Abstract
Background: In mammals, lymph is propelled centrally primarily via the phasic contractions of collecting lymphatic vessels, known as lymphatic pumping. Electrophysiological studies conducted in guinea pig and sheep mesenteric lymphatic vessels indicate that contractions are initiated in the lymphatic muscle by nifedipine-sensitive action potentials (APs). Lymphatic pumping is highly sensitive to luminal fluid loading and the mechanical properties of this stretch-induced pumping have been consistently studied, in particular in rat mesenteric lymphatic vessels. However, membrane potential (Vm) and the electrophysiological events underlying stretch-induced lymphatic pumping have not been investigated in the rat. The aim of this study was thus to examine the properties of rat mesenteric lymphatic muscle Vm under resting conditions and to assess changes in Vm caused by distension.
Methods and Results: Lymphatic muscle Vm was measured with sharp intracellular microelectrodes either in unstretched conditions or under isometric tension provided by a wire-myograph. In unstretched vessels, Vm was −48±2 mV (n=30). APs (amplitude ∼25 mV) were observed at a frequency of ∼8/min and were abolished by nifedipine. Under isometric tension, Vm was less polarized (-36±1 mV, n=23), even at minimum tension. Increase in tension led to increase in contraction strength and contraction/AP frequency, while Vm was slightly hyperpolarized and AP amplitude not markedly altered.
Conclusions: In our experimental conditions, rat lymphatic muscle has electrophysiological characteristics similar to that in other species. It responds to an increase in isometric tension with an increase in AP frequency, but resting Vm is not significantly affected.
Introduction
In most mammals, including humans, transient and phasic constrictions of collecting lymphatic vessels allow fluids to be removed from tissues, propelled along the lymphatic tree and returned back to the blood circulation. This function, or lymphatic pumping, is essential to tissue fluid homeostasis, macromolecule transport and immune cell trafficking and if impaired, leads to profound swelling and edema and inadequate immune response.1–5 The underlying mechanism of this contractile process, as revealed in bovine, sheep, and guinea-pig lymphatics, is intrinsic to the lymphatic muscle present in the vessel wall and consequent to action potentials (APs) mediated by voltage-activated Ca2+ channel opening. Investigations, undertaken in guinea pig and sheep mesenteric lymphatic vessels to further characterize the electrical basis of APs and consequent phasic contractions, have revealed the occurrence of spontaneous transient depolarizations (STDs).6–8 STDs do not directly cause contractions, but occasionally under resting conditions or rhythmically upon chemical stimulation, these events summate to trigger APs. STDs depend on the presence of cytosolic and extracellular Ca2+ and have been shown to be to the voltage signature of Ca2+-activated chloride channel opening.8 The occurrence of STDs is increased by agonists using the Gq/InsP3 signaling pathway and reduced by agonists/agents increasing cAMP or cGMP cytosolic concentration.9–11 Although STD involvement in lymphatic contraction is well established under resting conditions (mostly quiescent vessels), this correlation needs to be tested in functionally relevant physiological conditions, where vessels are actively contracting in response to vessel filling to propel fluid forward. While strong correlations between the frequency of lymphatic vessel contractions and the distension of the vessel wall have been amply demonstrated in lymphatic vessels from several species,1–3 including the rat,12–15 the ionic mechanisms underlying stretch-induced lymphatic contractions in this animal have not been examined. The aims of our study were two-fold. First, given the importance of the rat model in our understanding of the lymphatic contractile function and its sensitivity to distension, we wanted to investigate the membrane potential (Vm) properties of lymphatic muscle in rat mesenteric vessels under resting conditions and compare them to those of the better described guinea pig lymphatic muscle. Second, we sought to evaluate how rat mesenteric lymphatic muscle Vm was affected during increases in stretch provided by isometric distension.
Materials and Methods
Ethical approval
Male Sprague Dawley rats (150–250 g, n=60) and Hartley guinea pigs (7–15 days of age, n=13, Charles River, QC) were used to perform all experiments. Animals were allowed full access to food and water; both normal circadian rhythms and body temperatures were maintained. All animal protocols were reviewed and approved by the University of Calgary Animal Care and Ethics Committee, the Texas A&M University, and University of Missouri Institutional Animal Care and Use Committees and were conducted in accordance with the guidelines of the Canadian Council on Animal Care and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Tissue preparation
Lymphatic vessels were isolated in two ways to allow investigations under resting and stretched conditions. For protocols conducted under resting conditions, rats and guinea pigs were sacrificed by decapitation during deep anesthesia induced by inhalation of isofluorane. The small intestine with its attached mesentery was rapidly dissected and placed in a physiological saline solution (PSS) of the following composition (mM): NaCl, 120; KCl, 5; CaCl2, 2.5; MgCl2, 2; NaHCO3, 25; NaH2PO4, 1; glucose, 11. The pH was maintained at 7.4 by constant bubbling with 95% O2/5% CO2. Lymphatic tissue (typically 1–2 mesenteric preparation per animal) was prepared as previously described.16,17 Briefly, a small ileal piece of mesentery containing collecting lymphatic vessels with their associated artery and vein was dissected, pinned down to the Sylgard-covered bottom of a small organ bath (volume 100 μL), placed on the stage of an inverted microscope and continuously superfused with PSS heated to 36°C at a flow rate of 3 mL min−1, causing a change-over time of <7 sec. In rats, the layer of mesenteric fat covering the lymphatic was carefully and gently cleaned to make a small (0.5–1 mm) opening through which the lymphatics could be visualized and accessed. Vessels mounted in such manner (i.e., pinned down) will be referred to as “unstretched vessels” in the remainder of the text.
To investigate the effect of stretch and prepare vessels for wire-myography experiments (see below), rats were fasted overnight and anesthetized with an intramuscular injection of Fentanyl/Droperidol solution (0.3 mL/kg IM) and Diazepam (2.5 mg/kg IM).18 A laparotomy was performed to gain access to the mesenteric lymphatics and loop of small intestine, 3–5 cm long, was gently exteriorized onto a specially designed preparation board. The exposed gut and mesentery were continuously moistened with Dulbecco's phosphate buffer saline (DPBS, Sigma). With the assistance of a stereomicroscope, a suitable unbranched collecting lymphatic vessel (typically 1.7–2.0 mm in length) running along a blood vessel arcade was identified, carefully cleared of connective tissue and fat,18,19 while being continuously superfused with DPBS. The vessel segment to be studied was then dissected free and transferred to a 35-mm Petri dish containing DPBS. After the tissue was removed, the animal was killed by cervical dislocation.
Wire-myography
Two 25-mm long, 25 μm OD, stainless steel wires were carefully passed, one at a time, through the lumen of the vessel segment. The vessel was then transferred to the organ chamber of a single-channel, wire-myograph (Model 310A, Danish Myo Technology, Aarhus, Denmark). The wires were secured to the myograph jaws and minimal force was applied during a 30-min warming step (36°–37°C). During the process, DPBS was replaced with warm albumin phosphate saline solution (APSS) of the following composition (mM): NaCl 145; KCl 5; CaCl2 2.5; MgSO4 1; NaH2PO4 1; EDTA 0.02; pyruvate 2; glucose 5, MOPS (3-(N-morpholino) propanesulfonic acid) 3; purified bovine serum albumin (BSA) 0.5 g/100 mL), with pH adjusted to 7.4 at 37°C. Passive force (preload) was then set to 0.2 mN.
Spontaneous force transients typically started after 5–10 min, with stable frequency and amplitude occurring over the course of a 60-min equilibration period. Step-increase and ramp protocols were applied to vary vessel tension and spontaneous force transients were recorded19 together with lymphatic muscle Vm (see below). To perform a ramp increase in preload, a wire myograph modified with a servo-control system for force was used. This system had a software-based servo-control with a direct output option (modified DMT Model 310A). The force signal was amplified, filtered and digitized. This system allowed us to precisely control and change preload while recording force and to generate ramp increases in passive force at constant speed.19
Electrophysiology
Impalements of lymphatic muscle were obtained from the adventitial side of the vessels. Vm was recorded under resting conditions (unstretched vessels) and during changes in preload (wire-myograph mounted vessels) with conventional glass intracellular microelectrodes filled with 0.5 M KCl (resistance 150–250 MΩ), connected to an amplifier (Intra 767, World Precision Instruments, Sarasota, FL) through an Ag–AgCl half-cell and recorded on a computer via an analog-digital converter (PowerLab/4SP, ADInstrument, Mountain View, CA). Lymphatic muscle impalements were characterized by a sharp drop in potential that settled after 10–15 sec to −40 to −45 mV in unstretched conditions.16,17
When experiments were performed on vessels mounted on the wire-myograph, impalements could only be successfully obtained and maintained when a minimal preload (≥0.1 mN) was applied to the myograph, and as a consequence, the vessel was slightly stretched. Under these conditions, the resting Vm value recorded was usually not more negative than −40 mV (typically between −30 to −35 mV). The underlying causes for the differences in Vm between unstretched and myograph-mounted vessels are described in the Results section. Vm was corrected at the end of the impalement by subtracting the voltage value obtained after pulling the electrode out of the muscle to that of the measured intracellular value. Spontaneous depolarizing events greater than 1 mV were considered as STDs,8,11 and their activity assessed by manually measuring their frequency and amplitude. These two measurements can be co-dependent because STDs encompass a wide spectrum of amplitudes from clearly distinct depolarizations to smaller events, which merge into the baseline noise. Therefore, a change in amplitude is likely to correlate with a change in frequency.8,11 STD frequency and amplitude were measured in quiescent segments or between action potentials over intervals of 30–60 sec (depending on the stability of the recording). When blockers or agonists were tested, this baseline measurement was compared with that occurring over a period of the same duration during the maximum response to the chemicals. This protocol was usually performed during the same impalement. However, in some instances, successive impalements from neighboring cells were needed to complete the experiment.16,17
Chemicals and drugs
The following drugs were used: indomethacin, NG-nitro-l-arginine (l-NNA), niflumic acid (NFA), and thimerosal from Sigma/Aldrich, and nifedipine from Calbiochem. NFA and nifedipine were dissolved in dimethylsulfoxide, indomethacin in ethanol, l-NNA in 0.1 M HCl, and thimerosal in distilled water to give 10 mM stock solutions (100 mM for l-NNA), which were then diluted in APSS to achieve the appropriate concentration. The final concentrations of the vehicles were less than 0.1% and had no effects on lymphatic Vm and contractile activity.
Data and statistical analysis
Experimental data are expressed as means±one S.E.M. Statistical significance was assessed using two-tailed paired or unpaired Student's t-test, or one-way ANOVA with Dunnett's post-hoc test as indicated in the text. P value below 0.05 was considered significant.
Results
Resting membrane potential characteristics of rat lymphatic muscle
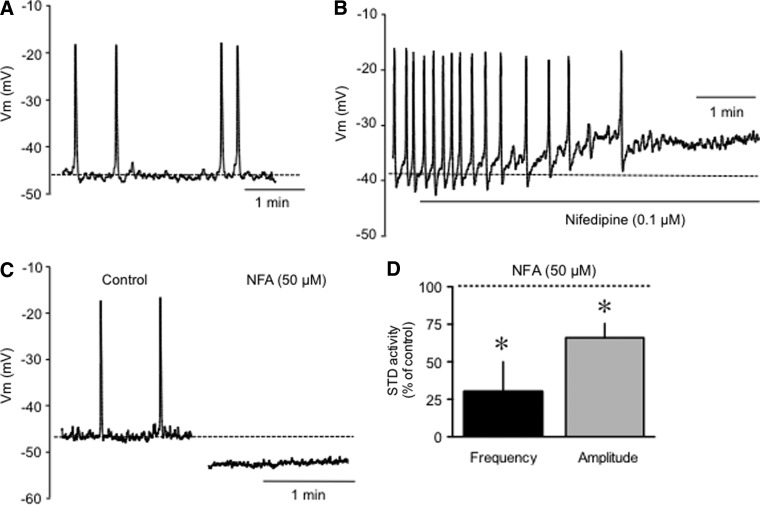
The first set of experiments was designed to examine rat lymphatic muscle Vm characteristics under resting conditions (unstretched vessels), where the vessels were simply maintained against the chamber bottom by pinning down the surrounding mesentery. We compared rat data to those obtained under the same conditions from guinea pig vessels, which have been characterized previously.6,8,9,17 The resting Vm recorded in unstretched rat lymphatic muscle was −48±2 mV (n=30, Fig. 1 and Table 1). Rat mesenteric lymphatics displayed AP-like depolarizations with similar amplitudes but higher frequencies than those observed in guinea pig vessels (Table 1). AP-like depolarizations were inhibited by nifedipine (1–10 μM, n=3, Fig. 1B), a treatment that also unmasked spontaneous transient depolarizations (STDs). In guinea pig mesenteric lymphatic vessels where they were first described,6,8 these events are characteristic features of lymphatic muscle Vm necessary for AP initiation. They were shown to be the voltage signature of Ca2+-dependent Cl- channel openings upon intracellular Ca2+ release from inositol 1,4,5-trisphosphate (IP3) stores.8 Data presented in Figure 1 illustrates that STDs also occur in rat lymphatics, with frequency and amplitude patterns similar to that of STDs in guinea pig vessels (see also Table 1). Rat STDs were further characterized in the presence of the Ca2+-dependent Cl- channel blocker niflumic acid (NFA) and the IP3 receptor sensitizer thimerosal, compounds previously shown to respectively inhibit and increase guinea pig STD activity.8 NFA (50 μM) markedly reduced STD frequency and amplitude (Fig. 1D) while hyperpolarizing the lymphatic muscle by 7±2 mV (n=3) and abolishing randomly occurring APs (Fig. 1C). Thimerosal (1 μM) increased STD frequency to 177±23% and STD amplitude to 113±4 of control, while depolarizing Vm by 4 mV (n=2).
FIG. 1.
Electrophysiological characteristics of rat mesenteric lymphatic muscle in an unstretched vessel in the isolated mesentery. (A) Original membrane potential recording displaying AP-like depolarizations (large upward deflections) and STDs (small upward deflections). (B) Abolition of AP-like depolarizations by nifedipine (1 μM). (C) Abolition of APs and STDs by niflumic acid (NFA, 50 μM). (D) Graph summary of the effect of NFA on STD frequency and amplitude expressed as percent of control. Columns are mean±S.E.M. of three experiments. *p<0.05 (two-tailed paired Student's t-test).
Table 1.
Comparison of Membrane Potential Characteristics Between Unstretched Rat and Guinea Pig Mesenteric Lymphatic Vessels
| Rat | Guinea pig | |
|---|---|---|
| Vm | −48±2 mV (30) | −50±1 mV (13) |
| AP-like spikes | ||
| observed in | 67% of the cells | 30% of the cells |
| Amplitude | 25±1 mV (8) | 25±1 mV (12) |
| Frequency | 8±2/min (8) | 3±1/min (10) |
| STDs | ||
| observed in | >90% of the cells | >90% of the cells |
| Amplitude | 1.4±0.1 (15) | 1.6±0.1 mV (13) |
| Frequency | 28±3/min (15) | 29±3/min (13) |
Membrane potential in wire-myograph mounted vessels
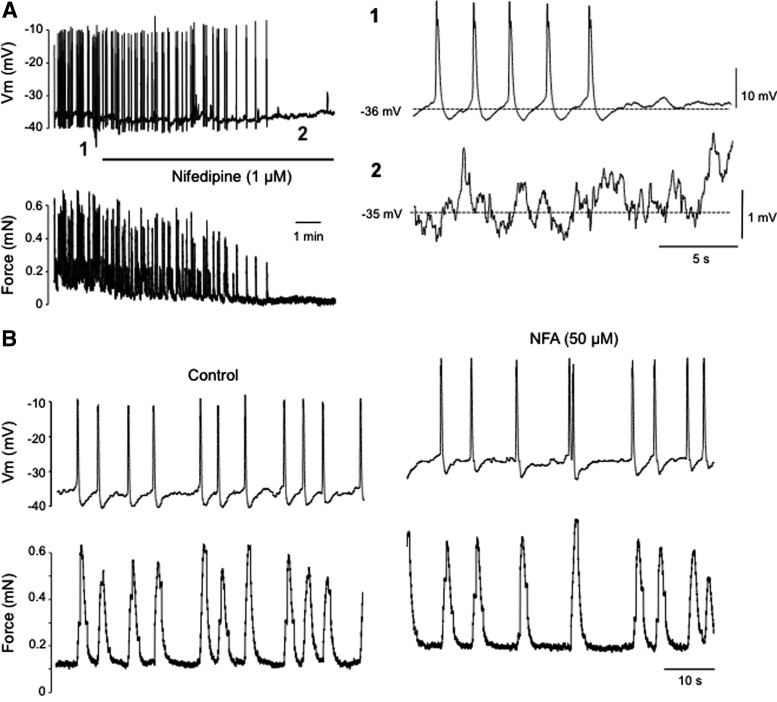
We then investigated how stretch affected Vm by mounting rat lymphatic vessels in a wire-myograph. Using this method, impalements could successfully be obtained under minimal preload of 0.1–0.2 mN, but lowering this initial preload further caused the recording electrode to be dislodged. Under these conditions, Vm recordings displayed frequent and regularly occurring AP-like spikes at a rate 50% higher than in unstretched vessels (frequency 12±2/min, n=20), which preceded each phasic contraction (Fig. 2). The AP amplitude was similar to that in unstimulated vessels (26±1 mV, n=23; compare to Table 1), but resting Vm values measured between APs were markedly less polarized (−36±1 mV, n=23) than in unstretched vessels. During these intervals, STDs could usually be observed. Like in unstretched vessels, they became totally unmasked after AP inhibition by nifedipine (1–10 μM; Fig. 2A), a treatment that did not significantly change STD frequency and amplitude (87±8% and 84±4% of control, respectively, n=5). Interestingly, however, and in contrast with what occurred in unstretched vessels, application of NFA failed to inhibit APs/contractions in vessels mounted in the wire-myograph (frequency 18±3/min in control and 17±3/min in 50 μM NFA, n=4; Fig. 2B). Basal tone of the vessels under minimal (∼0.2 mN) preload was increased by 227±95% during the first 5 min of NFA exposure (n=4).
FIG. 2.
Electrophysiological characteristics of rat mesenteric lymphatic muscle in a wire myograph-mounted isolated vessel. (A) Original recordings of membrane potential (top trace) displaying APs (illustrated on an expanded scale in 1) and correlated isometric contractions in a vessel stretched to 0.2 mN preload (bottom trace). Administration of nifedipine (1 μM, horizontal bar) inhibited the contractions and APs, unmasking STDs (as evident on an expanded scale in 2). (B) Original recordings of membrane potential (top trace) and force (bottom trace) under control conditions (left) and in the presence of niflumic acid (NFA, 50 μM). Traces are representative of 4–10 experiments.
Integrity of the lymphatic endothelium in wire-myograph-mounted vessels
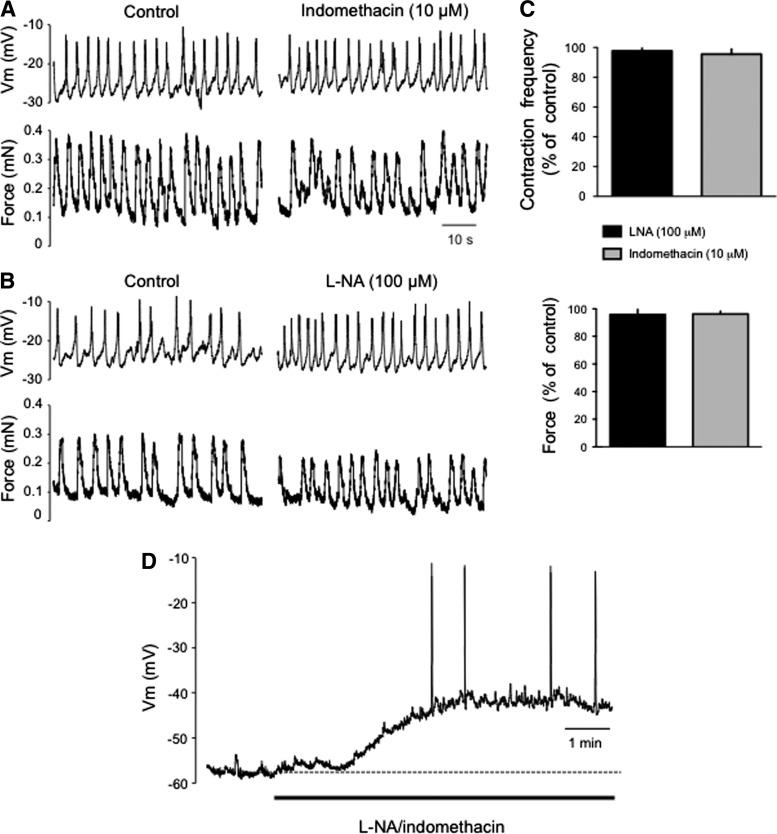
In wire-myograph-mounted vessels, the introduction of two 25-μm wires into the lumen very likely damaged the endothelium, a layer which plays a critical role in the modulation of lymphatic muscle Vm and contractile activity via release of diffusible factors, such as NO and prostaglandins.11,20–22 We assessed the integrity of the endothelium in the wire-myograph-mounted vessels by measuring Vm and contraction frequency while nitric oxide synthases and cyclooxygenases were pharmacologically inhibited. In the wire-myograph-mounted vessels, neither l-NNA (100 μM) nor indomethacin (10 μM) altered the resting Vm, the frequency of contractions, nor the force generated per contraction (Fig. 3A, B and C). This contrasts with data obtained in unstretched vessels, where administration of the two inhibitors markedly depolarized Vm (11±2 mV, n=4) and increased AP frequency (Fig. 3D)11,20
FIG. 3.
Assessment of endothelial function on lymphatic contractile activity and membrane potential. (A and B) Original recordings of membrane potential (top traces) and force (bottom traces) obtained from an isolated vessel mounted on a wire-myograph under control condition (left) and in the presence of indomethacin (10 μM, A) or L-NA (100 μM, B). (C) Bar graph summary of the frequency of contractions and APs (top) and force (bottom) in the presence of the inhibitors. Data are expressed as percentages of values during the same impalement before application of the inhibitors. Columns are mean±S.E.M. of four experiments. (D) Original membrane potential recording, representative of four experiments, from an unstretched lymphatic vessel still embedded in the mesentery, illustrating the depolarization induced by the administration of L-NA and indomethacin. Note the occurrence of APs at depolarized potentials.
Membrane potential responses to changes in preload
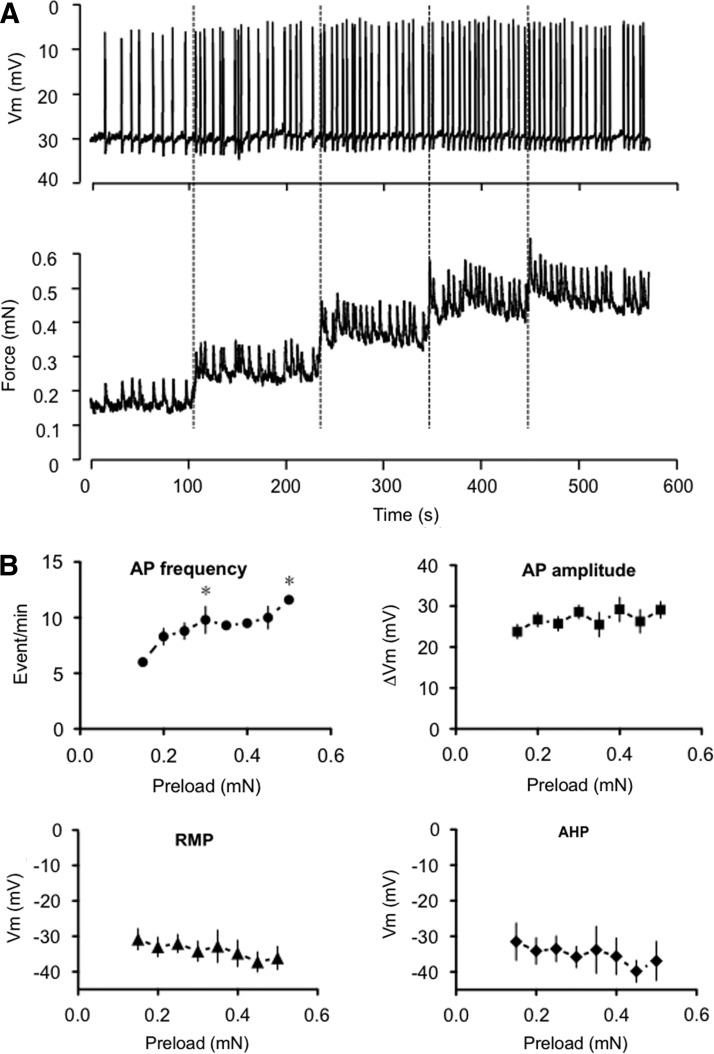
Lymphatic vessels responded to a stepwise rise in preload with an increase in the amplitude of spontaneous force transients and an increase in contraction frequency (Fig. 4). Consistently, the AP frequency increased in a similar fashion with increased preloads (Fig. 4B). The AP amplitude was minimally affected at all preloads. In our hands, the increase in preload did not cause significant change in the lymphatic muscle Vm (Fig. 4B). The hyperpolarization following the AP (after-hyperpolarization, AHP) was minimally affected by the preload increase, reaching a value of ∼2 mV at each preloads (Fig. 4B).
FIG. 4.
Effect of a step-increase in passive force on lymphatic muscle membrane potential in isolated vessels. (A) Original membrane potential and force recordings during a step-increase in preload. (B) Summary graphs (mean±S.E.M.; n=2–7) illustrating changes in AP frequency and amplitude, resting membrane potential (RMP), and after-hyperpolarization (AHP) occurring during step-increase in preload. *p<0.05 (one-way ANOVA with Dunnett's post-hoc).
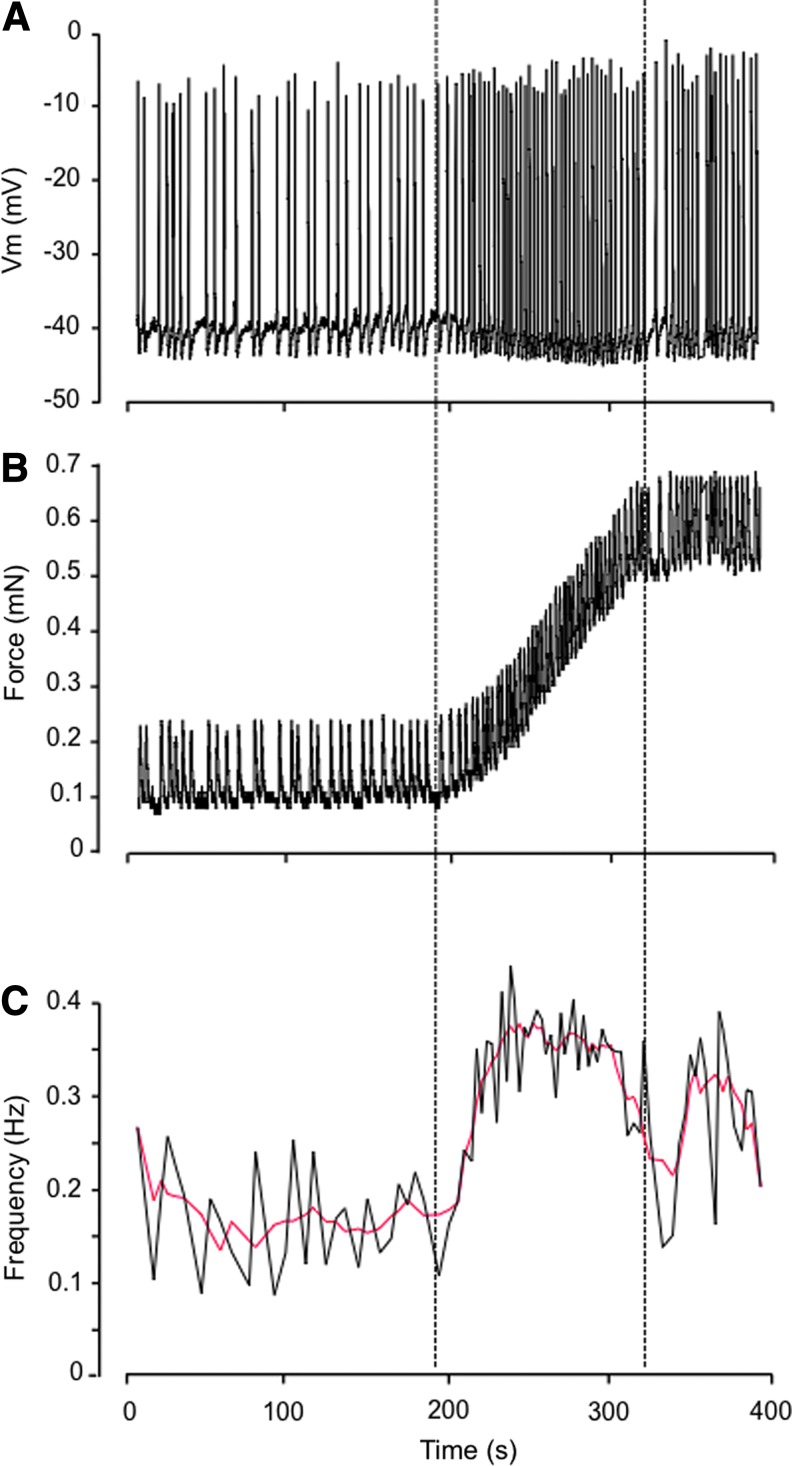
Comparable increase in AP frequency was noted during ramp increases in preload (n=6; Fig. 5), which also hyperpolarized Vm by ∼3 mV. While elevated AP frequency was usually maintained as preload stabilized at the highest level, Vm typically returned close to its initial value.
FIG. 5.
Effect of ramp-increase in passive force on lymphatic muscle membrane potential in isolated vessels. Representative experiment (out of 6) illustrating changes in membrane potential (A), force (B), and frequency of AP (C) during a ramp-increase in preload.
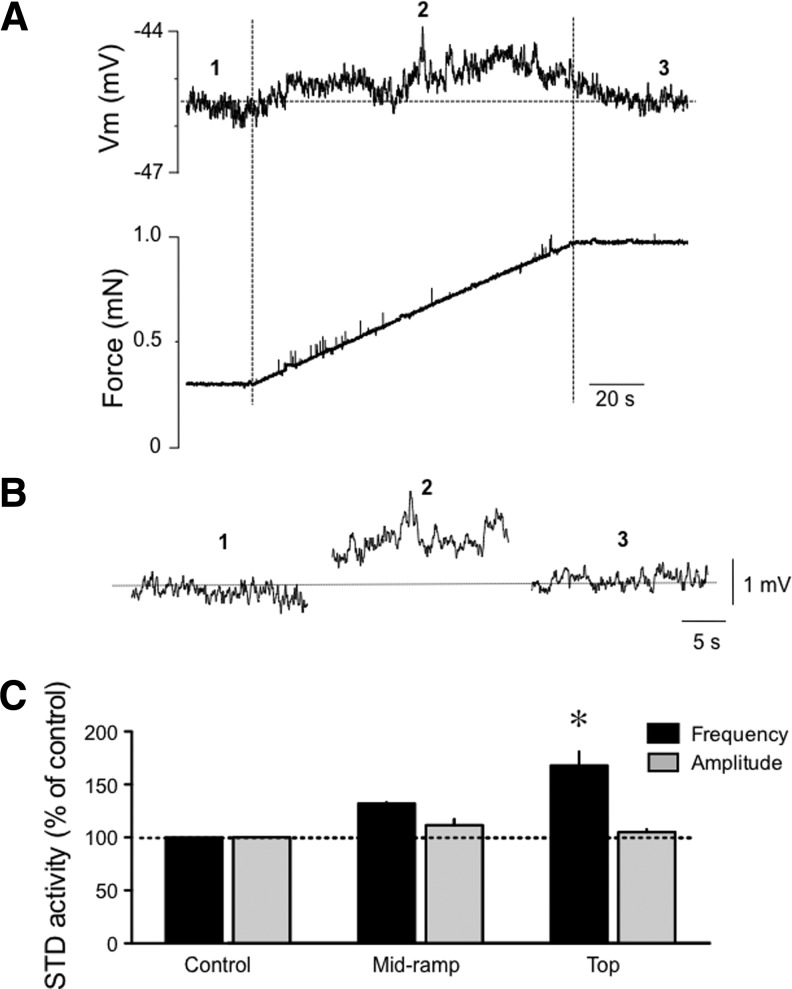
In order to assess more accurately whether Vm varies with stretch, we repeated this last set of experiments with vessels treated with nifedipine (1 μM) to inhibit APs and contractions. Ramp increase in preload did not cause significant changes in Vm, which varied from −40±3 mV before, to −38±2 mV during the ramp (n=4, p=0.058), and −39±2 mV at maximum preload (p=0.135). With APs inhibited by nifedipine, we had the opportunity to investigate the influence of stretch on STD activity. As illustrated in Figure 6, a small increase in STD frequency was observed during the ramp, with significance reached at the top of the ramp (n=4, p<0.05). STD amplitude was not significantly affected.
FIG. 6.
Effect of a ramp-increase in passive force on STD activity in isolated lymphatic vessels. (A) Original recordings, representative of four experiments, of membrane potential and force generated in a wire myograph-mounted lymphatic vessel treated with nifedipine (1 μM) to block APs and contractions in response to a ramp increase in preload. (B) Illustration of the STD activity recorded before (1), during (2) and at the end of the ramp (3). (C) Bar graph summary of STD frequency and amplitude (n=4) expressed in percent of control before, during, and at the end of the ramp. *p<0.05 (one-way ANOVA with Dunnett's post-hoc test).
Discussion
Although muscular rat mesenteric lymphatics are widely used to study the properties of their phasic rhythmical contractile activity and despite the fact that electrical events are causative to these mechanical changes, Vm measurements from rat vessels have not been reported. In our hands, intracellular microelectrode recordings from muscle in isolated “unstretched” rat vessel preparations, revealed Vm values and electrical characteristics similar to those previously reported from guinea pig mesenteric lymphatic vessels.6,8,23 In particular, we observed the occasional occurrence of APs, which lead to transient vessel contractions typical of lymphatic pumping. We also recorded STDs, which frequency, amplitude, and basic pharmacological characteristics were shown to be similar to those described in guinea pig mesenteric lymphatics.8
Lymphatic pumping is very sensitive to changes in transmural pressure and many studies using isolated lymphatic vessel preparations under luminal pressure or isometric tension have demonstrated a tight correlation between increase in stretch and increase in contraction frequency. Whether and how changes in Vm underlie the stretch-induced increase in contraction frequency has however not been addressed. We thus obtained sharp microelectrode Vm recordings from lymphatic muscle in isolated rat mesenteric vessels mounted on a wire-myograph. Under these isometric conditions, impalements were maintained during increases in preload, making possible to record Vm and APs simultaneously with contractile force. At low isometric tension, APs occurred more regularly than in unstretched vessels and the frequency of the events augmented with increases in preload, as was the force generated during each contraction. The amplitude of the AP was not affected during this process.
The resting Vm values recorded in the wire-myograph-mounted vessels were markedly more depolarized than those recorded in unstretched vessels still embedded in the mesentery. This depolarization could have resulted from the initial tension applied to the vessel wall that was necessary to successfully impale the muscle and maintain a reliable Vm recording. Indeed, Vm values measured in vascular smooth muscles when blood vessels are pressurized to normal diastolic values are depolarized compared to values obtained in unpressurized vessels, with the largest Vm changes occurring in the pressure range of 40–80 mmHg.24–27 While it is expected that in lymphatic vessels, the application of the initial stretch depolarized the muscle cells, Vm could have also been affected by another confounding factor inherent to the wire-myography. Indeed, this procedure requires two 25 μm diameter wires to be passed through the lumen of the lymphatic vessel with an inner diameter of 80–100 μm, very likely damaging the endothelium in the process and compromising the endothelium's vasoactive function. The lymphatic endothelium has been shown to constitutively release vasoactive substances that contribute to modulation of the contractile activity of the lymphatic muscle, as well as its resting Vm. In line with their vasodilatory/relaxing actions typically observed in other smooth muscles, nitric oxide and prostanoids, such as prostaglandin E2 and prostacyclin, cause the lymphatic muscle to hyperpolarize and the pumping activity to slow down.17,20,22,28,29 Endothelium-derived thromboxane A2, on the other hand, induces a depolarization as well as an increase in electrical activity (STDs and APs) and lymphatic vessel contraction frequency.11,30–32 We confirmed that, in wire-myograph-mounted vessels, endothelial function was compromised, as contractile and Vm responses were not affected by treatment with nitric oxide synthase and cyclooxygenase inhibitors, while the same inhibitors depolarized the Vm in unstretched vessels by about 10 mV. This value certainly accounts for at least part of the difference in Vm between stretched and unstretched vessels. The endothelial dysfunction is thus likely to be the main reason for the depolarized Vm recorded in wire-myograph-mounted vessels and is probably also responsible for the higher-than-normal frequency of contractions that we recorded.33
Another possibility for the differences we recorded between unstretched lymphatics still contained within a section of the mesentery and those that have been isolated from the mesentery and mounted onto the wire-myograph is the loss of any potential influence of cells nearby but outside of the lymphatic vessel wall. For example, it has been reported that mesenteric lymphatic vessels have a population of mast cells that are closely associated but outside of the lymphatic vessel wall.31,34 These mast cells (or other closely associated immune cells) could release a host of agents able to potentially alter the electrophysiological activities of the lymphatic vessel.31 It is known that the vessel isolation procedure eliminates or destroys most of these cells.34 Thus removing the vessels from the mesenteric cellular environment in order to mount the vessels in the wire-myograph may alter the contribution of these cells to the lymphatic muscle electrical activity.
Under the relatively depolarized conditions observed in wire-myograph-mounted vessels, increases in passive tension caused the frequency of contractions and APs to increase as expected. However, only small changes in baseline Vm were observed. Specifically, a slight hyperpolarization occurred during ramp-increase in preload, which was less marked and insignificant during step-increase in preload. Furthermore, we were not able to demonstrate significant changes in Vm during ramp increase in preload, while APs were abolished by nifedipine. In this situation, STD frequency was shown to increase, staying significantly elevated at maximum preload.
The use of the Ca2+-activated Cl− channel blocker NFA revealed a striking difference between unstretched and wire-myograph-mounted vessels. While NFA inhibited APs in unstretched vessels, it did not alter the AP frequency in wire-myograph-mounted vessels. This difference correlates well with several of our unpublished observations and suggests that while STDs are involved in the initiation of spontaneous APs and contractions in unstretched vessels,6,8 they do not seem to play such a significant role in initiating contractions caused by an active distension of the vessel wall. These observations suggest the involvement of different mechanisms requiring further investigations.
Although wire-myography, by minimizing vessel movements, allowed us to record Vm changes associated with variations in vessel stretch, we have to take into account that this method may alter the normal contractile function of the lymphatic vessels in several ways. The first one concerns the destruction of the endothelium. As discussed above, the endothelium only modulates lymphatic vessel contractility, so that this alteration should not affect the way the lymphatic muscle responds to stretch, but its disruption could lead to a confounding depolarization that might interfere with the pacemaker mechanism. Along the same line, the careful dissection of the vessels mounted on the wire-myograph would free them from the mesentery but also from any cells that it may contain and that could potentially modulate the lymphatic muscle electrical activity. The last issue relates to the spatially non-uniform tension applied to the vessels. Increasing the distance between the wires causes a flattening of the vessel and the stretch to be applied laterally, contrasting with the more uniform circumferential distension resulting from a physiological increase in transmural pressure.33 Furthermore, the isometric tension provided by the wire-myograph, while suitable to study tonic contractions, is less adapted to phasic activities, as it compromises the contraction and transient reduction in vessel diameter that has been shown to be an important regulator in the contractile cycle.14,22 Thus the range of preloads over which frequency and amplitude of contractions are modulated is narrower than the corresponding range under isobaric conditions.33 Whether this limitation is affecting the electrical response to stretch requires further investigation.
In conclusion, the present findings suggest that under our experimental conditions, the increase in contraction/AP frequency in response to an increase in preload is only accompanied by subtle changes in Vm. Whether Vm modulation is of limited importance in the increase in contraction frequency in response to stretch or compromised in our experimental conditions could not be solved, prompting more investigation to determine the identity and importance of the ionic conductances potentially involved in the stretch-induced increase in lymphatic pumping.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Florey HW. Observations on the contractility of lacteals. Part I. J Physiol 1927;62: 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florey HW. Observations on the contractility of lacteals. Part II. J Physiol 1927;63:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RO. Lymphatic contractility: A possible mechanism of lymphatic vessels for the transport of lymph. J Exp Med 1949;90:497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor AE. The lymphatic edema safety factor: The role of edema dependent lymphatic factors (EDLF). Lymphology 1990;23:111–123 [PubMed] [Google Scholar]
- 5.Yoffey JM, Courtice FC. Lymphatics, Lymph and the Lymphomyeloid Complex. 1970, London: Academic Press [Google Scholar]
- 6.van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 1993;471: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Helden DF, von der Weid P-Y, Crowe MJ. Electrophysiology of lymphatic smooth muscle, in: Interstitium, Connective Tissue, and Lymphatics, Bert J, Laine GA, McHale NG, Reed R, Winlove P. Eds. 1995, Portland Press: London: p. 221–236 [Google Scholar]
- 8.von der Weid P-Y, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: Pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 2008;295: H1989–2000 [DOI] [PubMed] [Google Scholar]
- 9.von der Weid P-Y. ATP-sensitive K+channels in smooth muscle cells of guinea-pig mesenteric lymphatics: Role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol 1998;125:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von der Weid P-Y, Zawieja DC. Lymphatic smooth muscle: The motor unit of lymph drainage. Int J Biochem Cell Biol 2004;36:1147–1153 [DOI] [PubMed] [Google Scholar]
- 11.von der Weid P-Y, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol 2001;280: H2707–2716 [DOI] [PubMed] [Google Scholar]
- 12.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol 1989;257:H2059–2069 [DOI] [PubMed] [Google Scholar]
- 13.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol 1977;233: H57–65 [DOI] [PubMed] [Google Scholar]
- 14.Gashev AA, Zhang RZ, Muthuchamy M, Zawieja DC, Davis MJ. Regional heterogeneity of length-tension relationships in rat lymph vessels. Lymphat Res Biol 2012;10:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahbar E, Weimer J, Gibbs H, et al. . Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat Res Biol 2012;10:152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JL, von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 2002;136:1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von der Weid P-Y, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol 1996;493:563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty PJ, Davis MJ, Zawieja DC, Muthuchamy M. Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics. Am J Physiol Regul Integr Comp Physiol 2008;294:R1524–1532 [DOI] [PubMed] [Google Scholar]
- 19.Davis MJ, Lane MM, Scallan JP, Gashev AA, Zawieja DC. An automated method to control preload by compensation for stress relaxation in spontaneously contracting, isometric rat mesenteric lymphatics. Microcirculation 2007;14:603–612 [DOI] [PubMed] [Google Scholar]
- 20.Chan AK, Vergnolle N, Hollenberg MD, von der Weid P-Y. Proteinase-activated receptor 2 modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol 2004;560:563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 2002;540:1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: A self-regulatory mechanism in rat thoracic duct. J Physiol 2006;575:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Helden DF, von der Weid P-Y, Crowe MJ. Intracellular Ca2+ release: A basis for electrical pacemaking in lymphatic smooth muscle, in: Smooth Muscle Excitation Tomita T, Bolton TB, Eds. 1996, Academic Press: London: pp. 355–373 [Google Scholar]
- 24.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 1999;79:387–423 [DOI] [PubMed] [Google Scholar]
- 25.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res 1984;55:197–202 [DOI] [PubMed] [Google Scholar]
- 26.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol (Lond) 1998:508:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: Smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol 2005;289:H1326–1334 [DOI] [PubMed] [Google Scholar]
- 28.Rehal S, Blanckaert P, Roizes S, von der Weid PY. Characterization of biosynthesis and modes of action of prostaglandin E2 and prostacyclin in guinea pig mesenteric lymphatic vessels. Br J Pharmacol 2009;158:1961–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von der Weid PY. Review article: Lymphatic vessel pumping and inflammation–The role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther 2001;15:1115–1129 [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Zhao J, Rayner SE, van Helden DF. Evidence that the ATP-induced increase in vasomotion of guinea-pig mesenteric lymphatics involves an endothelium-dependent release of thromboxane A2. Br J Pharmacol 1999;127:1597–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaku KJ, von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation 2006;13:219–227 [DOI] [PubMed] [Google Scholar]
- 32.Rayner SE, van Helden DF. Evidence that the substance P-induced enhancement of pacemaking in lymphatics of the guinea-pig mesentery occurs through endothelial release of thromboxane A2. Br J Pharmacol 1997;121:1589–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, Gashev AA, Zawieja DC. Lane MM, Davis MJ. Length-dependence of lymphatic phasic contractile activity under isometric and isobaric conditions. Microcirculation 2007;14:613–625 [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 2012;303:H693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]