Summary
Background
Although colonoscopy is the accepted standard for detection of colorectal adenomas and cancers, many adenomas and some cancers are missed. To avoid interval colorectal cancer, the adenoma miss rate of colonoscopy needs to be reduced by improvement of colonoscopy technique and imaging capability. We aimed to compare the adenoma miss rates of full-spectrum endoscopy colonoscopy with those of standard forward-viewing colonoscopy.
Methods
We did an international, multicentre, randomised trial at three sites in Israel, one site in the Netherlands, and two sites in the USA between Feb 1, 2012, and March 31, 2013. Patients aged 18–70 years referred for colorectal cancer screening, polyp surveillance, or diagnostic assessment underwent same-day, back-to-back tandem colonoscopy with standard forward-viewing colonoscope and the full-spectrum endoscopy colonoscope. The patients were randomly assigned (1:1), via computer-generated randomisation with block size of 20, to which procedure was done first. The endoscopist was masked to group allocation until immediately before the start of colonoscopy examinations; patients were not masked. The primary endpoint was adenoma miss rates. We did per-protocol analyses. This trial is registered with ClinicalTrials.gov, number NCT01549535.
Findings
197 participants were enrolled. 185 participants were included in the per-protocol analyses: 88 (48%) were randomly assigned to receive standard forward-viewing colonoscopy first, and 97 (52%) to receive full-spectrum endoscopy colonoscopy first. By per-lesion analysis, the adenoma miss rate was significantly lower in patients in the full-spectrum endoscopy group than in those in the standard forward-viewing procedure group: five (7%) of 67 vs 20 (41%) of 49 adenomas were missed (p<0·0001). Standard forward-viewing colonoscopy missed 20 adenomas in 15 patients; of those, three (15%) were advanced adenomas. Full-spectrum endoscopy missed five adenomas in five patients in whom an adenoma had already been detected with first-pass standard forward-viewing colonoscopy; none of these missed adenomas were advanced. One patient was admitted to hospital for colitis detected at colonoscopy, whereas five minor adverse events were reported including vomiting, diarrhoea, cystitis, gastroenteritis, and bleeding.
Interpretation
Full-spectrum endoscopy represents a technology advancement for colonoscopy and could improve the efficacy of colorectal cancer screening and surveillance.
Funding
EndoChoice.
Introduction
Colonoscopy and polypectomy prevent incident cases of colorectal cancer by detection at an early and curable stage, and by identification and removal of colorectal adenomas—the precursor lesions of most colorectal cancers.1–11 However, this protection is imperfect and is less effective in the proximal than the distal colon,12–16 largely resulting from missed cancers and precancerous lesions (eg, adenomas) during colonoscopic examinations.17 Adenoma miss rates during colonoscopy have become widely acknowledged,18–24 which has spawned an extensive effort within the gastroenterology community to improve the quality of colonoscopy examinations by measurement of quality indicators.25–28 Additionally, new colonoscope technologies have been tested for their ability to better detect flat or subtle lesions, or to improve visualisation of the mucosa behind colonic folds (eg, with cap-fitted or retroscopic colonoscopes) where adenomas might be hidden. Until now, these technological changes have been minimally effective or impractical for improvement of adenoma detection.29,30
Nowadays, standard forward-viewing colonoscopes visualise the colon from the flexible tip of the instrument, with an angle of view up to 170°. The full-spectrum endoscopy colonoscope (Fuse, EndoChoice, GA, USA) is a new endoscopic platform that has imagers on not only the forward tip of the colonoscope, but also on both sides of the tip.31,32 Together three imagers provide a 330° angle of view of the colon displayed to the endoscopist on three side-by-side, contiguous video monitors. In preliminary testing, the full-spectrum endoscopy colonoscope provided far better detection of all polyps and of hidden polyps in an in-vitro colon model than the standard forward-viewing colonoscopies.31 Moreover, in the first ever pilot and feasibility study of the full-spectrum colonoscope in 50 participants, the device had a 100% caecal intubation rate and provided high evaluation scores from patients and endoscopists, with no adverse events.32
We postulated that full-spectrum endoscopy would have a significantly lower adenoma miss rate than the standard forward-viewing procedure.
Methods
Study design and patients
We did this international, multicentre, randomised, tandem colonoscopy trial at three sites in Israel, one site in the Netherlands, and two sited in the USA between Feb 1, 2012, and March 31, 2013. We enrolled patients aged 18–70 years who had been referred for colorectal cancer screening, polyp surveillance, or diagnostic assessment. We excluded individuals with a history of colonic resection; inflammatory bowel disease; polyposis syndrome; lower gastrointestinal bleeding; colonic stricture; acute diverticulitis or toxic megacolon; or a history of radiation therapy to the abdomen or pelvis. Institutional review board or medical ethics committee approval was obtained at each site. Informed written consent was obtained from all participants.
Randomisation and masking
Patients were randomly assigned (1:1), by computer-generated randomisation with block design (20 patients per block), to receive same-day, back-to-back tandem colonoscopy with either full-spectrum colonoscopy or standard forward-viewing colonoscopy, followed immediately by the other procedure. Immediately before start of the colonoscopy examinations, the site study coordinator opened the concealed allocation card to reveal group allocation. Until that moment, the endoscopist was masked to group assignment; patients were not masked.
Procedures
We used same-day, back-to-back tandem colonoscopy to assess adenoma miss rates. Each participant had both colonoscopy examinations done by the same gastroenterologist. All polyps were removed as they were identified, other than diminutive (1–2 mm) rectal polyps thought by the gastroenterologist to be hyperplastic in nature, and therefore would not falsely increase polyp detection. The Fuse full-spectrum endoscopy platform comprises a video colonoscope and a processor. The full-spectrum colonoscope is a standard adult (168 cm working length, 12·8 mm scope outer diameter), flexible, reusable, reprocessable colonoscope intended for repeated clinical use (diagnostic visualisation and therapeutic interventions). The device provides a high-resolution, 330° field of view while maintaining standard colonoscope capabilities and manoeuvrability, including full tip deflection (scope up or down 180° and left or right 160°), working channel (3·8 mm), air or CO2 insufflation options, suction, and forward water jet irrigation—identical technical features to the present industry-standard, forward-viewing colonoscopes. Full-spectrum endoscopy is achieved by use of three imagers and light-emitting diode (LED) groups positioned at the front and on the sides of the distal tip of the colonoscope. Figure 1 shows the field of view of the standard forward-viewing and full-spectrum colonoscope displayed on three contiguous video monitors. The left, centre, and right video monitors correspond with the colonic images transmitted from the left-facing, forward-facing, and right-facing lenses, respectively (video).
Figure 1. Standard forward-viewing versus full-spectrum endoscopy.
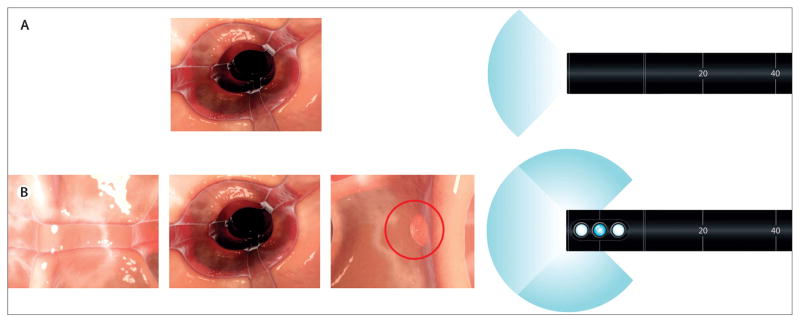
The standard forward-viewing endoscope has a 170° field of view that is shown on one screen (A). A full-spectrum endoscope has additional imagers and provides a 330° field of view that is viewed on three contiguous screens (B).
All patients underwent preparation for standard colonoscopy with either a polyethylene glycol-based solution or a sodium picosulfate preparation, both of which are commercially available and approved for use as colonoscopy preparations. The choice of colon preparation was at the discretion of the gastroenterologist. We measured the level of bowel cleanliness at the time of colonoscopy with the Ottawa Bowel Preparation Scale.33 Conscious sedation was delivered by the gastro-enterologist or anaesthesiologist and included midazolam, fentanyl, propofol, or a combination thereof during both colonoscopy examinations. Standard forward-viewing colonoscopy was done with Olympus (Evis Exera II 160 and 180 series) or Pentax (Pentax EPKi) adult colonoscopes. All colonoscopy exams were done with white light only with no other electronic imaging technologies. On colonoscope withdrawal, the endoscopist was instructed to use their usual colonoscope withdrawal technique and asked to spend a minimum of 6 min withdrawing and examining the colon.34 Water-jet irrigation was used to wash residual fluids and liquid stool to improve visualisation. Insertion time to the caecum, withdrawal time, and total procedure time were all recorded with a stopwatch during each colonoscopy procedure. The stopwatch was paused during polypectomy or diagnostic biopsy, then restarted after completion of these interventions. The endoscopist estimated polyp size with the open biopsy forceps technique. A polyp found proximal to the splenic flexure was a priori defined as located in the right colon; all other more distal polyps were regarded as being located in the left colon.20,22,23 Retroflexion of the colonoscope in the rectum was requested in each patient. The time between the tandem back-to-back colonoscopy examinations was less than 5 min and both examinations were done in the same endoscopy suite.
All polyps detected during first-pass colonoscopy were completely removed. Any additional polyps detected on second-pass colonoscopy were also completely removed. All removed polyps were sent to the pathology department at each study site. Histology results were reported to the attending gastroenterologist and to the study coordinator. Polyps were categorised as adenomatous, hyperplastic, or other. If a polyp was reported to be adenomatous on the basis of pathology, then the adenoma subtype was also recorded—ie, tubular, tubulovillous, villous, or serrated. We also used histological analysis to detect the presence of low-grade and high-grade dysplasia within adenomas.
Outcomes
The primary endpoint was adenoma miss rates. Secondary endpoints included polyp miss rates, advanced adenoma miss rates, time to caecal intubation, colonoscope withdrawal time, total procedure time, and adverse events. We defined advanced adenomas as any adenoma of 10 mm or greater in size, containing villous histology, or with high-grade dysplasia.6,9
Statistical analysis
We prospectively designed this study to allow for 80% power or more to detect a 20% difference (35% vs 15%) in adenoma miss rates, per lesion analysis, between colonoscopy procedures with a two group χ2 test with a two-sided α level of 0·05. A sample size of 178 participants was needed; therefore, the overall participant enrolment goal was 196 to allow for potential exclusions or dropouts, with each participant undergoing same day, back-to-back colonoscopy (356 tandem colonoscopies in total). Descriptive statistics were calculated for all measured variables and derived parameters. For continuous variables, time to reach the caecum, colonoscope withdrawal time, and total procedure time, we calculated means, medians, IQRs, SDs, and minimums and maximums. For categorical variables, summary statistics were counts and percentages. Because each participant was his or her own control in this study, we used paired t tests to compare continuous variables. For categorical variables, we used Fisher’s exact test or χ2 test to compare detection rates between groups. For estimates of proportions, we calculated 95% exact binomial CIs. All tests applied were two-tailed. We analysed data with SAS (version 9.1). This trial is registered with ClinicalTrials.gov, number NCT01549535.
Role of the funding source
IMG and PDS developed the study protocol in collaboration with the original study sponsor Peer Medical, Israel (now EndoChoice, GA, USA). Data collection was done by the local study sites with a study coordinator supplied by the sponsor. IMG, PDS, and DKR did all data analyses and data interpretation in collaboration with the independent contracted statistician (RBD’A). The sponsors had no role in writing of the report. IMG, PDS, DKR, and RBD’A had access to the raw data. IMG had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
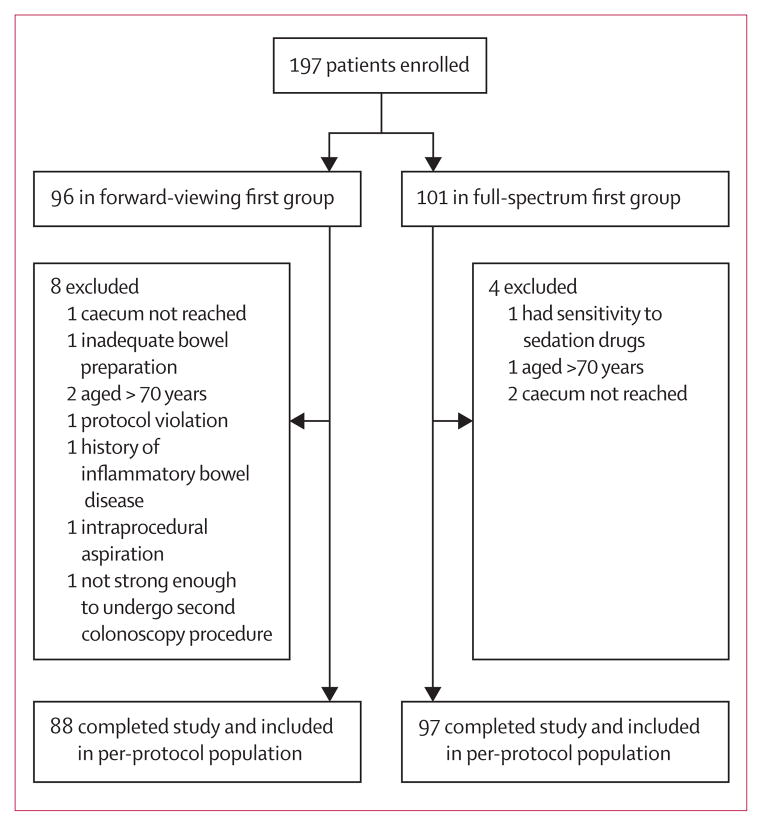
Figure 2 shows the trial profile. 197 participants were randomly assigned to the forward-viewing first group (n=96) or the full-spectrum first group (n=101), of whom 88 (92%) versus 97 (96%) completed the study and were included in the per-protocol analysis (figure 2). Excluded participants did not differ significantly from the included individuals in terms of age, sex, indication for colonoscopy, and randomisation group, but did differ on the Ottawa Bowel Preparation Scale (data not shown). The number of procedures done by each endoscopist is shown in table 1. Baseline and demographic characteristics were similar between groups (table 2).
Figure 2.
Trial profile
Table 1.
Number of endoscopists and patients completing analysis at each study site
| Patients (N=185) | Endoscopists per study site | Number of procedures per endoscopist | |
|---|---|---|---|
| Site one (Elisha Hospital, Haifa, Israel) | 53 (29%) | 2 | Endoscopist number 1=38 Endoscopist number 2=15 |
| Site two (North Shore Gastroenterology Associates, Great Neck, NY, USA) | 30 (16%) | 5 | Endoscopist number 1=11 Endoscopist number 2=9 Endoscopist number 3=5 Endoscopist number 4 =2 Endoscopist number 5=3 |
| Site three (South Shore Gastroenterology, Cedarhurst, NY, USA) | 3 (<1%) | 1 | Endoscopist number 1=3 |
| Site four (Utrecht University Medical Center, Utrecht, Netherlands) | 43 (23%) | 3 | Endoscopist number 1=18 Endoscopist number 2=16 Endoscopist number 3=9 |
| Site five (Lady Davis Carmel Medical Center, Haifa, Israel) | 6 (3%) | 1 | Endoscopist number 1=6 |
| Site six (Tel Aviv Sourasky Medical Centre, Tel Aviv, Israel) | 50 (28%) | 3 | Endoscopist number 1=32 Endoscopist number 2=16 Endoscopist number 3=2 |
Table 2.
Baseline and demographic characteristics of randomised groups completing tandem colonoscopy examinations
| Standard forward-viewing colonoscopy first (n=88) | Full-spectrum colonoscopy first (n=97) | |
|---|---|---|
| Age (years) | 56 (22–70) | 57 (21–70) |
|
| ||
| Sex (female) | 46 (52%) | 55 (57%) |
|
| ||
| Ottawa Bowel Preparation Scale | 3 (2–5) | 3 (0–5) |
|
| ||
| Indication for colonoscopy | ||
| Screening | 53 (60%) | 50 (52%) |
| Surveillance | 16 (18%) | 20 (21%) |
| Diagnostic assessment | 19 (22%) | 27 (28%) |
| Total adenoma detection | 30 (34%) | 34 (35%) |
Data are median (IQR) or n (%), unless otherwise indicated.
In patients who had standard forward-viewing colonoscopy first, 29 adenomas were identified on first-pass examination in 25 patients; full-spectrum colonoscopy detected 20 additional adenomas in 153 patients on second-pass examination (a 69% increase; table 3). In the full-spectrum colonoscopy first group, 60 adenomas and two cancers in 33 patients were identified initially. On second pass with standard forward-viewing colonoscopy, five additional adenomas were detected. The proportion of missed adenomas was significantly lower with full-spectrum colonoscopy (table 3). The proportion of patients with at least one adenoma detected at colonoscopy was lower in the standard forward-viewing first group than in the full-spectrum first group (p=0·41; table 4). Five (6%) patients in the standard forward-viewing first group had no adenomas detected at first-pass examination, then had adenomas detected by full-spectrum endoscopy (table 4). By contrast, no patients in the full-spectrum endoscopy first group had additional adenomas detected by standard forward-viewing colonoscopy (table 4). Although this study was not a priori powered towards per-participant analyses, we assessed false-negative detection at the time of initial colonoscopy examination. Full-spectrum colonoscopy led to significantly fewer false-negative examinations than did standard forward-viewing colonoscopy (table 4; p=0·02). Furthermore, we assessed patients with false-negative colonoscopy examinations as a proportion of all patients with adenomas detected, thereby providing an assessment of sensitivity. 30 patients assigned to receive standard forward-viewing colonoscopy followed by full-spectrum colonoscopy had adenomas detected. From this cohort, 25 (83%) patients had adenomas detected by standard forward viewing and five (17%) had adenomas detected by full-spectrum endoscopy. 33 patients assigned to receive full-spectrum endoscopy first had adenomas detected. From this cohort, 33 (100%) patients had adenoma detected by full-spectrum endoscopy; no additional unique patients with adenomas were detected by second-pass standard forward-viewing colonoscopy (p=0·02).
Table 3.
Adenomas detected and missed with standard forward-viewing and full-spectrum colonoscopy
| Adenomas detected with standard forward-viewing colonoscopy | Adenomas detected with full-spectrum colonoscopy | Total number of adenomas identified | Adenoma miss rate with standard forward-viewing colonoscopy* | Adenoma miss rate with full-spectrum colonoscopy* | |
|---|---|---|---|---|---|
| Standard forward-viewing colonoscopy first (n=88) | 29 | 20 | 49 | 20/49 (41%);† 27·0–56·0 | .. |
| Full-spectrum colonoscopy first (n=97) | 5 | 62‡ | 67 | .. | 5/67 (7%); 2·5–16·6 |
Data are n or n/N (%) with 95% CI.
Full-spectrum colonoscopy vs standard forward-viewing colonoscopy adenomas missed, p<0·0001.
Includes three advanced adenomas (two adenomas with villous histology and one adenoma ≥10 mm in size).
Includes two cancers.
Table 4.
Adenomas detected and missed per patient
| Patients with adenomas detected by standard forward-viewing colonoscopy or full-spectrum endoscopy | Patients with adenomas detected by full-spectrum or standard forward-viewing colonoscopy | Unique patients with adenomas detected by full-spectrum or standard forward-viewing colonoscopy | Total patients with adenomas | Patients with false-negative results from standard forward-viewing colonoscopy or full-spectrum colonscopy* | Patient miss rate (adenomas) with standard forward-viewing colonoscopy or full-spectrum colonscopy† | |
|---|---|---|---|---|---|---|
| Standard forward-viewing colonoscopy first (n=88) | 25 (28%) | 15 (17%) | 5 (6%) | 30 (34%) | 5/88 (6%); 1·9–12·8 | 5/30 (17%); 5·6–34·7 |
| Full-spectrum colonoscopy first (n=97) | 33 (34%) | 5 (5%) | 0 | 33 (34%) | 0/97; 0–3·7 | 0/33; 0–10·6 |
Data are n (%), or n/N (%) with 95% CI. A per-patient analysis.
p=0·02 Fisher’s exact test
p=0·02 Fisher’s exact test.
Standard forward-viewing colonoscopy identified 50 polyps on first-pass examination; on second-pass examination with full-spectrum endoscopy, 38 additional polyps were detected (a 76% increase; table 5). Full-spectrum endoscopy identified 102 polyps and, on second-pass colonoscopy with standard forward viewing, 11 additional polyps were detected (table 5). The overall polyp miss rate was significantly lower with full-spectrum colonoscopy than with the standard forward-viewing technique (p<0·0001; table 5).
Table 5.
Polyps detected and missed
| Polyps detected with standard forward-viewing colonoscopy | Polyps detected with full-spectrum colonoscopy | Total polyps identified | Incremental polyps detected with full-spectrum colonoscopy* | Incremental polyps detected with standard forward-viewing colonoscopy | Polyp miss rate with standard forward-viewing colonoscopy | Polyp miss rate with full-spectrum colonoscopy† | |
|---|---|---|---|---|---|---|---|
| Standard forward-viewing colonoscopy first (n=88) | 50 | 38 | 88 | 38/50 (76%); 61·8–86·9 | .. | 38/88 (43%); 32·7–54·2 | .. |
| Full-spectrum colonoscopy first (n=97) | 11 | 102 | 113 | .. | 11/102 (11%); 5·5–18·5 | - | 11/113 (10%); 5·0–16·8 |
Data are n or n/N (%) with 95% CI.
Full-spectrum endoscopy vs standard forward view additional polyps detected, p<0·0001.
Full-spectrum endoscopy vs standard forward view polyps missed, p<0·0001.
Of the 20 adenomas missed by standard forward-viewing colonoscopy, 18 (90%) were sessile and two (10%) pedunculated; 14 (70%) were 1–5 mm in size, five (25%) were 6–9 mm, and one (5%) was 10 mm or larger; 18 (90%) were tubular, one (5%) was tubulovillous, and one (5%) was villous adenoma. Thus, three (15%) of the 20 adenomas missed with standard forward-viewing colonoscopy were regarded as advanced.6,9 14 (70%) of the missed adenomas were in the right colon and six (30%) were in the left colon. Of the five adenomas missed by full-spectrum colonoscopy, all were sessile, 1–5 mm in size, tubular adenomas without high-grade dysplasia, thus none of the missed tumours were advanced. Two missed adenomas were in the right colon and three were in the left colon.
The median time to the caecum did not significantly differ between the procedures (p=0·84; table 6). Median time for colonoscope withdrawal and total procedure duration were significantly shorter with standard forward-viewing colonoscopy (both p<0·0001; table 6). The appendix has details about the time endpoints analyses based on group randomisation.
Table 6.
Time to the caecum, withdrawal time, and total procedure time
| Time to caecum (min) | Withdrawal time (min) | Total procedure time (min) | |
|---|---|---|---|
| Standard forward-viewing colonoscopy | 5·1 (3·2–7·6) | 5·6 (4·1–6·8) | 12·2 (9·2–16·5) |
| Full-spectrum colonoscopy | 4·8 (3·1–8·0) | 6·2 (5·1–8·3) | 14·5 (10·8–20·2) |
| p value* | p=0·84 | p<0·001 | p<0·001 |
Data are median (IQR), unless otherwise indicated.
From Wilcoxon signed-rank tests.
One patient from the forward-viewing colonoscopy group was admitted to hospital for colitis detected at colonoscopy. We recorded five minor adverse events: vomiting, cystitis, bleeding, and gastroenteritis in the full-spectrum colonoscopy followed by standard forward-viewing colonoscopy group, and diarrhoea in the forward-viewing colonoscopy first group.
Discussion
Our findings show that compared with standard forward-viewing colonoscopy, full-spectrum colonoscopy had a significantly lower adenoma miss rate (panel). Because significantly more adenomas were detected with full-spectrum colonoscopy than with the standard forward-view procedure, changes were made in patient management, with alterations to surveillance colonoscopy recommendations. In patients with adenomas missed by standard forward-viewing colonoscopy, the adenomas subsequently detected led to a shortening of the recommended post-polypectomy surveillance inter val for eight patients on the basis of present US (American Gastroenterological Association Institute, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology) and five patients on the basis of European (European Society of Gastrointestinal Endoscopy) guidelines.9–11
Panel: Research in context.
Systematic review
We searched Pubmed and Medline between 1980 and 2014 to identify relevant published scientific literature. We restricted our search to English-language articles. Keywords included “colonoscopy”, “colonoscope technology”, “endoscopy technology”, “colorectal cancer screening”, “colorectal cancer surveillance”, “interval colon cancer”, “colonoscopy quality indicators”, “adenoma detection rate”, and “adenoma miss rates”.
Interpretation
Our findings show that compared with standard forward-viewing colonoscopy, full-spectrum colonoscopy missed significantly fewer adenomas. These results suggest that full-spectrum colonoscopy improves visualisation of the colonic mucosa and might improve the effectiveness of colorectal cancer screening and surveillance colonoscopy.
When full-spectrum colonoscopy was used first, five adenomas were missed in five patients, although each of these patients had other adenomas detected during full-spectrum colonoscopy. The five adenomas missed by full-spectrum colonoscopy were not advanced adenomas. Two adenomas missed by full-spectrum colonoscopy were in the right colon and three were in the left colon; none of the adenomas missed by full-spectrum colonoscopy would have altered the post-polypectomy surveillance colonoscopy recommendations by US or ESGE guidelines.9,11 On the basis of European guidelines, one patient was reclassified into the high-risk category because they went from four to five to total small (<10 mm) adenomas detected after second-pass standard forward-viewing colonoscopy.10
70% of the adenomas missed by standard forward-viewing colonoscopy, and which were subsequently detected by full-spectrum colonoscopy, were in the right colon. This finding might have clinical significance in view of the known reduced protective effect of colonoscopy for proximal, compared with distal, colon cancers.12–16 Moreover, although this trial was not statistically powered for per-patient analyses, we noted that full-spectrum colonoscopy yielded significantly fewer false-negative examinations than did standard forward-viewing colonoscopy. However, because of the small sample size of the study, the adenoma detection rate was non-significant for standard forward-viewing versus full-spectrum colonoscopy.
One of the main solutions to significantly reduce the adenoma miss rate of standard forward-viewing colonoscopy is to improve on present colonoscope technology with more advanced optics and wider-angle visualisation combined with a user-friendly, intuitive platform interface.29 Findings from several studies in diverse patient populations have shown that adenomas are missed with present standard forward-viewing colonoscopy.18–24 Many previous developments have had no, or only a small, effect on adenoma miss rates, including colonic dye spraying (chromoendoscopy), virtual chromoendoscopy, autofluorescence, and cap-fitted colonoscopy.29,30 Although the individual adenomas missed in this study were mostly diminutive, in clinical practice, the detection of more adenomas of all sizes is strongly correlated with clinically important endpoints, including detection of large adenomas and of more patients with more than one adenoma.34,35 This point suggests that techniques and emerging technologies that detect more adenomas overall, will also detect more large adenomas. Furthermore, improved detection of several small adenomas in individual patients results in improved recognition of this well-known risk factor for subsequent advanced lesions, and might result in shorter and more protective surveillance intervals.9–11
The most recent technological advancement in colonoscopy is the Third-Eye Retroscope (Avantis Medical Systems, CA, USA).36,37 This device is an auxilliary, through-the-scope, optical technology intended to detect polyps located on the proximal side of colonic folds and at the anatomic flexures of the colon. In an international, multicentre, randomised trial of 349 participants, Leufkens and colleagues reported reductions in the proportion of missed adenomas, albeit more modest than those described here for full-spectrum colonoscopy.23 Furthermore, the retroscope technology is probe based, requiring the accessory channel of the colonoscope, thus increasing the time needed for scope withdrawal, biopsies, and polypectomies. By contrast, the full-spectrum colonoscope platform does not interfere with any of the standard operating features of colonoscopes.
We recorded that the time to reach the caecum with full-spectrum colonoscopy was less than 5 min. This time efficiency might have been aided by the improved driveability of the colonoscope on insertion because blind corners and angles within the colon lumen were largely eliminated by the greatly increased field of view provided by the three imagers. The first colonoscopy done, with either procedure, took longer (total procedure time) than did the second procedure. This finding might be partly explained by the straightening and shortening of the colon that happens during the first-pass examination thereby easing scope passage during the second examination. Moreover, although full-spectrum colonoscopy was significantly slower on both colonoscope withdrawal and total procedure times, these time differences are not likely to be clinically meaningful or relevant in daily endoscopy practice. Despite the recorded 30 s longer withdrawal time for full-spectrum colonoscopy (an estimated 10% longer than for standard forward-viewing colonoscopy), we do not believe this slight time differential explains the significantly higher proportion of adenomas detected.
Our study has some limitations. First, the endoscopist could not be masked to which colonoscope they were using and one endoscopist did both back-to-back colonoscopy examinations. Additionally, one endoscopist might not have used the same effort when trying to detect adenomas in both examinations and might have favoured the new technology. Although use of one endoscopist might create bias, this methodology has been the standard one used in most tandem, back-to-back colonoscopy trials.19,21,23,35,36 Use of the same endoscopist for both examinations has some advantages because they act as their own control with use of an identical withdrawal technique and baseline adenoma detection rate. Second, the study was statistically powered to be a per-lesion and not a per-patient analysis. However, published scientific literature about polyp and adenoma miss rates with tandem colonoscopy design have all used per-lesion analyses.18–23 Third, we did not restrict the study population to screening only participants. Nevertheless, in the patients who were specifically referred for screening colonoscopy, the study findings remained consistent. Fourth, we did not force colonoscopy withdrawal times to be equal in the two study agroups. Despite this, withdrawal times and total procedure times using the two colonoscope types were similar. Fifth, we recorded protocol violations and exclusions that led to the withdrawal of patients.
In conclusion, these data suggest that full-spectrum colonoscopy improves visualisation of the colonic mucosa during colonoscopy and could improve the efficacy of screening of colorectal cancer and surveillance colonoscopy.
Acknowledgments
We thank the physicians and study site staff who assisted in the undertaking of this study: Howard Rattner, Arthur Talansky, Mordecai A Dicker, Chaim S Abittan, David Milkes, and Sveta Domanov; and Prof Edmond Sabo for his histopathology work for this study. RBD’A Jr was supported by an NIH-NCI P30 CA012197 (biostatistics core) grant.
Footnotes
Contributors
IMG and PDS designed the study; collected, analysed, and interpreted data; and wrote and reviewed the manuscript. ZH and OS collected and interpreted data and reviewed the manuscript. AM, ASu, ES, ASl, JF, LMGM, and VKD collected data and reviewed the manuscript. RBD’A analysed and interpreted data, and wrote and reviewed the manuscript. DKR collected, analysed and interpreted data, and wrote the manuscript
Declaration of interests
This study was funded by PeerMedical, Caesaria, Israel (now EndoChoicec, Alpharetta, GA, USA). IMG, OS, ASu, RBD’A, and DKR were consultants for PeerMedical whilst the study was being done. PDS, ZH, OM, AM, ES, ASl, JF, LMGM, and VKD declare that they have no competing interests.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–68. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 3.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 4.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 5.Kahi CJ, Imperiale T, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–75. doi: 10.1016/j.cgh.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopy polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner H, Chang-Claude J, Jansen L, Seiler CM, Hoffmeister M. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med. 2012;157:225–32. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61:1180–86. doi: 10.1136/gutjnl-2011-300295. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Atkin WS, Valori R, Kuipers EJ, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44:SE151–63. doi: 10.1055/s-0032-1309821. [DOI] [PubMed] [Google Scholar]
- 11.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–51. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 12.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–74. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 13.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127:452–56. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;39:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: a population-based study. J Natl Cancer Inst. 2010;102:8–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 16.Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664–69. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–64. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Hixson LJ, Fennerty MB, Sampliner RE, Garewal HS. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–27. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–59. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 21.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 22.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–90. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 23.Leufkens AM, DeMarco DC, Rastogi A, et al. Effect of retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480–89. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Leufkens AM, van Oijen MGH, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470–75. doi: 10.1055/s-0031-1291666. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the US multi-society task force on colorectal cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 28.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with post-colonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Rex DK. Update on colonoscopic imaging and projections for the future. Clin Gastroenterol Hepatol. 2010;8:318–21. doi: 10.1016/j.cgh.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 30.ASGE Technology Committee. Report on emerging technology: devices to improve colon polyp detection. Gastrointest Endosc. 2011;73:1092–97. doi: 10.1016/j.gie.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 31.Gralnek IM, Carr-Locke DL, Segol O, et al. Comparison of standard forward viewing mode versus ultra-wide viewing mode of a novel colonoscopy platform: a prospective, multicenter study in the detection of simulated polyps in an in vitro colon model. Gastrointest Endosc. 2013;77:472–79. doi: 10.1016/j.gie.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Gralnek IM, Segol O, Suissa A, et al. A prospective cohort study evaluating a novel colonoscopy platform featuring full spectrum endoscopy. Endoscopy. 2013;45:697–702. doi: 10.1055/s-0033-1344395. [DOI] [PubMed] [Google Scholar]
- 33.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosci. 2004;59:482–86. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 34.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 35.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 36.DeMarco DC, Odstrcil E, Lara LF, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the third eye retroscope study group. Gastrointest Endosc. 2010;71:542–50. doi: 10.1016/j.gie.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Waye JD, Heigh RI, Rex DK, et al. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation. Gastrointest Endosc. 2010;71:551–56. doi: 10.1016/j.gie.2009.09.043. [DOI] [PubMed] [Google Scholar]