Abstract
Today, there is neither an effective nor an active treatment for food allergy. Allergy immunotherapy has been proposed as an attractive strategy to actively treat food allergy. Oral immunotherapy (OIT), also known as oral desensitization, is a method of inducing the body's immune system to tolerate a food that causes an allergic overreaction. It has been studied for the use in treatment of immunoglobulin E-mediated food allergy to the most common foods, including milk, egg, and peanut. OIT has been able to desensitize subjects to varying degrees. However, many questions remain unanswered, including efficient formulation, optimal dosing, and induction protocol to achieve full tolerance, transition of OIT to clinical practice, and maximal safety profile. This review focuses on the use of OIT as a new and active treatment for food allergy. The possibility of transition of OIT to clinical practice represents, in this field, the next pivotal step with the goal of improving the quality of life of patients with food allergy and their families.
Over the past few decades, the prevalence of allergic diseases has increased, especially in developed countries. While the prevalence of asthma has stabilized and the prevalence of eczema appears to be slowly decreasing, the prevalence of food allergy and anaphylaxis continues to rise.1
At present, there is no effective long-term treatment for food allergy. Supportive management of food allergy, consisting of avoidance of offending foods, early recognition, and treatment of anaphylaxis, is currently the standard of care for affected individuals.
Avoidance of food allergens is, to some extent, difficult to achieve, particularly with commercially prepared foods. Furthermore, 40%–100% of deaths from food anaphylaxis involved ingestion of foods catered or prepared away from home.2–4
Therefore, a safe and affordable therapeutic approach is needed, for at least patients who are at risk for anaphylaxis. Allergen-specific immunotherapy is capable of modifying the immunoglobulin E (IgE)-mediated immune response and of achieving a long-term protection against allergy. It has been demonstrated to be an effective treatment for both respiratory and hymenoptera venom allergies.5 For this reason, allergy immunotherapy was proposed as an attractive strategy to treat food allergy. The first attempt with food immunotherapy was made in the 1980s with peanut extracts administered subcutaneously. An unacceptable rate of severe adverse events prompted the abandonment of further development of this route of administration for the active treatment of food allergy.6,7 Thus, the oral administration of foods to achieve a tolerance or desensitization was proposed. This appears particularly suitable for cow's milk (CM), hen's egg (HE), and peanut allergies.8–27 Several clinical studies on the oral administration of allergy foods are available, and some trials are currently under way to better define indications, contraindications, and practical aspects. Of note, there is no specific study for non-IgE-mediated food allergy, such as food protein-induced enterocolitis syndrome. Recently, multiple studies have been published, and others are under way at various stages of research on the practice of oral immunotherapy (OIT).
OIT, sublingual immunotherapy (SLIT), and epicutaneous immunotherapy have been studied for the treatment of IgE-mediated food allergy, although reports on OIT thus far have been more extensive. Although clinical trials with OIT have been encouraging, additional studies are required before it can be recommended for incorporation into clinical care standards (Table 1).
Table 1.
Key Issues in Oral Immunotherapy
| Reaction severity and food type that drive the need for active treatment. |
| “Shared Schedules” for desensitization and extent of protection. |
| Adverse events with allergy foods during OIT and/or following OIT with previously tolerated foods. |
| Desensitization versus (or toward) tolerance? |
OIT, oral immunotherapy.
The Reaction Severity and Food Type that Drive the Need for Active Treatment
Allergic reactions to food proteins can range from immediate, potentially life-threatening reactions to chronic or long-lasting diseases, thus seriously impacting on patients and their families. On the other hand, some reactions to foods such as oral allergy syndrome caused by peach or apple are, frequently, mild and not life-threatening. The risk–benefit of OIT should be carefully considered in individuals with no life-threatening food allergies.27–30 Therefore, these allergic individuals with such symptoms should be considered cautiously regarding the risk–benefit ratio.
Moreover, a large number of children with allergy to CM or HE develop tolerance spontaneously. Therefore, in these patients, waiting until at least age 3 before starting OIT seems reasonable. The question of whether OIT has been adequately studied to be used in routine clinical practice has led to some disagreements between academic allergists and allergists in practice.31 Concerning atopic dermatitis (AD), ∼35% of children with AD sensitized to foods will have symptoms of food allergy upon challenge.32 Elimination of identified foods has been shown to provide improvement of AD symptoms. It should be combined with good skin care and pharmacotherapy when needed.33 Since AD may be unrelated to food allergies, appropriate skin care remains a cornerstone of care. Patients who respond well to skin care therapy with minimal topical steroid treatment are not likely to benefit from dietary interventions when no history of immediate and severe food allergy reactions are reported.
Therefore, children with mild-to-moderate AD without systemic symptoms could be carefully considered over OIT. Appropriate patient selection is pivotal for identifying individuals who worth OIT in terms of efficacy, adherence to treatment, and risk–benefit ratio.
Schedules for Desensitization and Degree of Protection
The first report of successful OIT in a child with HE-induced severe food allergy was published in 190834 with few sporadic cases following the initial report. Initial attempts to use traditional subcutaneous immunotherapy were short lived because injection therapy caused severe adverse reactions in the majority of treated patients. Patriarca et al. (1998)35 and Meglio et al. (2004)36 described 2 protocols for desensitizing 2 groups of children with IgE-mediated food allergy. Since then, numerous reports and trials have been published on the use of OIT as an active treatment for food allergy.
In addition, review articles37–39 and 4 meta-analysis on OIT have been published,40–43 including 2 recent Cochrane reports focusing on peanut–OIT42 and CM–OIT,43 respectively. The published trials are heterogeneous in terms of dose, duration of updosing regimen(s), maintenance dose(s), and severity of food allergy.
Currently, OIT is not standardized but instead is tailored to individual patient with consideration of the patient's age as well as type and severity of food allergy. Therefore, this condition makes a difference with patients allergic to environmental allergens. The published trials are different in terms of schedules, selection of enrolled patients, and form of treatment, such as OIT or SLIT (Table 2).
Table 2.
Clinical Trials of Oral Desensitization for Cow's Milk, Egg, and Peanut Allergies
| Author | Design | Number of patients | Age range, years | Female patients | Duration of induction | Initial dose | Tolerated dose | OIT discontinued for side effects |
|---|---|---|---|---|---|---|---|---|
| Staden et al.,8 milk | Randomized open controlled study | 25 | 1–13 | 16/25 (64%) | 67 days | 0.02 mg CM protein | Full tolerance (250 mL): 9/25 (36%); tolerant with regular intake: 3/25 (12%); partial tolerance: 4/25 | 9 (36%) |
| Skripak et al.,9 milk | Randomized DBPC | 13 OIT 7 Avoidance |
6–17 | 5 (38%) OIT 3 (43%) avoidance |
8 weeks | 0.4 mg of CM protein | 12/20 (37%) full tolerance (5,140 mg); 16/30 (53%) partial tolerance (5–150 mL) | 1/20 (5%) |
| Zapatero et al.,10 milk | Prospective study | 18 | >4 (mean age, 5.05 years) | 3 (17%) | 2 days | 0.05 mL | Full tolerance (200 mL): 15/18 (83%); partial tolerance (40 mL): 1/18 (5.5%) | 1/18 (11%) 1/18 (11%): failed |
| Longo et al.,11 milk | Randomized open controlled study | 30 OIT 30 Avoidance |
5–17 | 9 (30%) OIT 12 (40%) avoidance |
10 days (hospital) +3 months (home) | 1 drop of CM in 10 mL of water | Full tolerance (150 mL): 11/30 (37%); partial tolerance (5–150 mL): 16/30 (53%) | 3/30 (10%) |
| Pajno et al.,12 milk | Randomized, single-blind, soy milk-controlled trial | 15 OIT 15 Avoidance |
4–10 | 7 (46.7%) OIT 6 (40%) avoidance |
18 weeks | 1 drop of CM diluted 1:25 | Full tolerance (200 mL): 10/15 (77%); partial tolerance (40 mL): 1/15 (6.6%) | 2/15 (13%) 2/15 (13%) failed |
| Martorell et al.,13 milk | Randomized open controlled study | 30 OIT | 2 | 11 (36.7%) OIT | 2 days | 1 mL of diluted CM 1/100 | Full tolerance (200 mL): 27/30 (90%); partial tolerance (20–200 mL): 1/30 (3%) | 1/30 (3%): no tolerance |
| 30 Avoidance | 24–36 months | 15 (50%) avoidance | 1/30 (3%): abandoned study | |||||
| Salmivesi et al.,14 milk | Randomized, DBPC | 16 OIT 8 Avoidance |
6–14 | Not detailed | 24 weeks | 0.06 mg of CM protein | 16 (89%) OIT and 8 (80%) in the placebo group successfully completed the OIT protocol | 0% |
| Buchanan et al.,15 egg | Open study | 7 | 1–16 | Not detailed | 1 days (rush phase); 2 weeks (build-up phase) | 0.1 mg of powdered egg white | Full tolerance (300 mg): 2/7 (28.5%); partial tolerance (24 mg and 2 g): 2/7 (28.5%) | 3/7 (43%) |
| Itoh et al.,16 egg | Open study | 6 | 7–12 | 2 (33%) | Variable (9–18 days) | One-tenth of the threshold dose for each patient | 6/6 (100%) full tolerance (60 g) | None (0%) |
| Vickery et al.,17 egg | Open study | 8 | 1–16 | 3 (37.5%) | Rush phase (1 day), followed by build-up phase | 0.1 mg of powdered egg white | 4/8 (50%) full tolerance (300 g); 3/8 (37.5%) partial tolerance | None (0%) |
| Garcia Rodriguez et al.,18 egg | Prospective open study | 23 | 5–17 | 6 (26%) | 5 days | 0.001 mL of egg white | 20/23 (86.9%): full tolerance (cooked egg, omelette); 2/23 (8.6%) partial tolerance | 1/23 (4.3%) |
| Burks et al.,19 egg | Randomized DBPC | 40 OIT 15 Placebo |
5–11 | Not detailed | 22 months | Not detailed | 11/40 (27.5%): full tolerance | 5/40 (12.5) |
| Meglio et al.,20 egg | Randomized, controlled open study | 10 OIT 3 Placebo |
>4 (median age, 8.7) | 5 OIT 10 Placebo |
181 days | 0.27 mg of HE proteins (1 drop of raw HE diluted 1:100) | 8/10 (80%): full tolerance; 1/10 (10%): partial tolerance | 1/10 (10%) |
| Dello Iacono et al.,21 egg | Randomized controlled open study | 10 OIT 10 Placebo |
5–11 | 10 (50%) OIT | 176 days | 1 drop of a blended emulsion of 45 mL of raw HE and 150 mL of amino acid-based infant formula (Nutricia), corresponding to 0.015 mL of HE emulsion | 9/10 OIT (90%): partial tolerance (at least 10 mL, but <40 mL of raw HE emulsion, in a single dose) and 1/10 (10%) no tolerance (<5 mL) | None (0%) |
| Vazquez-Ortiz et al.,22 egg | Nonrandomized controlled parallel group intervention study | 50 | 5–18 | Not detailed | 16 weeks | 1/100 diluition with water: 0.1 mL (0.083 mg EW protein) | 40 (80%) full tolerance, 1 (2%) partial desensitization | 9 (18%) |
| Clark et al.,23 peanut | Open study | 4 | 9–13 | None | 6 weeks | 5 mg | 4/4 (100%) tolerated 2.38 g protein (equivalent to 10 peanuts) | None |
| Jones et al.,24 peanut | Open study | 39 | 1–16 | 25 (64%) | 1 day | 0.1 mg peanut protein | 27/29 (93%): full tolerance (3.9 g); 3/29 (7%) partial tolerance (2.1 g) | 4/39 (10%) 6/39 (15) |
| Blumchen et al.,25 peanut | Randomized open controlled study | 23 | 3–14 | Not detailed | 7 days | 0.03 g | 14/23 (61%): full tolerance (0.5–2 g); 1/23 (4%) partial tolerance (0.2 g) | 1/23 (4%) |
| Varshney et al.,26 peanut | Randomized DBPC | OIT 19 Placebo 9 |
1–16 | OIT 10/19 (60%); placebo: 0/9 (0%) | 1 day | 0.1 mg peanut protein | 16/19 (84%): full tolerance (4 g) | 3/19 (16%) |
| Fleischer et al.,27 peanut | Randomized DBPC multicenter trial SLIT | 40 | 12–37 (median, 15 years) | 13 (32%) | 44 week. The first phase of the study was a randomized, DBPCFC peanut SLIT trial through 44 weeks. The second phase was an unblinded additional 120 weeks of lower dose peanut SLIT treatment for the initial active therapy-treated subjects and 164 weeks of higher dose peanut SLIT for the placebo-treated subjects after crossover to active therapy | 0.000165 mg | 14/20 (70%): tolerated dose 500 mg for 8 subjects, 996 mg for 2 subjects, 1,996 mg for 1 subject, and 3,256 mg for 3 subjects | 4/40 (10%) |
CM, cow's milk; DBPC, double blind placebo controlled; DBPCFC, double blind placebo controlled food challenge; HE, hen's egg; OIT, oral immunotherapy; SLIT, sublingual immunotherapy.
The success rates of OIT vary from 36% to 90% with a wide range of outcomes. There are 4 distinct patterns of responders, which are as follows: responders without therapy or natural responders, partial responders, responders with daily exposure to the food allergen, and nonresponders. Responders may improve with the natural course of the food allergy, as with CM or HE. Partial responders can eat the culprit allergic food within other food products. Responders with daily exposures are represented by those who require daily ingestion of the culprit food. Nonresponders are represented by OIT failure.8 Some studies have been carried out using the sublingual route (SLIT), for example, hazelnut44 peanut,45 CM,46 and peach.47 When SLIT is compared with oral route, OIT was more efficacious for desensitization to CM than SLIT alone but was accompanied by more systemic side effects.48,49 A retrospective comparison study of patients with peanut allergy, treated with either peanut OIT or SLIT, indicated that after 12 months of therapy, patients who received SLIT reacted at lower eliciting dose thresholds and have been less likely to pass food challenges evaluation desensitization.50 Thus far, different schedules were used for clinical trials: rush immunotherapy,10,11,13,15,18,24–26 slow up dosing regimen,8,9,14,17,19,20–23,27 and weekly schedule.12 Altogether, the amount of tolerated dose(s) of foods is marginally affected by the different regimens.51
Therefore, the quality of the allergen vaccines is critical for both diagnosis and treatment. Standardized vaccines of known potency and shelf life should be used; currently, the vaccines containing food protein and those prepared by pharmaceutical companies or hospital pharmacies are not available as standardized products. Both the bacteriological load and biological activity of these products are still undetermined. Therefore, the use of fresh material or native foods for OIT is, at the moment, advisable to achieve the goal of desensitization. Altering the immune environment to prevent T helper 2 cell (Th2)-mediated responses directed against immunotherapeutic agents is another approach for increasing the efficacy and safety during OIT or SLIT. Therefore, using an anti-IgE monoclonal antibody (eg, omalizumab) as an adjunct to OIT or SLIT may be a safer and effective strategy. The utility of using omalizumab to facilitate desensitization in a small group of children undergoing high-dose milk OIT was recently investigated.52 After 9 weeks of pretreatment with omalizumab, 9 of the 11 patients initially enrolled were able to rapidly reach the maintenance dose with minimal adverse events.
Desensitization Versus (or Toward) Tolerance?
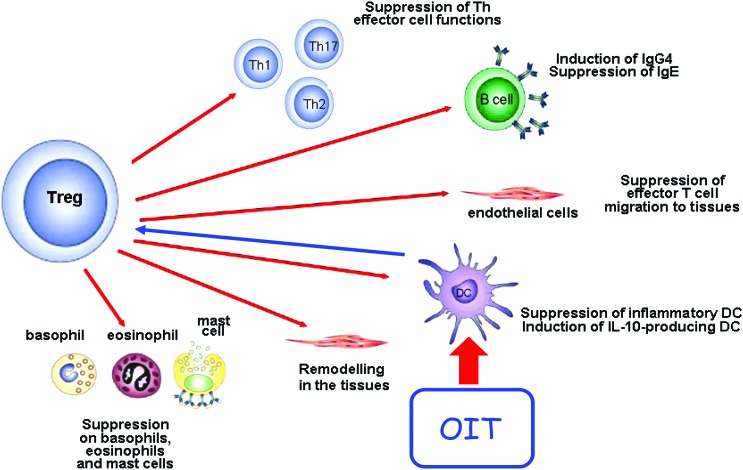
In a strict sense, allergen immunotherapy describes the administration of gradually increasing doses of the allergen to reduce symptoms due to natural allergen exposure, to induce measurable immunological changes [eg, IgE, immunoglobulin G4 (IgG4), T helper 1 cell (Th1)/Th2 balance, T regulatory cells (Treg)], and possibly to maintain the tolerance status with periodic delayed administration of offending allergens.53 The presumed mechanism of action for OIT is the activation of gut mucosal dendritic cells, which affect the allergic response through immunomodulation of circulating effector cells.54 Other mechanisms have been shown to be important including the increase in specific IgG412 and IgE receptor pathway suppression for basophils.49 For other immunologic variables in some studies, there is no change in specific IgE levels,9,12,24 the regulation of antibody isotypes demonstrating late decrease in specific IgE.17 In allergic diseases, including IgE-mediated food allergy, the balance between Treg and disease-promoting Th2 appears to be decisive in the development of an allergic versus a nondisease promoting or healthy immune response against allergen. As with other forms of immunotherapy, Treg appears to have likely a pivotal role in various immunosuppressive pathways (Fig. 1). According to this concept in the case of food allergy, a permanent tolerance should be expected, which implies that the food can be ingested without the appearance of allergic symptoms despite periods of withdrawal. In contrast, the term desensitization refers to a reversible state after short-term exposure to incremental doses of an allergen that renders effector cells less reactive, but once the administration of the allergen is discontinued, the previous level of clinical reactivity returns. Currently, it is still unclear whether oral desensitization represents the first step toward permanent oral tolerance. As with other kinds of immunotherapy (ie, for environmental allergens), the duration of desensitization could be pivotal for achieving tolerance. Although clinical desensitization and immune modulation have been demonstrated with OIT, the strength of the current evidence from clinical trials is insufficient concerning the induction of tolerance.54 Several uncontrolled studies have previously been reported on the development of sustained unresponsiveness following egg,8,17 milk,8,48 and peanut55 OIT. The only report of sustained unresponsiveness from a placebo-controlled trial of food OIT (egg) was recently published in an article from the Consortium of Food Allergy Research.19 This large multicenter study demonstrated that sustained unresponsiveness occurred in 27.5% of subjects actively treated for 22 months with none in the placebo group. It is worth noting that the development of sustained unresponsiveness most likely represents the achievement of a true tolerance to previously offending allergens. However, additional trials are warranted to further investigate the long-term effects of OIT.
FIG. 1.
Schematic representation of the putative mechanisms of action of oral immunotherapy (OIT), with the various pathways that T regulatory cells (Treg) can exert on cells of the innate and adaptive immune systems, leading to the suppression of a variety of effector cell functions. OIT works primarily through allergen activation of dendritic cells (DC) in the gut mucosa, resulting in effector cell modulation. (Adapted with permission from Dr. C. Akdis.) Color images available online at www.liebertpub.com/ped
In developed countries, the continuous or frequent ingestion of foods such as CM or HE, usually present in the diet, after the achievement of desensitization is per se easy to do by patients; therefore, active specific therapy could be successful for some food allergies, even without the induction of a true permanent tolerance. Regarding this issue, a very recent trial showed that the achieved tolerance to CM can be maintained without a mandatory daily consumption.56
Adverse Events
In all immunotherapy trials, safety is of the paramount importance. The appearance of adverse reactions during OIT is reported frequently; in some studies, 100% of patients experienced adverse reactions during desensitization with CM,8,11 but OIT had to be discontinued in <20% of subjects.39
The rate of adverse events with HE OIT is also high (78%), with a study dropout rate of 10%.19 Recently, a study confirmed the efficacy of OIT performed with raw eggs in 40 (80%) children allergic to HE, the discontinuation of OIT in remaining 10 (20%) subjects was associated with underlying asthma, higher specific IgE, and lower threshold of oral food challenge.22 A large peanut OIT study57 examined clinical reactions throughout all stages of the protocol and revealed that the frequency and severity of reactions were greatest on the initial rush induction days and least during the home dosing phases. Ninety-three percent of subjects experienced some symptoms during the initial rush induction, mostly upper respiratory (79%) and abdominal (68%) symptoms, although 4 patients withdrew (12%) because of persistent adverse reactions. During the subsequent build-up phase, adverse reactions occurred after 46% of the build-up doses, with 29% experienced upper respiratory tract symptoms and 24% skin symptoms. Severe systemic side effects have been reported independent of the schedule, that is, with rush,13 weekly,12 or slow up dosing regimen.9 Mild reactions, such as abdominal pain, throat pruritus, gritty eyes, watery eyes, transient erythema, and sneezing, usually do not require stopping desensitization.
On the contrary, when rhinitis, dyspnea, asthma, generalized urticaria, and hypotension occur alone or in combination, OIT should be stopped and reevaluated. Adverse reactions are largely unpredictable, and they can occur during home dosing. In addition, systemic reactions have occurred with previously tolerated doses in the occasion of exercise,58 viral illness, or suboptimal controlled asthma.59 Of note, these reactions are usually well controlled by antihistamines, steroids, or epinephrines.
Conclusion
IgE-mediated food allergy represents both a promising and an intriguing field of application for allergen immunotherapy especially in the oral form. The published study41 along with the recently published meta-analysis confirmed the overall benefit of OIT.42,43 However, the procedure is time-consuming and not devoid of side effects, whereas we know that many children with CM allergy or HE allergy develop tolerance spontaneously, and they can be easily managed with avoidance of regimen.
For these children, waiting for the natural history of their allergies, before starting OIT, represents a convincing option. In contrast, the avoidance of regimen may be insufficient in children with severe systemic reactions because of the risk of inadvertent food intake or of the assumption of foods containing proteins as hidden allergens. In such patients, an effective tolerance induction would represent a life-saving treatment. Thus, more information on indications for OIT, such as the appropriate patients and the predictive factors to identify responders and subjects at risk for serious side effects, is urgently needed.
Another aspect that must be clarified is if the tolerance status achieved by OIT is permanent or if it needs to be maintained with a regular intake of culprit food(s).
Nowadays, it seems that ad libitum consumption of known food allergens to maintain tolerance does not appear to be required in all cases. Therefore, more flexible maintenance of regimens is possible at least for children who have been successfully desensitized to CM.56 The clinical trials have shown that OIT can successfully desensitize a large number of individuals without major morbidity or mortality. Ultimately, the majority of patients experienced a greater tolerance to the offending foods compared to pretreatment. The ultimate goal is to be able to extend OIT protocols to primary care practices as standard medical therapy. However, at this time, OIT remains in the purview of allergists and immunologists because of the associated safety concerns. Recently, anti-IgE monoclonal antibody (omalizumab) was used in combination therapy with OIT in individual with severe food allergy to CM. This combination may enhance both safety and efficacy of OIT.60
SLIT appears to be safer but less effective than OIT, which may be related to the lack of standardization of available sublingual extracts. Among patients who have undergone active treatment with SLIT, response has been variable. Therefore, the applicability of SLIT in the patients with food allergy remains unclear.49 On the other hand, OIT represents an emerging reality that provides both hope and optimism for patients with food allergy; it represents the active treatment for allergies caused by foods with the goal of improving the quality of life of patients and their families. Of note, selecting patients for OIT based on the presence of allergy documented by history, laboratory parameters, and oral food challenge(s) is not sufficient to ensure the success of OIT. Because of the length of the protocol, patients and their families must be extremely compliant, reliable, and committed to the treatment. So far, the clinical studies carried out with OIT have some limitations, such as the uncontrolled nature of most of the trials, variety of parameters included in the methods, and the heterogeneity in protocols.42,43 However, in our opinion, the time is ripe for the practice of OIT in selected medical centers and under strict medical supervision: Longum iter est per praecpta, breve et efficax per exempla (Far-reaching is the way of precepts, short and effective is the one of models)—Lucius Annaeus Seneca.
Author Disclosure Statement
Dr. Cox has received one or more consulting fees or honoraria from Stallergenes; has received one or more fees for participation from Novartis, Circassia, and Biomay; is a Board Member for and has received one or more payments for travel/accommodation/meeting expenses from the American Academy Allergy, Asthma Immunology, and the American Board of Allergy and Immunology. Dr. Pajno is a Board Member (Pediatric Section) of and received one or more payments for travel/accommodation from the European Academy of Allergy and Clinical Immunology. The remaining authors declare that they have no relevant conflicts of interest.
References
- 1.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax 2007; 62:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allergy Clin Immunol 2007; 119:1018–1019 [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol 2007; 119:1016–1018 [DOI] [PubMed] [Google Scholar]
- 4.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009; 123:434–442 [DOI] [PubMed] [Google Scholar]
- 5.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy 2011; 41:1235–1246 [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer JJ, Nelson HS, Bock SA, Chritensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin Immunol 1992; 90:256–262 [DOI] [PubMed] [Google Scholar]
- 7.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 1997; 99:744–751 [DOI] [PubMed] [Google Scholar]
- 8.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns reaction. Allergy 2007; 62:1261–1269 [DOI] [PubMed] [Google Scholar]
- 9.Skripak JM, Nash SD, Rowley H, Brereton NH, Hamilton RG, Matsui EC, et al. A randomized, double blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol 2008; 122:1154–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zapatero L, Alonso E, Fuentes V, Martinez MI. Oral desensitization in children with cow's milk allergy. J Investig Allergol Clin Immunol 2008; 18:389–396 [PubMed] [Google Scholar]
- 11.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol 2008; 121: 343–347 [DOI] [PubMed] [Google Scholar]
- 12.Pajno GB, Caminiti L, Ruggeri P, De Luca R, Vita D, La Rosa M, et al. Oral immunotherapy for cow's milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol 2010; 105:376–381 [DOI] [PubMed] [Google Scholar]
- 13.Martorell A, De la Hoz B, Ibanez MD, Bone J, Terrados MS, Michavila A, et al. Oral desensitization as a useful treatment in 2-year-old children with cow's milk allergy. Clin Exp Allergy 2011; 41:1297–1304 [DOI] [PubMed] [Google Scholar]
- 14.Salmivesi S, Korppi M, Mäkelä MJ, Paassilta M. Milk oral immunotherapy is effective in school-aged children. Acta Paediatr 2012; 102:172–176 [DOI] [PubMed] [Google Scholar]
- 15.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in non anaphylactic children with egg allergy. J Allergy Clin Immunol 2007; 119:199–205 [DOI] [PubMed] [Google Scholar]
- 16.Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow-up. Allergol Int 2010; 59:43–51 [DOI] [PubMed] [Google Scholar]
- 17.Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development tolerance. Ann Allergy Asthma Immunol 2010; 105:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Rodriguez R, Urra JM, Feo-Brito F, Galindo PA, Borja J, Gomez E, et al. Oral rush desensitization to egg: efficacy and safety. Clin Exp Allergy 2011; 41:1289–1296 [DOI] [PubMed] [Google Scholar]
- 19.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SM, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012; 367:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen's egg allergy: a new protocol with raw hen's egg. Pediatr Allergy Immunol 2013; 24:75–83 [DOI] [PubMed] [Google Scholar]
- 21.Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen's egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol 2013; 24:66–74 [DOI] [PubMed] [Google Scholar]
- 22.Vazquez-Ortiz M, Alvaro M, Piquer M, Dominguez O, Machinena A, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin Exp Allergy 2014; 44:130–141 [DOI] [PubMed] [Google Scholar]
- 23.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy 2009; 64:1218–1220 [DOI] [PubMed] [Google Scholar]
- 24.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol 2009; 124:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LCL, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol 2010; 126:83–91 [DOI] [PubMed] [Google Scholar]
- 26.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 2011; 127:654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013; 131:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson NE, Möller C, Werner S, Magnusson J, Bengtsson U, Zolubas M. Self-reported food hypersensitivity in Sweden, Denmark, Estonia, Lithuania, and Russia. J Investig Allergol Clin Immunol 2004; 14:70–79 [PubMed] [Google Scholar]
- 29.Gamboa PM, Cáceres O, Antepara I, Sánchez-Monge R, Ahrazem O, Salcedo G, et al. Two different profiles of peach allergy in the north Spain. Allergy 2007; 62:408–414 [DOI] [PubMed] [Google Scholar]
- 30.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic. Ann Allergy Asthma Immunol 2010; 104:101–108 [DOI] [PubMed] [Google Scholar]
- 31.Thyagarajan A, Varshney P, Jones SM, Sicherer SH, Wood RA, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol 2010; 126:31–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of Ig-E mediated food allergy among children with atopic dermatitis. Pediatrics 1998; 101:E8. [DOI] [PubMed] [Google Scholar]
- 33.Greenhawt M. The role of food allergy in atopic dermatitis. Allergy Asthma Proc 2010; 31:392–397 [DOI] [PubMed] [Google Scholar]
- 34.Schofield AT. A case of egg poisoning. Lancet 1908; 1:716 [Google Scholar]
- 35.Patriarca G, Schiavino D, Nucera E, Schinco G, Milani A, Gasbarrini GB. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology 1998; 45:52–58 [PubMed] [Google Scholar]
- 36.Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE mediated cow's milk allergy. Allergy 2004; 59:980–987 [DOI] [PubMed] [Google Scholar]
- 37.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol 2011; 127:558–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail IH, Tang ML. Oral Immunotherapy for the treatment of food allergy. Isr Med Assoc J 2012; 14:63–69 [PubMed] [Google Scholar]
- 39.Passalacqua G, Landi M, Pajno GB. Oral immunotherapy for cow's milk allergy. Curr Opin Allergy Clin Immunol 2012; 12:271–277 [DOI] [PubMed] [Google Scholar]
- 40.Fisher HR, Du Toit TG, Lack G. Specific oral tolerance induction in food allergic children: is oral desensitization more effective than allergen avoidance? A meta-analysis of published RCTs. Arch Dis Child 2011; 259–264 [DOI] [PubMed] [Google Scholar]
- 41.Brozek JL, Terracciano L, Hsu J, Kreis J, Compalati E, Santesso N, et al. Oral immunotherapy for IgE-mediated cow'smilk allergy: a systemic review and meta-analysis. Clin Exp Allergy 2012; 42:363–374 [DOI] [PubMed] [Google Scholar]
- 42.Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev 2012; 9:CD009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung JP, Kloda LA, Mc DeDevitt J, Ben-Shoshan M, Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev 2012; 11:CD009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enrique E, Pineda F, Malek T, Batra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double blind, placebo controlled study with a standardized hazelnut extract. J Allergy Clin Immunol 2005; 116:1073–1079 [DOI] [PubMed] [Google Scholar]
- 45.Kim EH, Bird J, Kulis M, Laubach S, Pons L, Sheffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 127:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Bossieu D, Dupont C. Sublingual immunotherapy for cow's milk protein allergy: a preliminary report. Allergy 2009; 64:876–883 [DOI] [PubMed] [Google Scholar]
- 47.Fernandez–Rivas M, Garrido Fernandez S, Nadal JA, Diaz de Duana MD, Garcia BE, Gonzales-Mancebo E, et al. Randomized double-blind, placebo controlled trial of sublingual immunotherapy with Pru p3 quantified peach extract. Allergy 2009; 64:876–883 [DOI] [PubMed] [Google Scholar]
- 48.Keet CA, Frischmeyer-Guerriero PA, Tyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol 2012; 129:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol 2014; 133:318–323 [DOI] [PubMed] [Google Scholar]
- 50.Chin SJ, Vickery BP, Kulis MD, Kim EH, Varshney P, Steele P, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol 2013; 132:476–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narisety SD, Keet CA. Sublingual vs Oral Immunotherapy for food allergy: identifying the right approach. Drugs 2012; 72:1977–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol 2011; 127:1622–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol 2013; 131:1288–1296 [DOI] [PubMed] [Google Scholar]
- 54.Vickery BP, Scurlock AM, Jones SM, Burks AW. Mechanisms of immune tolerance relevant to food allergy. J Allergy Clin Immunol 2011; 127:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014; 133:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pajno GB, Caminiti L, Salzano G, Crisafulli G, Aversa T, Messina MF, et al. Comparison between two maintenance feeding regimens after successful cow's milk oral desensitization. Pediatr Allergy Imunol 2013; 24:376–381 [DOI] [PubMed] [Google Scholar]
- 57.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steel PH. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol 2009; 124:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caminiti L, Passalacqua G, Vita D, Ruggeri P, Barberio G, Pajno GB. Food-exercise induced anaphylaxis in a boy successfully desensitized to cow milk. Allergy 2007; 62:335–336 [DOI] [PubMed] [Google Scholar]
- 59.Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, et al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow's milk allergy. J Allergy Clin Immunol 2009; 124:610–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khoriaty E, Umetsu DT. Oral immunotherapy for food allergy: towards a new horizon. Allergy Asthma Immunol Res 2013; 5:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]