Abstract
We have recently reported that an immunotoxin targeting mesothelin produced durable major tumor regressions in patients with extensive treatment refractory mesothelioma. These unprecedented tumor responses have prompted us to review how mesothelin was discovered and the advances that led to these tumor responses. This review is not comprehensive but focuses on major developments over the past 20 years since mesothelin was first identified in our laboratory. Mesothelin is a cell-surface glycoprotein whose expression in normal human tissues is restricted to mesothelial cells. Because it is highly expressed by many solid tumors it is an attractive immunotherapy target. Antibody based therapies currently in clinical trials include an immunotoxin, a chimeric monoclonal antibody and an antibody drug conjugate. In addition, a mesothelin tumor vaccine and a mesothelin-CAR are being evaluated in the clinic. SS1P, an anti-mesothelin immunotoxin was the first mesothelin directed therapy to enter the clinic and its use showed that mesothelin targeted therapy was safe in patients. More importantly our recent work has shown that SS1P in combination with pentostatin and cyclophosphamide can result in durable tumor regression in patients with advanced mesothelioma and opens up the possibility that such an approach can benefit patients with many common cancers.
Discovery of Mesothelin
In the early 1990s Ira Pastan and Mark Willingham, realizing there were very few targets on the plasma membrane of solid tumors that were useful for antibody-based therapies, initiated a search for new antibodies that recognized cell-surface proteins highly expressed on cancers and not expressed on essential normal tissues so that undesirable side effects would not occur when antibodies were given to these patients. To make new monoclonal antibodies (mAbs), they used standard hybridoma methodology, but to prevent mice from making antibodies to normal tissue antigens, they added a step in which mice were tolerized to normal human proteins by first immunizing them with normal liver or kidney membranes and then treating with cyclophosphamide to kill the B cells activated by this immunization. In the experiment that led to the discovery of mesothelin, they were looking for a new antibody to ovarian cancer and thus the mice were immunized with an ovarian cancer cell line (OVCAR3). After isolation of candidate mAbs, they used immunohistochemistry on frozen sections of normal tissues to exclude mAbs reacting with essential organs.
In 1992 they reported on an antibody reacting with ovarian cancers named mAb K1 (1). Immunohistochemical studies performed on normal human and monkey tissues showed that the reactivity of mAb K1 was limited to the mesothelial cells of the pleura, peritoneum and pericardium, as well as cells of the fallopian tubes and tonsils (1). The mAb was subsequently shown to react with malignant mesotheliomas, as well as squamous cell carcinomas of the esophagus and cervix (2,3). The antibody was given the name K1, to acknowledge the contribution of Kai Chang, the postdoctoral fellow who worked on the project.
The K1 antibody has low affinity; it reacts with frozen tissues but not as well with formalin fixed tissues, presumably because the epitope it recognizes is destroyed by fixation. Subsequent studies using an antibody made to a peptide that reacts with fixed tissues showed mesothelin was also present in cancers of the pancreas, lung, stomach, bile ducts and triple-negative breast cancer (4–7). It was estimated that mesothelin is expressed in 30% of human cancers and is therefore a very important target for immunotherapy (8).
Protein Characterization and Cloning
To identify the protein reacting with mAb K1, proteins on the cell surface were labeled with 125I and the cells were treated with phospholipase C to release surface proteins. The proteins released were subjected to SDS PAGE followed by western blotting. The antibody recognized a protein with a molecular weight (M.W.) of 40-kDa on both OVCAR3 and Hela cells. The K1 mAb was then used to screen a lambda cDNA expression library made from Hela cells. The cDNA that was isolated encoded a 69-kDa protein, much larger than the 40-kDa protein detected on the surface of cells (9). When the cDNA was expressed in 3T3 cells, a major 40-kDa band and a minor 69-kDa band was detected indicating the 40-kDa band was derived from a larger protein. Furthermore analysis of the DNA sequence showed that the C terminus of the protein was characteristic of proteins, which are attached to the plasma membrane by phosphatidyl inositol. Since the protein was expressed in normal mesothelial cells, we named the gene and the protein it encoded mesothelin.
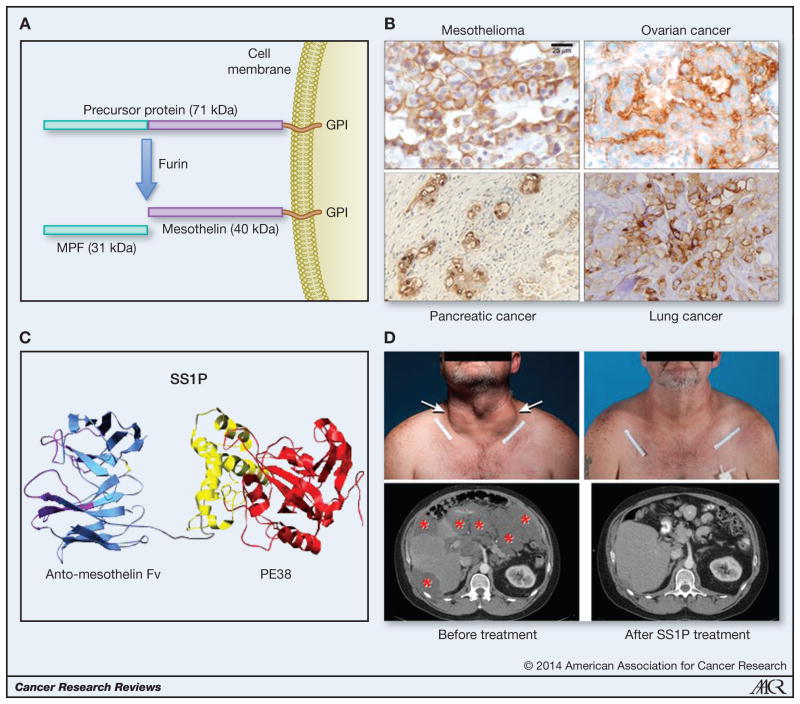
Cell-surface mesothelin is almost exclusively of the 40-kDa-glycosylated form. The amino terminal peptide named MPF (megakaryocyte potentiating factor) is released from cells by the action of the protease furin (Figure 1A). MPF was initially identified as a factor produced by a pancreatic cancer cell line that had the ability in the presence of interleukin 3 to stimulate megakaryocyte differentiation in mice (10). Its function in humans is not clear at this time.
Figure 1.
Processing of the 71-kDa mesothelin precursor protein to MPF and membrane-bound mesothelin by the protease furin. Mesothelin is attached to the cell membrane by a GPI anchor (A). Mesothelin expression detected in different human tumors by immunohistochemistry using an anti-mesothelin antibody. Mesothelin positivity is shown by brown staining of the tumor cells (B). Schematic representation of the immunotoxin SS1P consisting of an anti-mesothelin Fv linked to the truncated Pseudomonas exotoxin, PE38 (C). Major tumor shrinkage in the neck and abdomen of a patient with metastatic peritoneal mesothelioma treated with SS1P in combination with pentostatin and cyclophosphamide (D).
[A portion of this figure (B) was originally published in an article by Hassan and Ho in the Eur. J. Cancer 44:46–53, 2008, Elsevier publishers]
Immunotherapy Target
To determine if mesothelin would be a useful target for antibody based therapies, Hassan and colleagues carried out experiments in mice subcutaneously bearing grafted human tumors expressing mesothelin. In the first experiment the K1 antibody was labeled with indium111 and specific tumor uptake of the labeled antibody was demonstrated (11). To determine if the antibody could be used to kill tumor cells, a fragment of Pseudomonas exotoxin A (PE) was attached to the antibody and the resulting immunotoxin (K1-LysPE38QQR) was shown to kill mesothelin expressing cell lines and to cause regressions of a mesothelin-expressing tumor in mice (12). These two experiments plus the frequent distribution of mesothelin on cancers and the limited expression on normal tissues provided key evidence that mesothelin is an excellent target for antibody based therapies and encouraged the development of agents that could be used in patients.
Because the K1 antibody has a low affinity for mesothelin, a new antibody termed SS was produced by immunizing mice using a mesothelin-expressing cDNA construct. The technique of phage display was used to isolate an Fv that bound to mesothelin (13). The affinity of SS was low, but was improved to a Kd of about 1nM by mutagenizing residues in the CDR3 of the heavy chain of the Fv using a new technique called hot spot mutagenesis (14). The new Fv (SS1) was used to make a recombinant immunotoxin, termed SS1P, by fusing the Fv to a 38-kDa fragment of PE. Recombinant immunotoxins are chimeric proteins in which a tumor-specific Fv is attached to a portion of PE or other protein toxins (15). SS1P kills cells by binding to mesothelin, entering cells by receptor-mediated endocytosis, inactivating elongation factor 2, arresting protein synthesis and inducing apoptosis.
Blood Test for Mesothelin
In 1999 Scholler and colleagues reported the development of a blood ELISA test that measured “soluble member(s) of the mesothelin/megakaryocyte potentiating factor family” in sera from patients with ovarian carcinoma (16). They named this material SMRP (soluble mesothelin related peptide) and suggested it differed from mesothelin. We had also been developing a test to measure mesothelin levels in the blood using two new antibodies to mesothelin (MN and MB) made by our group and thought it very likely that SMRP was actually shed mesothelin (17). To demonstrate the nature of the protein shed from cells, we isolated the protein and determined its amino acid sequence. These studies showed that the shed protein is identical to mesothelin and established that SMRP is actually shed mesothelin (18).
Mesothelin levels are elevated in about 50% of patients with mesothelioma and ovarian cancer (17,19). Unexpectedly mesothelin levels are not elevated in patients with pancreatic cancer despite the fact that the majority of pancreatic adenocarcinomas highly express mesothelin on the cell surface (20). It is also not clear why mesothelin levels are not elevated in more patients with mesothelioma because the protein is highly expressed in almost all patients with epithelial-type mesothelioma. Possible reasons for the lack of detection of mesothelin in the blood are poor shedding, destruction in the cancer before entry into the blood, barriers to entry into the blood and small volume disease. Several ELISAs have also been developed to measure the levels of MPF in human serum (21). MPF levels, like mesothelin levels, are elevated in many patients with mesothelioma and ovarian cancer but are not elevated in patients with pancreatic adenocarcinoma (20).
Although mesothelin is attached to the cell membrane through its GPI anchor and phospholipase C can release the protein from the cell, it appears that phospholipase C does not play a significant role in the physiological shedding process. Mesothelin shedding is mediated by the sheddase TNF-α converting enzyme (TACE), which is a member of the matrix metalloproteinase/a disintegrin and metalloprotease (MMP/ADAM) family (22).
Mesothelin Function
Although many studies have been carried out on the possible function of mesothelin, its role in cancer is still unclear and may be cancer type-specific. Mice in which the mesothelin gene had been inactivated appeared perfectly normal. They bred normally, had normal blood counts and grew to normal size (23).
It was shown that cells expressing CA125 bind to cells expressing mesothelin (24). Furthermore the anti-mesothelin antibody MORAb-009 prevents this binding (25). Based on these studies it was suggested that mesothelin has a role in the spread of ovarian cancer cells in the peritoneal cavity and mesothelioma in the pleural and peritoneal cavity. However no direct evidence in support of this hypothesis was published, although an interaction of mesothelin and CA125 must occur in patients, since patients treated with the mesothelin-binding antibody MORAb-009 had an elevation in serum levels of CA125 (26).
A variety of studies have been done to evaluate the function of mesothelin. In Eker rats, the development of tuberous sclerosis-2-induced renal carcinoma was significantly reduced in the absence of a homologue of the MSLN gene (27). In pancreatic cancer cells, overexpression of MLSN was implicated in significant enhancement of tumor cell growth and migration in vitro, but the molecular mechanism that contributes to these phenotypes are not well understood (28). On the other hand human mesothelioma cells frequently lose mesothelin expression when placed in culture, indicating that mesothelin is not needed for growth of mesothelioma cells, although it may contribute to the aggressive behavior of some types of cancer in animals (29). Recently it was shown that in patients with early-stage lung adenocarcinoma overexpression of mesothelin is associated with decreased overall survival (30). However, the precise role of mesothelin in carcinogenesis is unclear and is an active area of investigation.
Clinical Trials
Because of the high expression of mesothelin in many malignancies (Figure 1B), a variety of agents are being developed to target mesothelin. Results of several ongoing clinical trials of immunotherapy agents directed against mesothelin have shown that targeting mesothelin is safe and does not result in toxicity to essential normal tissues. Both antibody-based therapies, as well as mesothelin vaccines, are being investigated.
Immunotoxins
The first mesothelin directed agent to enter the clinic was the immunotoxin SS1P (Figure 1C). Because the SS1P contains a bacterial protein, the goals of the phase I trials were to determine its safety profile, to determine a maximum tolerated dose (MTD) and to assess how many doses could be given before antibodies formed and inactivated the toxin. In one trial SS1P was given as a bolus infusion over 30 minutes and the dose-limiting toxicity was pleuritis. A second cycle of SS1P could only be given to 10% of patients due to neutralizing antibody formation (31). In another trial SS1P was given as a continuous infusion for 10 days. Dose-limiting side effects were very similar to the bolus infusion trial (32). In neither trial were major responses observed, although there was shrinkage of small volume disease in some patients.
One factor limiting the activity of large M.W. molecules like immunotoxins and antibody drug conjugates is penetration into solid tumors. To overcome this limitation we performed a clinical trial in which we combined SS1P with chemotherapy. We had shown in mouse studies that chemotherapy, by disrupting tumor cell packing and lowering intra-tumoral fluid pressure, allowed immunotoxin to reach more cells within the tumor and produced better anti-tumor responses (33). In the trial SS1P was combined with pemetrexed and cisplatin for the treatment of chemotherapy naïve patients with unresectable pleural mesothelioma. Results of this clinical trial showed that SS1P can be safely combined with pemetrexed and cisplatin and both chemotherapy and SS1P could be administered at their MTDs without overlapping toxicity. Out of the 13 patients treated at the MTD 10 patients had objective partial response. Although this was a small trial the objective response rate of 77% was significantly better than the 41% response rate seen in patients treated with chemotherapy alone (34). However, as seen in the phase I clinical trials of SS1P alone, 90% of patients developed neutralizing antibodies to SS1P after only one cycle of SS1P and limited re-treatment.
Development of human antibody response to immunotoxins in patients with solid tumors is a significant impediment to their clinical development and previous efforts to limit their immunogenicity by treatment with single agents such as steroids, cyclophosphamide, cyclosporine or rituximab have not been successful (35–37). However, we have recently shown that a regimen of two agents, pentostatin and cyclophosphamide to deplete T and B cells, completely abrogated the development of anti-SS1P antibodies in immune-competent Balb/C mice, which were immunized with SS1P (38).
This strategy of using pentostatin and cyclophosphamide to prevent human antibody response to SS1P was evaluated in a pilot study of heavily pre-treated chemotherapy refractory mesothelioma patients. Eleven patients were enrolled in the study and received pentostatin and cyclophosphamide prior to SS1P administration. This regimen was well tolerated and no patient developed opportunistic infections. As predicted this regimen decreased the development of human antibodies to SS1P; only two of ten patients developed antibodies after cycle 1 of therapy, which was significantly better than in prior clinical trials where about 90% of patients developed antibodies after one cycle of therapy (39). Unexpectedly we observed remarkable anti-tumor activity in these treatment refractory patients who had poor prognosis (Figure 1D). Three of the ten evaluable patients with extensive tumor burden had durable partial response and two of these patients had complete metabolic response by PET scan. All three patients were alive after 27, 25 and 22 months of starting therapy. In addition, two patients who did not initially respond to SS1P had a dramatic anti-tumor response when treated with chemotherapy to which they had not previously responded. We have recently initiated additional clinical studies in which the pentostatin and cyclophosphamide regimen will be evaluated in more patients with mesothelioma, as well as in patients with pancreatic cancer.
Chimeric Anti-mesothelin Antibody
MORAb-009 (Amatuximab) is a chimeric antibody directed to mesothelin. In pre-clinical studies it kills mesothelin expressing cell lines by ADCC and also inhibits the interaction between mesothelin and CA-125 (25). In a phase I clinical trial this antibody was well tolerated and the MTD was established as 200 mg/m2 (40). A randomized phase II clinical trial of amatuximab plus gemcitabine versus gemcitabine alone in patients with pancreatic cancer failed to show any advantage to the combination therapy (41). In a non-randomized clinical trial of patients with advanced unresectable pleural mesothelioma, amatuximab was given in combination with pemetrexed and cisplatin. Although this trial did not achieve its primary objective, improvement in progression-free survival as compared to historical controls showed improvement of overall survival with a plateau at the end of the survival curve (42). These data are encouraging for patients with advanced mesothelioma and suggest that this regimen should be evaluated in a randomized clinical trial compared to pemetrexed and cisplatin alone.
Antibody Drug Conjugates (ADC)
BAY94-9343 is an ADC in which a humanized IgG1 anti-mesothelin mAb is linked to the maytansine derivative DM4; it is currently undergoing phase I evaluation in patients with advanced solid tumors. BAY94-9343 is given as an i.v. infusion every 3 weeks till disease progression or unacceptable side effects. Preliminary results of this trial show acceptable toxicity profile and the MTD has not yet been achieved (43).
Chimeric Antigen Receptor (CAR) T Cell Therapy
CAR T cells are engineered to express the Fv portion of a mAb fused to a T cell receptor. Binding of the CAR to the tumor antigen activates T cell signaling and results in cell killing. Since early clinical trials of SS1P showed no off target toxicity, there is a growing interest in exploiting mesothelin as a target for CAR T cell therapy. Currently, two anti-mesothelin CAR T cell approaches are being pursued (44,45). Preliminary results of mesothelin-specific CAR mRNA-engineered T cells show that this approach is safe and can induce anti-tumor responses (46).
Mesothelin Tumor Vaccines
The only mesothelin tumor vaccine currently in clinical development is CRS-207, which consists of a live-attenuated strain of the bacterium Listeria monocytogenes expressing human mesothelin. The safety of this vaccine was established in a phase I clinical trial of patients with mesothelin expressing refractory cancers (47). This vaccine has recently been evaluated in a randomized clinical trial of patients with advanced pancreatic cancer. These patients were randomized to six cycles of GVAX (allogeneic pancreatic cancer cell lines secreting GM-CSF) vaccine alone or two cycles of GVAX followed by four cycles of CRS-207 every 3 weeks. A total of 90 patients were enrolled with 61 treated with GVAX/CRS-207 and 29 with GVAX alone. After a median follow-up of 7.8 months the median overall survival of patients treated with GVAX/CRS-207 was 6.1 months versus 3.9 months for patients treated with GVAX alone (p=0.011) (48). Although a small study these results are impressive in this study population and need to be validated in a larger phase III setting. CRS-207 is also in early stages of clinical evaluation in patients with mesothelioma and is being tested in a front line setting in combination with pemetrexed and cisplatin.
Future Directions
Clinical trials of different agents targeting mesothelin have confirmed that it is an excellent target for cancer immunotherapy and much of the effort is now focused on larger trials to confirm the preliminary hints of anti-tumor activity seen in patients. Our studies have shown that combining SS1P with pentostatin and cyclophosphamide can delay antibody formation to SS1P and lead to durable anti-tumor activity in some patients. We have also employed protein engineering to identify and remove B and T cell epitopes in SS1P to make a less immunogenic immunotoxin (49,50). Based on these studies we have recently developed a new immunotoxin in which domain II of PE was deleted and six residues in domain III mutated to alanine. Because the deletion of domain II resulted in a protein that is small and rapidly filtered by the kidney, we collaborated with Roche Pharmaceuticals to replace the Fv with a Fab to make RG7787. Preclinical studies with RG7787 have shown that large doses can be safely given to mice, that it has a decreased capacity to cause capillary leak syndrome in rats and that it has significant anti-tumor activity in mice bearing several types of mesothelin expressing tumors. RG7787 is expected to undergo phase I clinical evaluation by the end of 2014.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and a CRADA with Roche Pharmaceuticals
Footnotes
Conflict-of Interest: The authors declare no conflict-of-interest.
References
- 1.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992 doi: 10.1002/ijc.2910500308. 50-73-81. [DOI] [PubMed] [Google Scholar]
- 2.Chang K, Pai LH, Pass H, Pogrebniak HW, Tsao MS, Pastan I, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–68. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Pastan I, Willingham M. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinoma. Int J Cancer. 1992;51:548–54. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 4.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 1993;27:1418–28. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is over-expressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 7.Tchou J, Wang L-C, Selven B, Zhang H, Conejo-Garcia J, Borghaei H, et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren YR, Patel K, Paun BC, Kern SE. Structural analysis of the cancer-specific promoter in mesothelin and in other genes overexpressed in in cancers. J Biol Chem. 2011;286:11960–9. doi: 10.1074/jbc.M110.193458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi N, Hattori K, Oh-eda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J Biol Chem. 1994;269:805–8. [PubMed] [Google Scholar]
- 11.Hassan R, Wu C, Brechbiel MW, Marguilies I, Kreitman RJ, Pastan I. 111Indium-labeled monoclonal antibody K1: biodistribution study in nude mice bearing a human carcinoma xenografts expressing mesothelin. Int J Cancer. 1999;80:559–63. doi: 10.1002/(sici)1097-0215(19990209)80:4<559::aid-ijc13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer cancer and malignant mesothelioma. J Immunother. 2000;23:473–9. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA. 1998;95:669–74. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotech. 1999;17:568–72. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 15.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 16.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellström, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–6. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan R, Remaley A, Sampson ML, Zhang J, Cox DD, Pingpank J, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–53. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 18.Ho M, Onda M, Wang QC, Hassan R, Pastan I, Lively MO. Mesothelin is shed from tumor cells. Cancer Epidemiol Biomarkers Prev. 2006;15:1751. doi: 10.1158/1055-9965.EPI-06-0479. [DOI] [PubMed] [Google Scholar]
- 19.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–15. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 20.Sharon E, Zhang J, Hollevoet K, Steinberg SM, Pastan I, Onda M, et al. Serum mesothelin and megakaryocyte potentiating factor in pancreatic and biliary cancers. Clin Chem Lab Med. 2012;50:721–5. doi: 10.1515/CCLM.2011.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onda M, Nagata S, Ho M, Bera TK, Hassan R, Alexander RH, et al. Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res. 2006;12:4225–31. doi: 10.1158/1078-0432.CCR-06-0472. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Chertov O, Zhang J, Hassan R, Pastan I. Cytotoxic activity of immunotoxin SS1P is modulated by TACE-dependent mesothelin shedding. Cancer Res. 2011;71:5915–22. doi: 10.1158/0008-5472.CAN-11-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–6. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–8. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 25.Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-09, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan R, Schweizer C, Lu KF Schuler B, Remaley AT, Weil SC, et al. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: implications for cancer therapy. Lung Cancer. 2010;68:455–9. doi: 10.1016/j.lungcan.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Kajino K, Abe M, Tan K, Maruo M, Sun G, et al. Suppression of cell death by the secretory form of N-terminal ERC/mesothelin. Int J Mol Med. 2010;26:185–91. doi: 10.3892/ijmm_00000451. [DOI] [PubMed] [Google Scholar]
- 28.Zheng C, Jia W, Tang Y, Zhao H, Jiang Y, Sun S. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and –independent signal pathway. J Exp Clin Cancer Res. 2012;31:84. doi: 10.1186/1756-9966-31-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Qiu S, Zhang Y, Merino M, Fetsch P, Avital I, et al. Loss of mesothelin expression by mesothelioma cells grown in vitro determines sensitivity to anti-mesothelin immunotoxin SS1P. Anticancer Res. 2012;32:5151–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20:1020–8. doi: 10.1158/1078-0432.CCR-13-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 32.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase 1 trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Xiang L, Hassan R, Paik CH, Carrasquillo JA, Jang BS, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 34.Hassan R, Sharon E, Schuler B, Mallory Y, Zhang J, Ling A, et al. Antitumor activity os SS1P with pemetrexed and cisplatin for front-line treatment of pleural mesothelioma and utility of serum mesothelin as a marker of tumor response. J Clin Oncol. 2011;29(suppl):abstr 7026. [Google Scholar]
- 35.Oratz R, Speyer JL, Wernz JC, Hochster H, Meyers M, Mischak R, et al. Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma: results of a phase II trial. J Biol Response Mod. 1990;9:345–54. [PubMed] [Google Scholar]
- 36.Selvaggi K, Saria R, Schwartz R, Vlock DR, Ackerman S, Wedel N, et al. Phase I/II study of murine monoclonal antibody-ricin A chain (xomazyme-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother. 1993;13:201–7. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Hassan R, Williams-Gould J, Watson T, Pai-Scherf L, Pastan I. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin Cancer Res. 2004;10:16–8. doi: 10.1158/1078-0432.ccr-1160-3. [DOI] [PubMed] [Google Scholar]
- 38.Mossoba ME, Onda M, Taylor J, Massey PR, Treadwell S, Sharon E, et al. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;17:3697–705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–8. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov [homepage on the internet]. Identifier: NCT00570713.
- 42.Hassan R, Jahan TM, Kindler HL, Bazhenova L, Reck M, Pastan I, et al. Amatuximab, a chimeric monoclonal antibody to mesothelin, in combination with pemetrexed and cisplatin in patients with unresectable pleural mesothelioma: results of a multicenter phase II clinical trial. J Clin Oncol. 2012;30(suppl):abstr 7030. [Google Scholar]
- 43.Bendell J, Blumenschein G, Zinner R, Hong D, Jones S, Infante J, et al. First-inhuman phase I dose escalation study of a novel anti-mesothelin antibody drug conjugate (ADC), BAY 94-9343, in patients with advanced solid tumors. AACR Annual Meeting 2013; Washington, DC. (Clinical Trials Symposium Abstract) [Google Scholar]
- 44.ClinicalTrials.gov [homepage on the internet]. Identifier: NCT01897415.
- 45.ClinicalTrials.gov [homepage on the internet]. Identifier: NCT01583686.
- 46.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–20. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le DT, Wang-Gillam A, Picozzi V, Jr, Greten TF, Crocenzi TS, Springett GM, et al. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: updated results. J Clin Oncol. 2014;32(suppl 3):abstr 177. [Google Scholar]
- 49.Liu W, Onda M, Lee B, Kreitman RJ, Hassan R, Xiang L, et al. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA. 2012;109:11782–7. doi: 10.1073/pnas.1209292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazor R, Vassall AN, Eberle JA, Beers R, Weldon JE, Venzon DJ, et al. Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc Natl Acad Sci USA. 2012;109:E3597–603. doi: 10.1073/pnas.1218138109. [DOI] [PMC free article] [PubMed] [Google Scholar]