce nociception(Chiu et al., 2013). It is still unclear, in homeostatic periods, whether and how luminal microbial antigens reach into muscularis mucosa and sub-mucosa, where the ENS resides and the physical contact with sensory neurons occurs.
In the endocrinal pathway, the gut microbiome plays a major role in the development and regulation of the HPA axis that is critical to stress responses. Studies in gnotobiotic mice showed that postnatal exposure to gut microbiome affected the set point of the HPA axis(Sudo, 2012). Enteroendocrine cells interspersed among gut epithelium, particularly enterochromaffin cells, can secrete neurotransmitters and other signaling peptides in response to luminal stimuli, and thus act as transducers for the gut-endocrine-CNS route(Rhee et al., 2009). Besides, the vasoactive intestinal peptide (VIP), a peptide hormone synthesized in the gut but also brain, could mediate immune-modulation during CNS inflammation(Gonzalez-Rey et al., 2006). While the direct impact of microbiome on VIP expression has not been identified, dietary intervention is able to increase intestinal VIP, which might hint the role of microbiome(Velickovic et al., 2013).
Since a main function of microbiome is to facilitate host metabolism, a metabolic pathway is naturally implicit in the microbiome-gut-CNS signaling. Examples of metabolites associated with microbial metabolism or microbial–host co-metabolism have been reviewed(Holmes et al., 2011). Dysregulation of serotonergic and kynurenine routes of tryptophan metabolism influences the CNS pathological conditions of dementia, Huntington's disease and Alzheimer's disease(Ruddick et al., 2006). Probiotic treatment could alter kynurenine levels and ameliorate CNS pathologies(Desbonnet et al., 2008). In addition, the metabolic pathway represents an important inter-kingdom communication as host signaling molecules can be fully synthesized or mimicked by microbiota-derived metabolites. Commensal organisms can produce a range of neuroactive molecules such as serotonin, melatonin, gamma-aminobutyric acid (GABA), catecholamines, histamine and acetylcholine(Barrett et al., 2012; Forsythe et al., 2010; Lyte, 2011).
The immunological pathway seems to be an independent mechanism in the microbiome-gut-CNS signaling. The CNS, though viewed as an immune-privileged site, is not devoid of immune cells. There is a regular presence of macrophages and dendritic cells (DCs) in the choroid plexus and meninges, microglial cells in the brain parenchyma, and leukocytes in the cerebrospinal fluid (CSF). Aberrant CNS autoimmunity arises as a consequence of direct immune disruption of neural tissues. Commensal microbiome, known to shape the host immune system, affects the auto-reactivity of peripheral immune cells to the CNS(Berer and Krishnamoorthy, 2012; Rook et al., 2011). Secondly, immune-to-CNS communication is also mediated by systemic circulation of immune factors, which is implicated in neuro-psychiatric disorders such as depression. Indeed, factors that increase peripheral inflammation markers such as C-reactive protein (CRP), IL-1, IL-6 and tumor necrosis factor (TNF-a), are also risk factors for depression(Dantzer et al., 2008; Rook et al., 2011). In both routes of the pathway, there are anti-inflammatory mechanisms that can counter-act immune-mediated CNS disease symptoms.
3. The role of microbiome in CNS disorders
As multiple mechanisms guide the impact of microbiome on the CNS, it is therefore of particular interest to explore the role of microbiome in the regulation of CNS disorders. While there is still a lack of epidemiological evidence to connect microbiome with CNS pathologies, accumulating studies have underscored the importance of microbiome in a range of CNS disorders (Ochoa-Reparaz et al., 2011). CNS disorders can be classified as immune-mediated (exemplified by CNS autoimmune diseases such as multiple sclerosis) and non-immune-mediated (exemplified by neuro-psychiatric disorders such as autism, depression, anxiety and stress) according to main etiologies. This dichotomy, however, is not arbitrary since there often exists a crosstalk of etiologies. We herein summarize how microbiome can affect both categories of CNS disorders.
3.1. How microbiome affects immune-mediated CNS disorders
3.1.1. Multiple sclerosis
Multiple sclerosis (MS) is a chronic CNS demyelinating disease mediated by auto-reactive immune attack against central neural tissues. EAE is a widely used animal model of MS induced by CNS-restrictive antigens. Although EAE might not recapitulate all the features of human MS, it simulates its core neuro-inflammation process(Baxter, 2007). Historically, viral infection, such as Epstein-Barr virus (EBV) or human herpes virus 6, has been suggested as the trigger for human MS(Brahic, 2010). Recent studies, however, have begun to elucidate the contribution of microbiome and its relevant factors to MS pathogenesis, with much of the work investigated in EAE models(Ochoa-Reparaz et al., 2011). It has been shown in MOG92-106 TCR transgenic (RR) mice that commensal microbiota are essential for the development of spontaneous EAE. Germ-free RR mice were prevented from sEAE as a result of attenuated Th17 and auto-reactive B cell responses(Berer et al., 2011). Commensal microbiota are also required for induced EAE model, as GF B6 mice developed less severe EAE accompanied with decreased IFN-γ and IL-17 responses and increased Foxp3+Tregs. Segmented filamentous bacteria (SFB) colonization restored EAE susceptibility in GF mice(Lee et al., 2011). Antibiotic modulation of gut microbiota controls EAE progression via diverse cellular mechanisms. Ochoa-Reparaz et al. demonstrated that IL-10-producing CD4+CD25+Foxp3+Tregs were required for oral antibiotic attenuation of EAE progression(Ochoa-Reparaz et al., 2009). In a following study, Ochoa-Reparaz et al. showed that oral antibiotic treatment of EAE mice systemically induced a regulatory CD5+B cell subset(Ochoa-Reparaz et al., 2010b). Yokote et al. found that iNKT cells, a CD1d-restricted T cell subset that shared properties of both T and NK cells, were necessary for oral antibiotics amelioration of murine EAE (Yokote et al., 2008). While it is unknown whether enteric microbiota affect human MS, a higher percentage of MS patients exhibited antibody responses against gastrointestinal antigens in contrast to healthy control, which could indicate altered gut microbiome and immune status(Banati et al., 2013).
Oral treatment with a single bacterium or bacteria mixture can modulate EAE as observed in a range of studies. Probiotic Bifidobacterium animalis reduced the duration of symptoms in a rat EAE model(Ezendam et al., 2008). Conversely, probiotic strain Lactobacillus casei Shirota (LcS) exacerbated EAE symptoms in rats(Ezendam and van Loveren, 2008). However, later studies indicated that probiotic Lactobacilli, inclusive of LcS, did not enhance but rather suppressed rat EAE(Maassen and Claassen, 2008). This has been corroborated by other studies using probiotic mixtures of strains under the Lactobacillus genus. Indeed, Lactobacilli (including LcS), either administrated alone or in combination with other strains of Bifidobacterium genus, tend to alleviate murine EAE symptoms via reciprocal regulation of pro- and anti-inflammatory cytokine responses(Kobayashi et al., 2010; Kobayashi et al., 2012; Kwon et al., 2013; Lavasani et al., 2010). Probiotic treatment with B. fragilis and Pediococcus acidilactici (strain R037) also significantly reduced mice susceptibility to EAE(Ochoa-Reparaz et al., 2010a; Takata et al., 2011). In the case of the human commensal B. fragilis, capsular PSA expression was critical for its immune-regulatory functions(Ochoa-Reparaz et al., 2010a). Further, engineered strains such as Salmonella-CFA/I and Hsp65-producing Lactococcus lactis can prevent EAE in mice via Tregs-associated TGFβ and IL-13 signals(Ochoa-Reparaz et al., 2007; Ochoa-Reparaz et al., 2008; Rezende et al., 2013).
Isolated commensal microbial products can often recapitulate the biological effects of their parent organisms on hosts. Some of these products have been found as potent therapeutics against EAE. Purified B. fragilis PSA, referred to as a symbiosis factor in other studies, conferred prophylactic as well as therapeutic protection against EAE via induction of tolerogenic CD103+DCs at CNS-draining lymph nodes, similar to the effects conferred by probiotic B. fragilis(Ochoa-Reparaz et al., 2010c). While PSA is a TLR2 ligand, its immune-regulatory functions against EAE are not seen as putative in other commensal-derived TLR2 ligands. Nichols, et al. reported that a unique lipid TLR2 ligand, phosphorylated dihydroceramide (PE DHC), derived from human oral commensal Porphyromonas gingivalis but also gut commensals, was able to exacerbate murine EAE via TLR2-dependent mechanisms(Nichols et al., 2009). Commensal-derived extracellular ATP can be viewed as a danger-associated molecular pattern (DAMP) by hosts and has been related to Th17 development. Accordingly, Entpd7-/- mice that are deficient of ATP hydrolyzing enzymes have displayed a more severe level of EAE(Kusu et al., 2013).
Finally, diet patterns have been reported to influence the development of EAE. Piccio et al. found that high-fat diet increased murine EAE severity. In contrast, calorie restriction diet attenuated EAE symptoms, which was associated with hormonal, metabolic and cytokine changes rather than immune suppression(Piccio et al., 2008). Kleinewietfeld et al. illustrated that mice fed with a high-salt diet developed a more severe form of EAE, in line with the ability of sodium chloride to activate Th17 cells(Kleinewietfeld et al., 2013). Recent developments may insinuate a central role of gut microbiome in linking diet with MS and EAE.
3.1.2. Neuromyelitis optica
Neuromyelitis optica (NMO), also known as Devic's disease, is a CNS autoimmune disease featured by immune-mediated demyelination of the optic nerve and spinal cord. It resembles multiple aspects of MS. Auto-reactive humoral and T cell-mediated immunity against aquaporin 4 (AQP4), a predominant CNS water channel protein, drives the NMO pathogenesis(Lennon et al., 2005; Varrin-Doyer et al., 2012). Like MS, no research so far has established a direct link between gut microbiome and NMO. Banati et al. found that patients of AQP4-seropositive NMO and NMO spectrum diseases showed much higher serum level of antibodies against gastrointestinal antigens (most frequently dietary proteins) than did healthy controls, insinuating the alteration of microbiota composition and consequent immune status in NMO patients(Banati et al., 2013). Varrin-Doyer et al. found that AQP4-specific T-cells in NMO patients showed cross-reactivity to a protein of the indigenous gut commensal species, Clostridium perfringens, supporting a microbiota-related molecular mimicry process in NMO pathogenesis(Varrin-Doyer et al., 2012).
3.1.3. Guillain–Barré syndrome
Guillain–Barré syndrome (GBS) is an autoimmune disease of the peripheral nervous system. Similar to MS, auto-reactive immune attack of myelin acts as the cause of neuro-degeneration in GBS(Nachamkin et al., 1998). Preceding infection with bacteria or virus, such as Haemophilus pneumoniae, Mycoplasma pneumoniae, influenza, and EBV, has been suggested as environmental triggers for GBS. Indeed, cross-reaction of pathogen-induced antibodies against neural surface antigens in a molecular mimicry process constitutes an important mechanism for GBS neuronal damage that leads to acute flaccid paralysis(Ochoa-Reparaz et al., 2011). Campylobacter jejuni, a gut commensal species found in poultry, is a major cause of human enteritis induced by food contamination. Tam et al. indicated a far greater risk of GBS among Campylobacter enteritis patients than previously reported by retrospective serological studies(Tam et al., 2007). Further, Campylobacter is associated with several pathologic forms of GBS. Different strains of Campylobacter, along with host factors, play an important role in shaping auto-reactive immune reactions during GBS development(Nachamkin et al., 1998). Therefore, C. jejuni represents a gut-associated pathogen that mediates neural autoimmunity.
3.1.4. Other immune-mediated conditions
The role of microbiome has been implicated in other immune-involved CNS diseases. Meningitis is inflammation of the protective membranes of the CNS. Viral or bacterial infection may lead to meningitis. Zelmer et al. reported that the adult gut commensal Escherichia coli K1 were able to cause meningitis via maternal transfer to newborn infants. The polysialic acid (polySia) capsule synthesized by E. coli K1 guided the critical process of blood-to-brain transit of this neuro-pathogenic strain(Zelmer et al., 2008). Chronic fatigue syndrome (CFS), also referred to as myalgic encephalomyelitis (ME), is so far of unknown etiology. Immune factors, such as chronic lymphocyte over-activation and cytokine abnormalities, contribute to its pathogenesis(Patarca-Montero et al., 2001). Maes et al found that increased IgA responses to commensal bacteria in CFS patients were associated with inflammation, cellular immune activation, and symptomatic severity. It was postulated that elevated translocation of commensal bacteria could be responsible for the disease activities in some CFS patients(Maes et al., 2012).
3.2. How microbiome affects non-immune-mediated CNS disorders
3.2.1. Autism and depression
Autism spectrum disorder (ASD) is a range of developmental neuro-behavioral disorders characterized by impaired social interaction and communication. Autism represents the primary type of ASD. Emerging data have indicated a link between gut microbiome and ASD, either as direct causality or as indirect consequences of atypical patterns of feeding and nutrition(Mulle et al., 2013). Disruption of gut microbiota might promote the over-colonization of neurotoxin-producing bacteria and thus contribute to autistic symptoms. It has been reported, however, that oral vancomycin treatment brings short-term benefit to regressive-onset autism children(Sandler et al., 2000). General gut microbiota alteration or specific gut commensal strains have been implicated in ASD. Bolte et al. postulated that Clostridium tetani could induce autism(Bolte, 1998). Indeed, two ensuing human gut microbiome studies illustrated a greater number of species under the Clostridium genus present in fecal samples of autistic children(Finegold et al., 2002; Parracho et al., 2005). An imbalance of Bacteroidetes and Firmicutes phyla also manifests in autistic children. Finegold et al. reported increased presence of Bacteroidetes in severe autistic group and predominant presence of Firmicutes in healthy controls(Finegold et al., 2010). Williams et al. revealed a reverse trend in comparing autism and GI disease co-morbid (AUT-GI) children and GI disease alone controls(Williams et al., 2011). In addition, altered levels of other gut commensals, including those of Bifidobacterium, Lactobacillus, Sutterella, Prevotella and Ruminococcus genera and of the Alcaligenaceae family, were correlated with autism(Adams et al., 2011; Kang et al., 2013; Wang et al., 2013; Williams et al., 2012). Nonetheless, there are studies refuting the microbiota alteration between autistic and healthy subjects(Gondalia et al., 2012). Variance in sampling strategies and techniques applied to microbiome assays may account for these differences. Further, gut microbiome-mediated metabolism also impacts autism. Metabolites profile gathered from both urinary and fecal samples differed in autistic patients and healthy control, potentially consequent of microbiota changes(Ming et al., 2012; Wang et al., 2012; Yap et al., 2010).
Depression is a major form of mood disorder that results from neuro-psychiatric disturbance or immunological deregulation(Dantzer et al., 2008). Probiotic treatment has shown efficacy in suppression of animal depression models. Species under Lactobacillus genus are particularly characterized as anti-depressant. Probiotic mixture comprising L. rhamnosus and L. helveticus strains ameliorated maternal separation-induced depression via normalizing corticosterone level(Gareau et al., 2007). Similarly, L. rhamnosus strain JB-1 reduced depression-related behavior through regulating corticosterone and GABA receptor in a vagal-dependent manner(Bravo et al., 2011). Species of Bifidobacterium are also potent anti-depressants. Bifidobacterium infantis alleviated depression as indicated by rat forced swim test (FST) and maternal separation models. Mechanisms involved include attenuation of pro-inflammatory cytokines, regulation of tryptophan metabolism and CNS neurotransmitters(Desbonnet et al., 2008; Desbonnet et al., 2010). Probiotics combining Lactobacilli and Bifidobacteria were tested in post-myocardial infarction depression models. L. helveticus and Bifidobacterium longum together ameliorated post-MI depression through reduction of pro-inflammatory cytokines and restoration of barrier integrity at GI tract(Arseneault-Breard et al., 2012; Gilbert et al., 2013). In addition, gut microbial products, such as sodium butyrate (salt formed from butyrate acid, a type of SCFA) have been explored in animal depression model, without showing anti-depressant effects(Gundersen and Blendy, 2009). Further, a diet formulation containing high levels of polyunsaturated fatty acids (PUFAs) n-3 attenuated rat post-MI depression via similar mechanisms as did L. helveticus and B. longum(Gilbert et al., 2013).
3.2.2. Anxiety and stress
Anxiety and stress are common forms of mood disorders with nervous, endocrinal and immunological basis. Exposure to stressors such as chemical, biological or environmental stimuli can trigger stress and anxiety responses, which involves activation of the HPA axis. As aforementioned, co-morbidity with anxiety and stress has been perceived in drastic and mild types of intestinal dysfunctions, underscoring the role of gut-brain signals such as neurotransmitters and immune factors(Diamond et al., 2011; Dinan and Cryan, 2012; Fukudo and Kanazawa, 2011; Konturek et al., 2011; O'Malley et al., 2011; Reber, 2012).
GF mice showed increased motor activity and reduced anxiety, compared to SPF mice with normal gut microbiota. This behavioral phenotype was associated with higher levels of neurotransmitters and reduced synaptic long-term potentiation in the CNS of GF mice(Diaz Heijtz et al., 2011). Reduced anxiety-like behavior in GF condition has been confirmed by later studies, which are explained by other neurochemical changes such as decreased neurotransmitter receptors and increased tryptophan metabolism. It is therefore postulated that gut microbiome regulates the set point for HPA axis(Clarke et al., 2013; Neufeld et al., 2011). Gut-associated pathogens can exacerbate anxiety. Infection with C. jejuni elevated anxiety-like behavior through induction of the c-Fos protein, a neuronal activation marker, in the CNS as well as ANS(Gaykema et al., 2004; Goehler et al., 2008). C-Fos protein induction was also indicated in Citrobacter rodentium exacerbation of anxiety, whereas Trichuris muris elevated anxiety via immunological and metabolic mechanisms(Bercik et al., 2010; Lyte et al., 2006). In contrast, beneficial probiotics can ameliorate anxiety. Specific species of Lactobacillus and Bifidobacterium genera have anxiolytic effects. Probiotic treatment with certain strains of B. longum, B. infantis, L. helveticus, or L. rhamnosus, either alone or in combination, normalized behavioral phenotypes in animal anxiety models(Bercik et al., 2010; Bravo et al., 2011; McKernan et al., 2010; Messaoudi et al., 2011; Ohland et al., 2013).
Programming of HPA axis by gut microbiome is also observed in stress condition. GF mice showed exaggerated HPA stress response, accompanied by increased circulatory neurotransmitters and decreased brain-derived neurotrophic factor (BDNF) expression in the CNS(Sudo et al., 2004). Altered gut microbiota composition has been associated with stress. O'Mahony, et al. reported changes in fecal microbiota in early life stress induced by maternal separation(O'Mahony et al., 2009). Murine exposure to the SDR stressor led to decreased abundance of Bacteroides, increased abundance of Clostridium, and changes of other bacteria genera, which were concurrent with enhanced circulatory pro-inflammatory cytokines(Bailey et al., 2011). The anxiolytic strains of Lactobacillus and Bifidobacterium genera that have anti-anxiety effects often display anti-stress effects as well. Ingestion with L. helveticus and L. rhamnosus reduced rat chronic psychological stress indicated by water avoidance test and improved intestinal barrier integrity(Zareie et al., 2006). Lactobacillus farciminis also suppressed stress-induced gut leakiness and attenuated HPA axis stress response(Ait-Belgnaoui et al., 2012). B. longum normalized anxiety-like behavior and CNS BDNF levels in mice co-morbid with infectious colitis through a vagal-dependent mechanism(Bercik et al., 2011b). A probiotic formulation consisting of L. helveticus and B. longum showed anxiolytic-like activities in rats and beneficial psychological effects in healthy human subjects(Messaoudi et al., 2011).
3.2.3. Pain
Nociceptive pain that is caused by peripheral nervous response to stimuli and signaling transduction to the CNS can be alleviated by probiotic modulation of microbiome. Antinociceptive effects are seen in species of Lactobacillus genus. L. farciminis ameliorated stress-induced hypersensitivity to colorectal distension (CRD), mediated by inhibition of colonic epithelial contraction and nitric oxide (NO)-related mechanisms(Ait-Belgnaoui et al., 2006). L. reuteri also attenuated visceral pain induced by CRD in normal rats(Kamiya et al., 2006). L. paracasei normalized visceral hypersensitivity to CRD in antibiotics-perturbed mice (Verdu et al., 2006). Lactobacillus acidophilus delivered analgesic effects in intestinal pain via induction of opioid and cannabinoid receptors(Rousseaux et al., 2007). Besides, two studies supported the anti-nociceptive effects of a specific B. infantis strain in the context of IBS. Probiotic B. infantis reduced CRD-induced pain in both visceral normal-sensitive and visceral hypersensitive rat strains, and also in a rat model of post-inflammatory colonic hypersensitivity(Johnson et al., 2011; McKernan et al., 2010). Recently, Chiu et al. reported that S. aureus triggered pain in mice through direct induction of calcium flux and action potentials in nociceptor neurons(Chiu et al., 2013).
3.2.4. Other neuro-psychiatric symptoms
Microbiome has been connected with other neuro-psychiatric disorders, where a mixture of immune- and non-immune-based etiologies often occurs. GF animals exhibit defective memory and cognitive abilities. Gareau et al. found that memory dysfunction occurred in GF mice regardless of exposure to stress(Gareau et al., 2011). Bercik et al. showed that re-colonization of GF mice with murine microbiota could either enhance or reduce exploratory behavior, depending on the strains of donor and recipient mice. Further, antibiotic treatment of SPF mice increased exploratory behaviors. Hippocampal levels of BDNF were positively correlated with exploratory behaviors, and regulated in both cases(Bercik et al., 2011a). Probiotics were able to improve infection-induced memory dysfunction and diabetes-induced cognitive defects(Davari et al., 2013; Gareau et al., 2011). Propionic acid, a type of SCFA, reduced murine social and cognitive abilities(MacFabe et al., 2011). Dietary alteration of gut microbiome also modulated murine cognitive and learning behaviors(Li et al., 2009). Microbiota alteration has been indicated in hepatic encephalopathy (HE). Different fecal and mucosal microbiota were found in HE patients as compared to healthy controls. In cirrhotic HE specifically, good cognition and decreased inflammation were linked with autochthonous and Prevotella genera as well as Alcaligenaceae and Porphyromonadaceae families, whereas poor cognition and increased inflammation were linked with over-represented Enterococcus, Megasphaera and Burkholderia genera(Bajaj et al., 2012a; Bajaj et al., 2012b; Bajaj et al., 2012c). Alteration of serum antibodies to oral microbiota and sub-gingival bacterial species was observed in Down's syndrome(Khocht et al., 2012; Morinushi et al., 1997). Oral microbiota changes were also observed in comatose patients(Cecon et al., 2010). A positive correlation between schizophrenia and serological surrogate markers of bacterial translocation was indicated(Severance et al., 2013).
4. Factors linking microbiome and the CNS
As microbiome refers to the collective genomes of total microbiota, microbiome research is broad in its scope, which incorporates general microbiota composition or specific bacterium, microbiota-generated products, external alteration of microbiota, and barrier integrity status that affects host-microbiota contact. It is thus worthy summarizing the factors that mediate the influence of microbiome on CNS disorders.
4.1. Hygiene
The hygiene hypothesis states that a lack of childhood exposure to infectious agents, parasites and commensals increases susceptibility to T helper 2 (Th2)-mediated allergic diseases. However, there also exists a correlation between improved sanitary conditions and increased incidences of T helper 1 (Th1)-mediated autoimmune diseases such as T1 diabetes and multiple sclerosis(Berer and Krishnamoorthy, 2012). Th1 response targets intracellular microbes, mediated by signature cytokine IFNγ; while Th2 response targets helminthes and allergens, characterized by signature cytokines IL-4 and IL-13. Aberrant immune development is therefore a potential mechanism that links hygiene and immune-mediated CNS disorders. GF mice displayed reduced EAE symptoms, concurrent with attenuated Th1, Th17 and B cell responses, which related to the hygiene hypothesis yet contradicted findings in human MS(Berer et al., 2011; Lee et al., 2011). This discrepancy might be explained by intricate etiologies underlying human MS and intrinsic differences between murine GF condition and human hygienic state. In murine models, GF condition is also linked to neuro-behavioral disorders. Total sterility results in reduction of BDNF levels and enhancement of HPA axis responses, correlated by elevated neurotransmitters in the plasma. GF animals displayed increased stress and impaired cognition(Gareau et al., 2011; Sudo et al., 2004). However, GF condition in other studies is identified as anxiolytic and can resolve anxiety, correlated by decreased neurotransmitter receptors levels(Kuss et al., 2011; Neufeld et al., 2011). Hence, hygiene exerts case-specific rather than universal influences on neuro-chemistry and neuro-behavioral manifestations.
4.2. Antibiotics usage
Antibiotics confer selective alteration of gut microbiota. Mice pre-conditioned with oral antibiotics are less susceptible to autoimmune models such as EAE. In studies conducted by Ochoa-Reparaz et al., amelioration of EAE was associated with reduced IFNγ and IL-17, increased IL-13 and IL-10, and systemic stimulation of Tregs and Bregs(Ochoa-Reparaz et al., 2009; Ochoa-Reparaz et al., 2010b). That antibiotics poise the Th1/Th2 equilibrium towards Th2 direction is consistent with hygiene hypothesis. An earlier study conducted by Yokote et al. also observed reduced pro-inflammatory cytokines, including IFNγ and IL-17, in antibiotic treatment of EAE. While iNKT cells were not induced by antibiotics, they were essential for protection against EAE(Yokote et al., 2008). Different antibiotic agents were utilized in these EAE studies, which could result in different gut microbiome profiles and explain the variability of immune mechanisms. Current studies support a beneficial role of antibiotic treatment of neuro-behavioral disorders. Antibiotic treatment reduced stress response and increased exploratory behavior in mice and offered short-term benefit to regressive-onset autism children. Underlying mechanisms may involve the reduction of luminal LPS concentration (and thus potentially reduced chronic inflammation) and changes of CNS signals, such as hippocampal expression of BDNF(Ait-Belgnaoui et al., 2012; Bercik et al., 2011a; Sandler et al., 2000). In sum, antibiotics might reset the default immune and neuro-hormonal status shaped by commensal microbiome and therefore alter predisposition to CNS disorders.
4.3. Microbiota composition
How microbiota composition impacts CNS disorders can be indicated by a variety of methodologies, including infection-induced microbiome perturbation, studies using SPF and gnotobiotic mice, mono-colonization of GF mice, and metagenomic approaches such as microbial microarray and 16S rRNA profiling. Further, compositional changes of microbiota can be indirectly reflected by profiling the metabolites and co-metabolites of microbiota and serum titers of antibodies against microbiota and diet components. As the study of enterotypes is still in its infancy, efforts to find disease-specific enterotypes are limited. Hildebrand et al. defined two murine enterotypes, ET1 and ET2 that bore striking similarity to Ruminococcus and Bacteroides enterotype in human, respectively. ET2 mice showed higher levels of fecal calprotectin, a biochemical marker for IBD(Hildebrand et al., 2013). For CNS disorders, a concrete link with enterotypes has yet to be established. While it is tempting to infer enterotypes from the scattered studies of certain disease type, opposing data often obstruct consensus. For instance, there are favorable and unfavorable results for the link between Bacteroides enterotype and autism(Finegold et al., 2010; Williams et al., 2011). Further, heed must be taken to clarify the cause and effect as CNS disorders could impact diet patterns or be concurrent with gut epithelial impairment, both scenarios affecting microbiota composition.
4.4. Probiotics
Ingestion of beneficial live bacteria, also know as probiotics, is a therapeutic way of using microbiota components for treatment. Probiotics can regulate immune subsets, especially in the case of CNS autoimmunity. B. fragilis is a prominent probiotic strain that promotes Foxp3+Treg quantity and functional maturation in both EAE and IBD(Mazmanian et al., 2008; Ochoa-Reparaz et al., 2010a). Lactobacilli and Bifidobacteria are key components of anti-inflammatory probiotic mixtures that can also function through stimulation of IL-10+Foxp3+Tregs(Kwon et al., 2013; Takata et al., 2011). Moreover, genetic modification of natural strains represents another potent probiotic approach. Fusing tolerogenic antigen into attenuated or innocuous strains has yielded oral therapeutics against EAE(Ochoa-Reparaz et al., 2007; Ochoa-Reparaz et al., 2008; Rezende et al., 2013). Probiotics can alleviate neuro-psychiatric disorders via hormonal and neuro-chemical mechanisms. For example, B. longum NCC3001 can normalize murine hippocampal BDNF expression and L. rhamnosus (JB-1) can exert differential regulation of GABA transcription in different CNS regions(Bercik et al., 2011b; Bravo et al., 2011). Particular probiotics may convey anxiolytic effects in multiple types of neuro-behavioral disorders, which indicates shared neural and endocrinal etiologies of these disorders. For example, L. helveticus R0052 and B. longum R0175 can ameliorate both anxiety and depression in rats(Gilbert et al., 2013; Messaoudi et al., 2011). Neural mechanisms that involve direct bacterial activation or inhibition of neurons may account for anti-nociceptive effects of probiotics.
4.5. Microbiota-derived products
Microbiota-derived products are often effective components responsible for microbiota-gut-CNS signaling. This is especially evident in the case of B. fragilis capsular PSA, where PSA can recapitulate the functions of its parent organism B. fragilis in regard to anti-inflammatory effects in EAE and activation of intestinal sensory neurons. PSA is a unique zwitterion and referred to as a symbiosis factor for commensalism(Mao et al., 2013; Ochoa-Reparaz et al., 2010c). Commensal-produced luminal extracellular ATP and LPS drive the chronic inflammation that contributes to the pathogenesis of neuro-immune and neuro-psychiatric disorders. Microbiota-derived metabolites and co-metabolites are critical intermediaries for microbiota-gut-CNS signaling. Commensals spawn a range of neuro-active substances. For example, Lactobacillus and Bifidobacterium species can produce the inhibitory neurotransmitter GABA(Barrett et al., 2012). The involvement of neuro-active metabolites in probiotic effects on neuro-psychiatric disorders remains unexplored. SCFAs, a group of fatty acids with aliphatic tails of 2 to 6 carbons, are fermentation products of dietary fibers by microbiota. While SCFAs have been found to be important immune regulators, there is a scarcity of studies that target at their impacts on CNS disorders(MacFabe et al., 2011; Thomas et al., 2012).
4.6. Diet
Diet patterns may modulate gut microbiome via alteration of nutrient availability. Recent developments have suggested that dietary intervention can impact gut microbial gene richness. Lower microbiome richness was identified as less healthy and associated with metabolic dysfunction and low-grade inflammation. Dietary formula with higher fiber contents can improve microbiome richness(Cotillard et al., 2013; Le Chatelier et al., 2013). Unhealthy diet patterns containing high levels of fat or salt could accelerate neuro-inflammation during EAE(Kleinewietfeld et al., 2013; Piccio et al., 2008). Western-style diet could negatively affect anxiety-like behavior and memory, depending on immune status(Ohland et al., 2013). Supplementation with high levels of PUPAs could alleviate depression(Gilbert et al., 2013). These experimental findings could indicate saturated fat as a risk factor for both neuro-immune and neuro-psychiatric disorders. Collectively, microbiome modulation is an integral mechanism underlying diet-based treatment.
4.7. Gut permeability
Gut permeability has been directly and indirectly associated with the role of microbiome in CNS disorders. Humoral and cellular immune reaction to microbiota in the circulation, persistent low-grade inflammation and neuro-psychiatric co-morbidity with IBD may hint the breach of mucosal epithelial barrier(Banati et al., 2013; Bercik et al., 2011b; Lyte et al., 2006; Maes et al., 2012; Severance et al., 2013; Varrin-Doyer et al., 2012). Probiotic treatment with several species of Lactobacillus genus restored the barrier integrity(Ait-Belgnaoui et al., 2012; Zareie et al., 2006). Dysbiosis and breakdown of mucosal barrier are interrelated phenomena. Microbiota and their ligands maintain the cell-cell junctions critical to barrier integrity(Hooper et al., 2001; Rakoff-Nahoum et al., 2004). Abnormal gut microbial composition is seen in IBD(Fava and Danese, 2011). In return, the cascade of inflammatory process during IBD may amplify intestinal dysbiosis. Although it is hard to determine the initial cause, dysbiosis and gut hyper-permeability orchestrate in driving CNS pathogenesis.
5. Conclusions and perspectives
Accumulating information of animal and human research strengthen the concept of microbiome-gut-brain axis. Microbiome controls canonical aspects of the CNS, immunity and behavior in health and disease. Still, unknowns abound regarding the detailed role of microbiome in CNS disorders. First, the relative contributions of immune, neural, and endocrine pathways in microbiome-CNS communications at pathological states need to be clarified. Second, it is crucial to elucidate the factors at play in microbiome-based therapeutics and further refine the effective components. Third, caution should be applied to the translation of animal data to human clinics using existing microbiome studies.
Microbiome research holds conceivable promise for the CNS disorder-relevant prognosis and therapeutics. Correlational studies that associate microbiota compositional patterns with specific disorders such as autism types contain prognostic value. Multitudes of commensal bacteria co-exist with hosts without incurring harmful immune responses. Symbiotic strains and their products are thus a precious mining pool that contains useful drug candidates with host-tolerated immune-modulatory functions. Innocuous commensal strains could also act as carriers for therapeutic substances when engineered. Finally, to restore the richness and functionality of gut microbial ecosystem by fecal transplantation has been proposed long time ago yet methodological and ethical obstacles remain.
Figure 1. Microbiome-gut-brain axis in relation to CNS disorders.
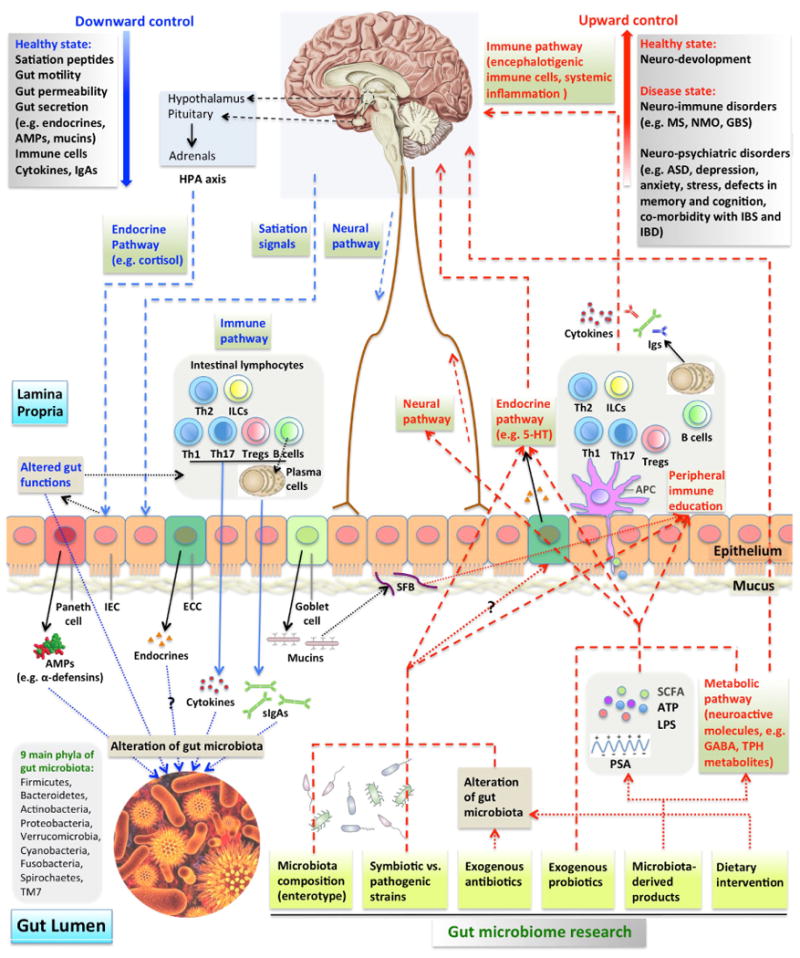
Multiple pathways guide the downward and upward directions of the microbiome-gut-brain axis in the contexts of health and disease. (A) Downwardly, CNS controls gut microbiome composition through satiation signaling peptides that affect nutrient availability, endocrines that affect gut functions and neural pathways. HPA axis release of cortisol regulates gut movement and integrity. Immune (cells, cytokines and sIgAs) pathways can be turned on in response to altered gut functions. Endocrine and neural pathways can also regulate the secretion from specialized gut epithelial cells, including paneth cells, enteroendocrine cells (ECC) and goblet cells. Their secretory products affect the survival and resident environment of microbiota. (B) Upwardly, gut microbiome controls CNS activities through neural (direct activation of neurons by microbiome), endocrine (e.g. ECC release of 5-HT), metabolic (microbiota synthesis of neuroactive molecules), and immune (CNS infiltrating immune cells and systemic inflammation) pathways. Microbiome influences CNS at healthy (neuro-development) and disease (a range of neuro-immune and neuro-psychiatric disorders) states. Gut luminal microbiota, their products sampled by APCs and epithelium-attaching SFBs mediate peripheral immune education. Gut microbiome composition, specific strains within microbiota, probiotic treatment, microbiota-derived products and other factors constitute the scope of microbiome studies.
Acknowledgments
We thank Dr. Pamela Bagley (Dartmouth College) for literature assistance.
Abbreviations
- AMPs
anti-microbial peptides
- TPH
tryptophan
- 5-HT
5-hydroxytryptamine
- SFB
Segmented filamentous bacteria
- PSA
polysaccharide A from B. fragilis
- ATP
adenosine-5′-triphosphate
- SCFA
short-chain fatty acid
- IEC
intestinal epithelial cell
- ILCs
innate lymphoid cells
- APC
antigen presenting cell
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- GBS
Guillain-Barré syndrome
- ASD
autism-spectrum disorder
- IBS
irritable bowel syndrome
- IBD
inflammatory bowel disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, Wherry EJ, Artis D. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC gastroenterology. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090–1094. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmakh M, Anuar F, Zadjali F, Rafter J, Pettersson S. Gut microbial communities modulating brain development and function. Gut microbes. 2012;3:366–373. doi: 10.4161/gmic.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. The British journal of nutrition. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M'Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, behavior, and immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, Schubert CM, Sikaroodi M, Heuman DM, Crossey MM, Bell DE, Hylemon PB, Fatouros PP, Taylor-Robinson SD. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metabolic brain disease. 2012a;27:205–215. doi: 10.1007/s11011-012-9303-0. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2012b;303:G675–685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. American journal of physiology. Gastrointestinal and liver physiology. 2012c;302:G168–175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati M, Csecsei P, Koszegi E, Nielsen HH, Suto G, Bors L, Trauninger A, Csepany T, Rozsa C, Jakab G, Molnar T, Berthele A, Kalluri SR, Berki T, Illes Z. Antibody response against gastrointestinal antigens in demyelinating diseases of the central nervous system. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013 doi: 10.1111/ene.12072. [DOI] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. Journal of applied microbiology. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nature reviews. Immunology. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nature immunology. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011a;141:599–609. 609 e591–593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011b;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112 e2101. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Berer K, Krishnamoorthy G. Commensal gut flora and brain autoimmunity: a love or hate affair? Acta neuropathologica. 2012;123:639–651. doi: 10.1007/s00401-012-0949-9. [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Bolte ER. Autism and Clostridium tetani. Medical hypotheses. 1998;51:133–144. doi: 10.1016/s0306-9877(98)90107-4. [DOI] [PubMed] [Google Scholar]
- Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Brahic M. Multiple sclerosis and viruses. Annals of neurology. 2010;68:6–8. doi: 10.1002/ana.22057. [DOI] [PubMed] [Google Scholar]
- Branton WG, Ellestad KK, Maingat F, Wheatley BM, Rud E, Warren RL, Holt RA, Surette MG, Power C. Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PloS one. 2013;8:e54673. doi: 10.1371/journal.pone.0054673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nature immunology. 2013;14:660–667. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]
- Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, Pizzuti D, Barbieri V, Rosato A, Sturniolo GC, Martines D, Zaninotto G, Palu G, Castagliuolo I. Toll-like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Cecon F, Ferreira LE, Rosa RT, Gursky LC, de Paula e Carvalho A, Samaranayake LP, Rosa EA. Time-related increase of staphylococci, Enterobacteriaceae and yeasts in the oral cavities of comatose patients. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2010;43:457–463. doi: 10.1016/S1684-1182(10)60071-0. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Wardenburg JB, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013 doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009;41:850–853. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Frontiers in immunology. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Dore J, Zucker JD, Clement K, Ehrlich SD, Blottiere H, Leclerc M, Juste C, de Wouters T, Lepage P, Fouqueray C, Basdevant A, Henegar C, Godard C, Fondacci M, Rohia A, Hajduch F, Weissenbach J, Pelletier E, Le Paslier D, Gauchi JP, Gibrat JF, Loux V, Carre W, Maguin E, van de Guchte M, Jamet A, Boumezbeur F, Layec S. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews. Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. The Journal of clinical investigation. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiology & behavior. 2011;105:100–105. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of psychiatric research. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Tracey K, Volpe BT. It takes guts to grow a brain: Increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:588–591. doi: 10.1002/bies.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA pediatrics. 2013;167:374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunological reviews. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- Ezendam J, de Klerk A, Gremmer ER, van Loveren H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clinical and experimental immunology. 2008;154:424–431. doi: 10.1111/j.1365-2249.2008.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezendam J, van Loveren H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. The British journal of nutrition. 2008;99:83–90. doi: 10.1017/S0007114507803412. [DOI] [PubMed] [Google Scholar]
- Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World journal of gastroenterology : WJG. 2011;17:557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A. Gastrointestinal microflora studies in lateonset autism. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain, behavior, and immunity. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- Frank DN, Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. Journal of gastroenterology and hepatology. 2011;26 Suppl 3:110–115. doi: 10.1111/j.1440-1746.2011.06631.x. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain, behavior, and immunity. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gilbert K, Arseneault-Breard J, Flores Monaco F, Beaudoin A, Bah TM, Tompkins TA, Godbout R, Rousseau G. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. The British journal of nutrition. 2013;109:50–56. doi: 10.1017/S0007114512003807. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain, behavior, and immunity. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism research : official journal of the International Society for Autism Research. 2012;5:419–427. doi: 10.1002/aur.1253. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. The American journal of pathology. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therapeutic advances in gastroenterology. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand F, Nguyen TL, Brinkman B, Yunta RG, Cauwe B, Vandenabeele P, Liston A, Raes J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome biology. 2013;14:R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends in microbiology. 2011;19:349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science (New York, NY) 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, Fernandez L, Rodriguez JM. Is meconium from healthy newborns actually sterile? Research in microbiology. 2008;159:187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Greenwood-Van Meerveld B, McRorie J. Effects of Bifidobacterium infantis 35624 on post-inflammatory visceral hypersensitivity in the rat. Digestive diseases and sciences. 2011;56:3179–3186. doi: 10.1007/s10620-011-1730-y. [DOI] [PubMed] [Google Scholar]
- Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PloS one. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Khocht A, Yaskell T, Janal M, Turner BF, Rams TE, Haffajee AD, Socransky SS. Subgingival microbiota in adult Down syndrome periodontitis. Journal of periodontal research. 2012;47:500–507. doi: 10.1111/j.1600-0765.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kato I, Nanno M, Shida K, Shibuya K, Matsuoka Y, Onoue M. Oral administration of probiotic bacteria, Lactobacillus casei and Bifidobacterium breve, does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis. Immunopharmacology and immunotoxicology. 2010;32:116–124. doi: 10.3109/08923970903200716. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Suzuki T, Kaji R, Serata M, Nagata T, Ando M, Iizuka R, Tsujibe S, Murakami J, Kiyoshima-Shibata J, Kato I, Nanno M, Shida K. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacology and immunotoxicology. 2012;34:423–433. doi: 10.3109/08923973.2010.617755. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2011;62:591–599. [PubMed] [Google Scholar]
- Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS computational biology. 2013;9:e1002863. doi: 10.1371/journal.pcbi.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. Journal of cellular and molecular medicine. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science (New York, N Y) 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K, Maeda Y, Nishimura J, Arima Y, Atarashi K, Honda K, Murakami M, Kunisawa J, Kiyono H, Okumura M, Yamamoto M, Takeda K. Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. Journal of immunology (Baltimore, Md: 1950) 2013;190:774–783. doi: 10.4049/jimmunol.1103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, Kim JE, Nam JH, Im SH. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clinical immunology (Orlando, Fla) 2013;146:217–227. doi: 10.1016/j.clim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Westrom B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS one. 2010;5:e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, Guedon E, Delorme C, Layec S, Khaci G, van de Guchte M, Vandemeulebrouck G, Jamet A, Dervyn R, Sanchez N, Maguin E, Haimet F, Winogradski Y, Cultrone A, Leclerc M, Juste C, Blottiere H, Pelletier E, LePaslier D, Artiguenave F, Bruls T, Weissenbach J, Turner K, Parkhill J, Antolin M, Manichanh C, Casellas F, Boruel N, Varela E, Torrejon A, Guarner F, Denariaz G, Derrien M, van Hylckama Vlieg JE, Veiga P, Oozeer R, Knol J, Rescigno M, Brechot C, M'Rini C, Merieux A, Yamada T. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. The Journal of experimental medicine. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiology & behavior. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxietylike behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiology & behavior. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Maassen CB, Claassen E. Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine. 2008;26:2056–2057. doi: 10.1016/j.vaccine.2008.02.035. [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behavioural brain research. 2011;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. Journal of affective disorders. 2012;136:909–917. doi: 10.1016/j.jad.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. The American journal of gastroenterology. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nature communications. 2013;4:1465. doi: 10.1038/ncomms2478. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:1029–1035, e1268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Ming X, Stein TP, Barnes V, Rhodes N, Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. Journal of proteome research. 2012;11:5856–5862. doi: 10.1021/pr300910n. [DOI] [PubMed] [Google Scholar]
- Morinushi T, Lopatin DE, Van Poperin N. The relationship between gingivitis and the serum antibodies to the microbiota associated with periodontal disease in children with Down's syndrome. Journal of periodontology. 1997;68:626–631. doi: 10.1902/jop.1997.68.7.626. [DOI] [PubMed] [Google Scholar]
- Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Current psychiatry reports. 2013;15:337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barre syndrome. Clinical microbiology reviews. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Nichols FC, Housley WJ, O'Conor CA, Manning T, Wu S, Clark RB. Unique lipids from a common human bacterium represent a new class of Toll-like receptor 2 ligands capable of enhancing autoimmunity. The American journal of pathology. 2009;175:2430–2438. doi: 10.2353/ajpath.2009.090544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO reports. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain, behavior, and immunity. 2011;25:1333–1341. doi: 10.1016/j.bbi.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Begum-Haque S, Kasper LH. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Annals of neurology. 2011;69:240–247. doi: 10.1002/ana.22344. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. Journal of immunology (Baltimore, Md: 1950) 2010a;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. Journal of immunology (Baltimore, Md: 1950) 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut microbes. 2010b;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 2010c;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. Journal of immunology (Baltimore, Md: 1950) 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. Journal of immunology (Baltimore, Md: 1950) 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science (New York, N Y) 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS biology. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of medical microbiology. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Patarca-Montero R, Antoni M, Fletcher MA, Klimas NG. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Applied neuropsychology. 2001;8:51–64. doi: 10.1207/S15324826AN0801_7. [DOI] [PubMed] [Google Scholar]
- Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. Journal of leukocyte biology. 2008;84:940–948. doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Reber SO. Stress and animal models of inflammatory bowel disease--an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37:1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Rezende RM, Oliveira RP, Medeiros SR, Gomes-Santos AC, Alves AC, Loli FG, Guimaraes MA, Amaral SS, da Cunha AP, Weiner HL, Azevedo V, Miyoshi A, Faria AM. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. Journal of autoimmunity. 2013;40:45–57. doi: 10.1016/j.jaut.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature reviews. Gastroenterology & hepatology. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn JA, Corssmit EP, Havekes LM, Pijl H. Gut-brain axis. Current opinion in clinical nutrition and metabolic care. 2008;11:518–521. doi: 10.1097/MCO.0b013e328302c9b0. [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL. Lymphocytes in neuroprotection, cognition and emotion: is intolerance really the answer? Brain, behavior, and immunity. 2011;25:591–601. doi: 10.1016/j.bbi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews Immunology. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature medicine. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert reviews in molecular medicine. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. Journal of child neurology. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nature immunology. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiological reviews. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia research. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, N Y) 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chemical immunology and allergy. 2012;98:163–175. doi: 10.1159/000336510. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, Sugimoto T, Kumanogoh A, Kayama H, Takeda K, Sakoda S, Nakatsuji Y. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PloS one. 2011;6:e27644. doi: 10.1371/journal.pone.0027644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CC, O'Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barre syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PloS one. 2007;2:e344. doi: 10.1371/journal.pone.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S, MacFabe DF. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. Journal of neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles Y, Gosalbes MJ, de Vries LE, Abellan JJ, Francino MP. Metagenomics and development of the gut microbiota in infants. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18 Suppl 4:21–26. doi: 10.1111/j.1469-0691.2012.03876.x. [DOI] [PubMed] [Google Scholar]
- Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, Nelson PA, Stroud RM, Cree BAC, Zamvil SS. Aquaporin 4-Specific T Cells in Neuromyelitis Optica Exhibit a Th17 Bias and Recognize Clostridium ABC Transporter. Annals of neurology. 2012;72:53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velickovic K, Markelic M, Golic I, Otasevic V, Stancic A, Jankovic A, Vucetic M, Buzadzic B, Korac B, Korac A. Long-term dietary L-arginine supplementation increases endothelial nitric oxide synthase and vasoactive intestinal peptide immunoexpression in rat small intestine. European journal of nutrition. 2013 doi: 10.1007/s00394-013-0585-8. [DOI] [PubMed] [Google Scholar]
- Verdu EF. Probiotics effects on gastrointestinal function: beyond the gut? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:477–480. doi: 10.1111/j.1365-2982.2009.01297.x. [DOI] [PubMed] [Google Scholar]
- Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Digestive diseases and sciences. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Molecular autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS one. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio. 2012;3 doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. Journal of proteome research. 2010;9:2996–3004. doi: 10.1021/pr901188e. [DOI] [PubMed] [Google Scholar]
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. The American journal of pathology. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelmer A, Bowen M, Jokilammi A, Finne J, Luzio JP, Taylor PW. Differential expression of the polysialyl capsule during blood-to-brain transit of neuropathogenic Escherichia coli K1. Microbiology (Reading, England) 2008;154:2522–2532. doi: 10.1099/mic.0.2008/017988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


