SUMMARY
Steroid receptors are found in discrete cellular locations but it is unknown whether extra-nuclear pools are necessary for normal organ development. To assess this we developed a point-mutant estrogen receptor alpha (ERα) knock-in mouse (C451A) that precludes palmitoylation and membrane trafficking of the steroid receptor in all organs. Homozygous knock-in female mice (nuclear-only ERα, NOER mice) show loss of rapid signaling that occurs from membrane ERα in wild type mice. Multiple developmental abnormalities were found including infertility, relatively hypoplastic uteri, abnormal ovaries, stunted mammary gland ductal development, and abnormal pituitary hormone regulation in NOER mice. These abnormalities were rescued in heterozygous NOER mice that were comparable to wild type mice. Messenger RNAs implicated in organ development were often poorly stimulated by estrogen only in homozygous NOER mice. We conclude that many organs require membrane ERα and resulting signal transduction to collaborate with nuclear ERα for normal development and function.
Introduction
Steroid receptors are found in discrete cellular locations in most organs. In female mammals, genetic deletion of ERα produces phenotypes of abnormal development in multiple organs (Lubahn et al., 1993). Deletion of only the nuclear ERα pool simulates the total ERα knockout mouse (Pedram et al., 2009), indicating the importance of nuclear ERα for normal organ development and function. However, it is unknown as to whether extra-nuclear receptor pools are also required. Palmitoylation of ERα at cysteine 447 (human)/451 (mouse) is required for trafficking of the endogenous receptor to the plasma membrane (Acconcia et al., 2004; Pedram et al., 2007), and acylation is also required for membrane localization of progesterone and androgen receptors (Pedram et al., 2007; Pedram et al., 2012). At the membrane, ERα acts as a G-protein coupled receptor, enacting multiple signal transduction pathways that impact genomic and non-genomic functions (Kumar et al., 2007). To assess importance, we developed a point-mutant ERα knock-in mouse (C451A) that precludes steroid receptor palmitoylation and membrane trafficking in all organs. Homozygous knock-in female mice (nuclear-only ERα, NOER mice) show loss of rapid signaling that occurs from membrane ERα in wild type mice. Multiple developmental abnormalities were seen in homozygous NOER mice and these abnormalities were rescued in heterozygous NOER mice that were comparable to wild type mice. Multiple mRNAs implicated in organ development were poorly stimulated by estrogen only in homozygous NOER mice. We conclude that many organs require membrane ERα and resulting signal transduction to collaborate with nuclear ERα for normal development and function.
Results
Loss of membrane ER signaling impacts transcription
NOER knock in (KI) mice that lack ERα palmitoylation were generated by inserting a cysteine 451 to alanine mutation into the esr1 locus in ES cells by homologous recombination, the cells then used to generate mice. Expression of the point mutant ERα transgene was found in all organs assessed (Figure S1A). Mammary gland epithelial cells and hepatocytes from intact WT and heterozygous KI mice showed comparable nuclear ERα expression and presence of membrane-localized steroid receptors (Figures 1A and 1B). However, homozygous KI cells showed complete absence of membrane-localized ERα, but strong nuclear receptor expression that was comparable to WT mouse cells. The nuclear ERα pool does not undergo palmitoylation (Pedram et al., 2009) and hence localization was not affected by the mutation (Figure 1B). Since ~90% of total ERα protein is in the nucleus, ER protein was similar in cells from all mice (Figure S1B).
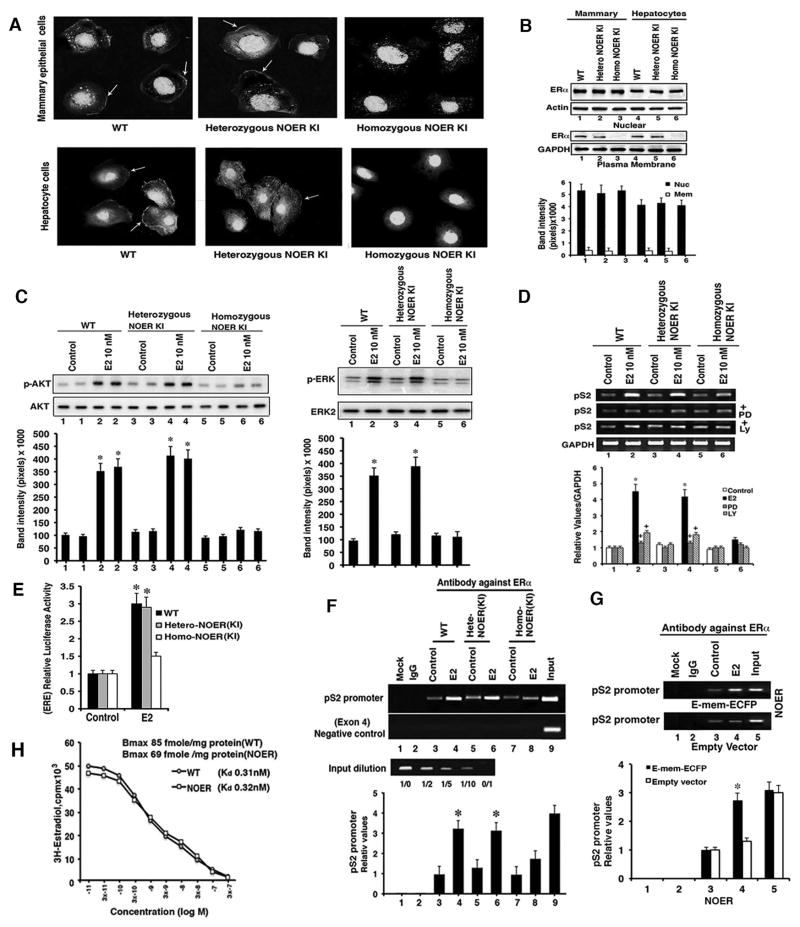
Fig. 1. NOER mouse characterization.
(A) Cellular ERα location by immuno-fluorescent microscopy of representative wild type (WT), heterozygous, and homozygous NOER mice cells. Arrows identify membrane receptors. (B) ERα protein blots in nuclear and membrane cell fractions. Graph is mean ± SEM from 5 mice each. (C) Representative kinase activity as AKT Ser473 phosphorylation (left) and ERKtyrosine 202/204 phosphorylation (right). Kinase total proteins are controls, graph data are from 2 individual mice per genotype in each of 3 exps combined. *p<0.05 by ANOVA + Schefe’s test for control vs E2 (D) pS2 mRNA in hepatocytes and regulation from ERK and PI3K signaling in response to 10nME2. RT-PCR results are 3 exps, GAPDH as control. *p<0.05 vs control, +p<0.05 E2 vs E2+PD or LY. (E) ERE-luciferase activity in hepatocytes from 5 mice each, in 3 separate exps. *p<0.05 vs E2. (F) Nuclear ERα occupancy of the pS2 promoter ERE. Graph is from cells of 5 mice each, in 3 exps. (G) Expression of the E domain of ERα targeted exclusively to the membrane promotes E2 recruitment of nuclear ERα to the pS2 promoter ERE. Graph is from cells of 5 mice for 3 exps *p<0.05 versus control in F and G. (H) Competition binding of increasing amounts of unlabeled E2 and H3-E2 to WT and homozygous NOER hepatocytes. Scatchard analysis of a representative experiment provides Kd and Bmax from cells from 5 livers per mouse type, the study done 3 times. See Figure S1 for additional results.
Membrane ER are solely responsible for rapid signal transduction stimulated by 17-β-estradiol (E2) (Pedram et al., 2006; Pedram et al., 2013). WT and heterozygous NOER hepatocytes responded to E2 with rapid activation of AKT and extracellular mitogen responsive protein kinase (ERK) (Figure 1C). In contrast, there was no significant signaling by E2 in homozygous NOER cells, consistent with lack of membrane ERα. We determined pS2 mRNA expression that is regulated by nuclear ERα binding estrogen response elements (ERE) (Won et al., 2012), and by membrane ER signaling through ERK and PI3K (Bjornstrom and Sjorberg, 2005; Madak-Erdogan et al., 2008; La Rosa et al, 2012). E2 stimulated comparably increased pS2 mRNA in WT and heterozygous NOER hepatocytes that depended upon ERK and AKT (Figure 1D). However, pS2 mRNA response to E2 was substantially less in homozygous NOER mouse hepatocytes, as was activation of an expressed EREx3/luciferase reporter (Figure 1E). After cell exposure to E2, nuclear ERα occupancy at an ERE within the pS2 gene promoter was robust in WT and heterozygous NOER hepatocytes but significantly less so in homozygous NOER cells (Figure 1F).
To determine whether decreased nuclear ERα recruitment to the pS2 promoter resulted from loss of signaling by membrane ERα, we expressed the E domain of ERα exclusively targeted to the plasma membrane in homozygous NOER hepatocytes. This portion of ERα is sufficient for numerous signaling pathways (Pedram et al., 2009) and we used this construct to derive the membrane ERα-only (MOER) mouse (Pedram et al., 2009, Pedram et al., 2013). pEmem-ECFP transfected homozygous NOER hepatocytes showed membrane localization from the expressed ERα E domain, E2 activation of ERK and PI3K-AKT, and restored E2-stimulation of pS2 mRNA that was ERK dependent (Figures S1C–S1E). Importantly, restoring E2 signaling from the membrane resulted in recruitment of nuclear ERα to the pS2 promoter ERE (Figure 1G). Thus, the C451A homozygous mutation prevents the collaboration between membrane and nuclear receptor pools from kinase activation by the membrane receptor. From Scatchard analysis of competition binding studies (Figure 1H), association, and dissociation studies (Figure S1F), labeled E2 binding to nuclear ER in WT and homozygous NOER hepatocytes was very similar.
Reproductive tract phenotypes in the homozygous NOER mice
The mating of proven breeder WT males with homozygous NOER female mice produced no pregnancies (Table S1). Breeding homozygous NOER male and female NOER mice was similarly not fruitful. This contrasted to matings between heterozygous male and female NOER mice (WT/KI) that were comparably successful to matings between WT male and female mice (12–18 weeks). Thus heterozygous expression of membrane ERα rescues the infertility phenotype of homozygous female NOER mice.
From vaginal smears, homozygous NOER female mice fail to ovulate, while heterozygous mice show epithelial cell cornification, indicating estrous cycling. To understand the basis for infertility, we determined that uteri from intact homozygous NOER mice were relatively hypoplastic compared to WT and heterozygous NOER mouse uteri that were comparable (n=5 each) (Figure 2A). Representative uterine tissue sections from the three types of mice showed that the luminal epithelium in particular was significantly thinner in the intact homozygous NOER females compared to WT and heterozygous NOER mice that were comparable (Figure 2B). This correlated to the complete loss of membrane ERα in isolated endometrial epithelial cells only from the homozygous KI mice (Figure S2A). WT, heterozygous, and homozygous NOER mice were ovariectomized and the responses to E2 for 21 days were determined (Figure 2C). E2 replacement was from estrogen pellets inserted under the skin, producing physiological levels of this sex steroid in mouse serum (Pedram et al., 2008). Homozygous NOER KI mice uterine epithelium showed little effects from ovariectomy and insignificant epithelial response to E2 (Figure 2C), despite abundant nuclear ERα(Figure 1B). In contrast, ovariectomy caused a marked loss of epithelial thickness in both WT and heterozygous NOER mice that was restored from E2 exposure (Figure 2C).
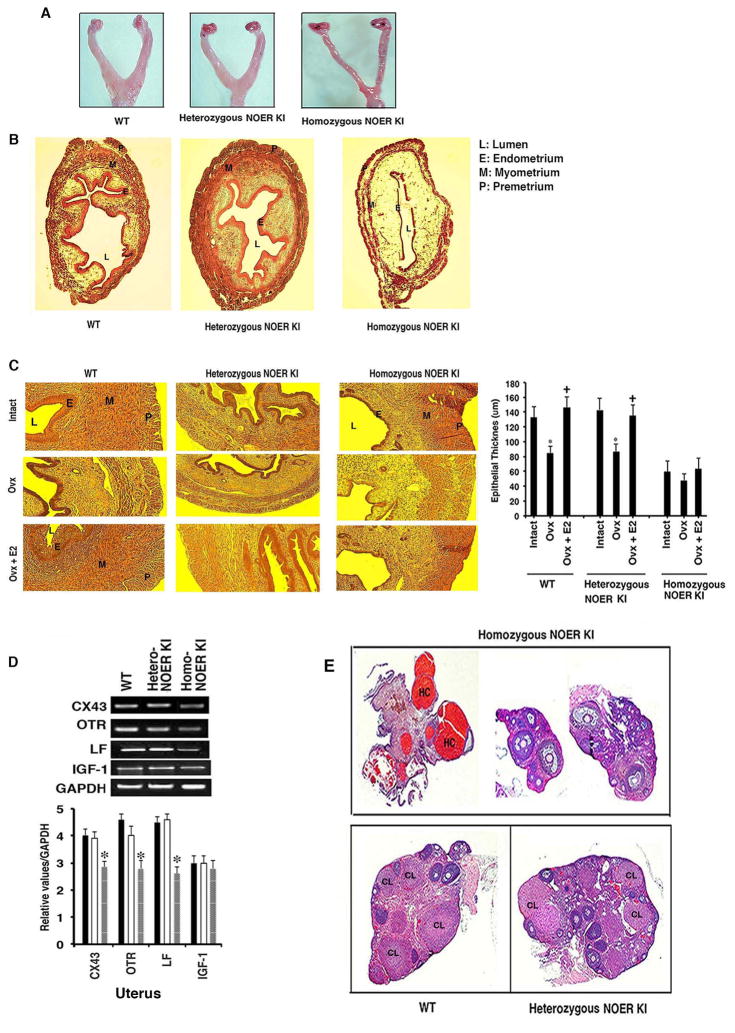
Fig. 2. Reproductive tract abnormalities in homozygous NOER mice.
(A) Representative reproductive tract development. (B) Cross sections of uterine layers from WT, heterozygous, and homozygous NOER mice. (C) Epithelial uterine thickness in intact or ovariectomized (Ovx) mice± E2 pellets (21 days) *p<0.05 vs intact, +p<0.05 vs Ovx (n=5). (D) Expression of key estrogen responsive mRNAs in uteri samples from intact WT, hetero, and homozygous NOER mice (bar graphs=5 mice each). *p<0.05 vs WT or heterozygous NOER organs. (E) Ovarian sections from WT and heterozygous NOER mice and 3 homozygous NOER mice (n=5). CL is corpus luteum, HC is hemorrhagic cysts. See also Figures S2 and S3.
Ovariectomized WT and homozygous NOER mice treated by E2 pellet for 12 days were further compared. Three representative homozygous KI mice uteri are shown to have little proliferative response to E2, and no significant change in uterine weight (Figure S2B). This is in contrast to the significant responses seen in WT mice. We also found decreased Ki67 staining in luminal epithelium of the homozygous NOER mice at 12 days exposure to the steroid, compared to WT mice (Figure S2C). These results indicate that nuclear ERα alone is not sufficient to maintain normal uterine epithelial proliferation and development.
We assessed mRNAs recognized as both important for uterine development and that are regulated by ERα (Grummer et al., 1999; Fleming et al., 2006; Teng, 1999). Expression of connexin 43, oxytocin receptor, lactoferrin, and insulin like-growth factor-1 (IGF-1) mRNAs were all comparable in uteri from intact WT and heterozygous NOER mice, but all except IGF1 were significantly reduced in intact homozygous NOER mice (Figure 2D) despite high serum E2 levels (Table 1). The IGF-1 results from intact mice suggest that nuclear ERα is sufficient for regulation of this mRNA in the endometrium. Ovariectomized WT mice responded to E2 pellets for 12 days with increases of all uterine mRNAs (Figure S2D). However, in homozygous NOER mice, E2 failed to significantly stimulate these mRNAs except for connexin 43. Thus, loss of membrane ERα compromises E2-induced expression of some important developmental mRNA(s) in the uterus. The reduced but present epithelial development in homozygous NOER mice probably reflects signaling by the IGF-1 receptor to trans-activate nuclear ERα in an E2-independent fashion (Winuthayanon et al., 2010). Nevertheless, growth receptor cross-talk to nuclear ERα is inadequate to fully compensate for the loss of membrane ERα function.
TABLE 1.
Adult female mouse serum hormone levels
| Mice | LH ng/ml | FSH ng/ml | E2 pg/ml | Prol ng/ml | IGF-1 ng/ml | GH ng/ml | Prog ng/ml |
|---|---|---|---|---|---|---|---|
| WT | 0.7±0.2* | 6.7±0.5 | 32.2±4.8 | 49.1±4.6* | 115±8.1 | 61±6 | 3.4±0.8* |
| WT (ovx) | 5.3±0.5 | 30.1±2.4 | 0.4±0.1 | 17±2.6 | 112±4.1 | 59±2.8 | 1.1±0.5 |
| Hetero -NOER KI | 1.2±0.3 | 6.1±0.6 | 37±3.9 | 52.3±4.7* | 120±9.3 | 59±3.9 | 3.0±0.5* |
| Hetero -NOER KI(ovx) | 4.8±1.0* | 29±2.1 | 0.8±0.2 | 21±2.6 | 109±5.1 | 52±2.8 | 0.8±0.3 |
| Homo -NOER KI | 2.3±0.5+ | 5.3±0.3 | 69±5.8+ | 32.6±1.9+ | 110±7.6 | 49±6.1 | 0.9±0.4+ |
| Homo -NOER KI (ovx) | 2.7±0.8+ | 24±1.5+ | 2.6±1.3 | 28±3.4 | 99±4.6 | 50±3.7 | 0.8±0.2 |
Data are from 6 mice per group/condition
p<0.05 by ANOVA + Schefe’s test for WT ovx or Hetero-NOER KI versus hetero-NOER KI ovx;
p<0.05 for Homo-NOER KI versus WT or hetero-NOER KI or same comparisons under ovx conditions.
Abnormalities of the ovary in homozygous NOER mice included large hemorrhagic cystic areas (HC), and small, malformed ovaries (Figure 2E). The ovaries lack corpora lutea (CL) that indicates anovulation, consistent with the vaginal smears and a basis for infertility. In contrast, heterozygote NOER and WT mice ovaries show normal development, consistent with their comparable fertility. Expression of the progesterone receptor (PR), transforming growth factor-β, and steroidogenic acute regulatory protein mRNAs were determined from pooled ovaries (Figure S3A) and individual mice (n=5, Figure S3A bar graph). For each, intact homozygous NOER mice showed significantly diminished mRNAs compared to WT and heterozygous NOER mice.
Hormone regulation is abnormal in homozygous NOER mice
We then determined sex steroids concentrations and impact on the hypothalamic-pituitary axis. Serum progesterone was measured without regard to estrus cycle phase, because homozygous NOER mice do not cycle. Progesterone in intact WT and heterozygous NOER mice was comparable and ~200% higher than homozygous NOER mice (Table 1). Very low progesterone in the latter reflects lack of ovarian corpora lutea, the source of post-ovulatory, circulating progesterone (Carr et al, 1981). Serum E2 was ~100% higher in homozygous NOER mice, compared to WT and heterozygous NOER mice that were comparable. Despite significantly higher E2 levels, serum LH was not suppressed in homozygous NOER mice. This is consistent with impaired negative feedback regulation of the hypothalamic-pituitary-ovarian axis, as reported in ERαKO mice (Couse and Korach, 1999), and indicates a role for membrane ERα action. The higher E2 levels did not stimulate LH secretion because, upon ovariectomy, serum LH was not diminished in the homozygous NOER mice and increased in WT and heterozygous NOER mice. IGF-1, growth hormone (GH), and FSH concentrations were not significantly different between the mice types but prolactin was significantly reduced in the intact homozygous KI mice. Upon ovariectomy, serum prolactin significantly decreased only in WT and heterozygous NOER mice. In-vitro, E2 signals to ERK activation from undetermined ER and ERK stimulates prolactin mRNA levels in cultured rodent pituitary cells (Watters et al., 2000). Our in-vivo findings are consistent with a role for membrane ERα signaling to increased prolactin production through ERK.
Restricted development of the mammary gland in homozygous NOER mice
Mammary gland development is rudimentary in ERαKO mice, mainly from loss of post-pubertal estrogen actions in the epithelium (Bocchinfuso et al., 1997). Whether membrane ERα signaling is required for glandular development is unknown. The mammary glands of pubertal, virgin WT and heterozygous NOER mice (~12 weeks old) were fully developed. In contrast, homozygous NOER female mice showed profoundly diminished ductal side branching and formation of blunted duct termini (Figure 3A and insets). Progressive development of post-pubertal mammary gland ducts results initially from estrogen action, followed by progesterone-induced ductal side-branching through its epithelial receptor, PR-B (Lydon et al., 1995; Brisken and O’Malley, 2010). The low serum progesterone in homozygous NOER mice therefore likely contributes to the abnormal mammary phenotype. ER stimulates PR transcription including in the mammary gland (Kastner P et al., 1990; Chauchereau et al., 1992), and membrane ERα signaling is important for PR expression in breast cancer epithelial cells (Pedram et al., 2012). WT and heterozygote NOER mice showed comparable mammary gland PR-B protein (left) and mRNA (right) expression (Figure 3B), markedly diminished in homozygous NOER glands.
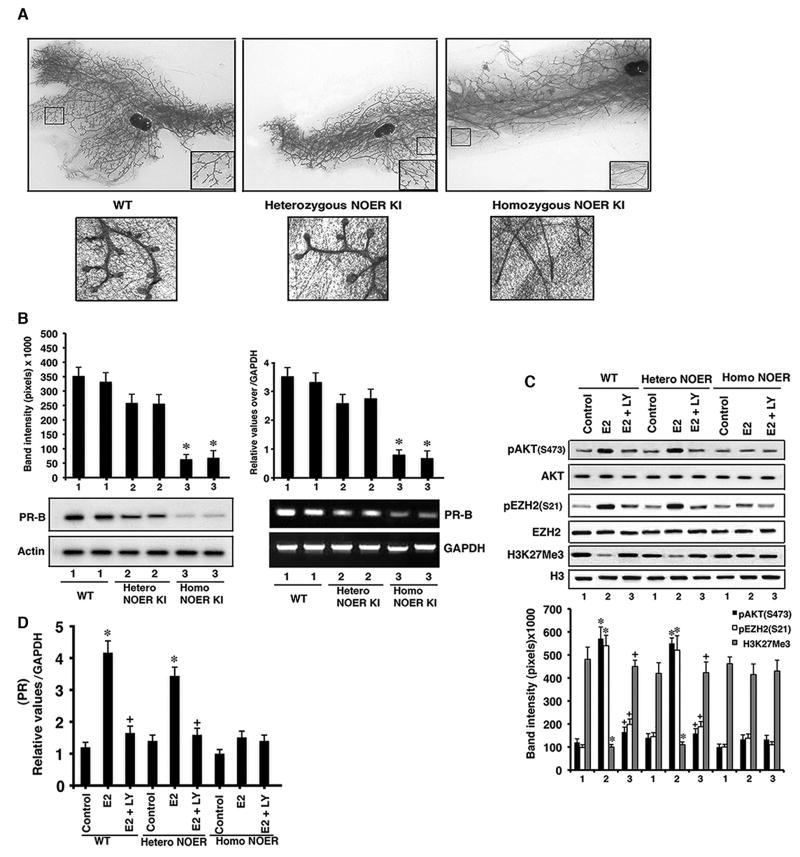
Fig. 3. Mammary gland development.
(A) Representative sections (n=5 mice per group) stained with carmine also show magnified inset. Terminal end bud formation is shown below. (B) PR-B proteins (left) and mRNAs (right) from mammary glands. Bar graphs are 5 mice each, *p<0.05 WT or heterozygous NOER vs homozygous NOER. (C) AKT activation by E2 in mammary epithelial cells results in EZH2 serine 21 phosphorylation and diminished H3K27methylation at the PR-B promoter, correlating to increased PR-B mRNA expression (bottom). Bar graphs are individual mouse data combined (n=5). *p<0.05 vs control, +p<0.05 vs E2. (D) PR-B mRNA expression in dissociated mammary epithelial cells exposed in culture to medium alone (control), E2, or E2±LY294002. *p<0.05 vs control, +p<0.05 vs E2. Combined data are from individual mouse cultures (n=5). See also Figure S3.
How might membrane ERα collaborate with nuclear ERα to up-regulate PR expression? Several possibilities may be relevant (Bjornstrom and Sjorberg, 2005), but others and we have recently shown that membrane ERα rapidly signals to the epigenetic regulation of genes in breast cancer cells (Bredfeldt et al., 2010; Pedram et al., 2012). In WT and heterozygous NOER mammary epithelial cells, E2 stimulated AKT-dependent phosphorylation of the histone methylase, EZH2, at an activity inhibitory site, serine 21 (Figure 3C). As a result, the H3K27me3 repressive mark at the PR promoter was significantly reduced. This correlated to E2-enhanced PR mRNA expression that was substantially reduced upon inhibition of PI3K-AKT (LY294002), further supporting the importance of rapid signaling from membrane ERα (Figure 3D). In contrast, H3K27 trimethylation was strong and PR mRNA was not stimulated by estrogen in homozygous NOER epithelial cells.
Amphiregulin is an important ligand for the epidermal growth factor receptor (EGFR) to stimulate mammary gland duct and terminal end bud development (Brisken and O’Malley, 2010; Ciarloni et al., 2007). mRNA for this EGFR ligand is induced by ERα and the protein has been implicated in estrogen-stimulated mammary epithelial proliferation and ductal elongation (Ciarloni et al., 2007). Amphiregulin, EGFR, and ErbB2 mRNAs in mammary glands from intact, homozygous NOER mice were significantly reduced compared to WT and heterozygous NOER glands (Figure S3A). Additionally, E2 or propyl-pyrazole-triol (PPT, ERα agonist) injection into ovariectomized mice resulted in stimulation of these and PR mRNAs, only in WT and heterozygous NOER mammary glands (Figure S3B). Thus, membrane ERα signaling is required for amphiregulin, EGFR, and ErbB2 mRNA expression, progesterone production, and mammary gland PR mRNA expression, thereby contributing to mammary gland ductal development. We also identify a potentially important mechanism where membrane ER signaling to the epigenetic regulation of PR mRNA impacts organ development.
Homozygous ERKO and MOER mice have comparable, rudimentary mammary development (Pedram et al., 2009). Comparing 10 week old, homozygous ERKO, MOER, and NOER mice, the phenotype in NOER mice is different. Homozygous NOER mice show greater extension of large ducts and filling in the mammary gland fat pad, compared to ERKO and MOER mice (Figure S3C). However, all three mice show the loss of ductal side branching and blunt end budding. We conclude that primary ductal extension through the mammary fat pad requires nuclear ERα action that is missing in ERKO and MOER mice, and is not rescued by only the membrane localization of expressed E domain of ERα (MOER). However, completely normal ductal development after puberty as seen in WT or heterozygous NOER mice requires both functional membrane and nuclear ERα pools.
Discussion
Important functional roles for extra-nuclear pools of ERα have increasingly been shown, particularly for membrane-localized receptors. In-vivo, membrane ERα signaling through AMP kinase suppresses key mRNAs and production of all lipids in liver, independently of nuclear ERα (Pedram et al., 2013). Administration to mice of an estrogenic compound that only binds membrane ERα prevents several forms of arterial injury in-vivo (Chambliss et al., 2010).
Here we provide evidence that membrane ERα is necessary for organ development and function. The ERα palmitoylation site mutant KI mouse shows nuclear ERα localization comparable to WT mice but E2-stimulated rapid signal transduction is markedly deficient, consistent with absence of membrane ERα. Importantly, loss of membrane ERα results in the markedly reduced expression of multiple mRNAs in response to E2 in several organs. Signaling from membrane ERα impacts transcription in several ways (Bjornstrom and Sjoberg, 2005) but our results indicate membrane ER signaling through MEK-ERK is important to recruit the nuclear receptor pool to the prototype pS2 promoter that is regulated from a recognized ERE. We also find epigenetic regulation of PR-B mRNA in WT and heterozygous NOER mammary epithelial cells results in part from membrane ERα activation of AKT, causing an inhibitory phosphorylation of EZH2 (Cha et al, 2005). Recently described, a mouse expressing inducible shRNA to EZH2 in the mammary epithelium showed diminished ductal elongation and impaired duct termini formation during puberty (Michalak et al., 2013), similar to the phenotype in homozygous NOER mice. Both mechanisms we describe provide further understanding of how membrane and nuclear ER integrate their functions but extensive investigation should uncover additional aspects. Abnormal development, mechanisms, and mRNA targets in homozygous NOER endometrium, ovary, and mammary glands are rescued in heterozygote KI mice that are comparable to WT mice.
Despite high estrogen levels in serum, only NOER female mice fail to show suppressed serum LH, indicating a necessary role of membrane ERα to participate in negative feedback regulation of LH by E2. Signaling by estrogen and ERα to kisspeptin/neurokinin B/dynorphin-expressing neurons inhibits GnRH production in the hypothalamus and suppresses serum LH (Rance, 2009; Navarro et al., 2009; Gottsch et al. 2009). However, our female MOER mice that lack nuclear ERα show non- suppressed serum LH despite high serum estradiol levels (Pedram et al., 2009). We therefore conclude that both receptor pools are involved.
While this paper was under revision, Adlanmerini et al characterized a newly developed mouse that also substituted alanine for cysteine at position 451 of ERα (Adlanmerini et al., 2014). These investigators described infertility and ovarian phenotypes, and loss of negative feedback regulation of LH that were very similar to our findings. However, in contrast to our studies, these investigators described similar uterine endometrial responses to E2 in homozygous WT and C451A mutant mice, and an 82% overlap of expressed genes by DNA microarray. Importantly, these investigators found that membrane ERα was only ~55% reduced in hepatocytes from their homozygous C451A mice, and did not characterize membrane ERα abundance in the endometrium. In contrast, we find the expected complete loss of membrane ERα in luminal epithelial cells from the endometrium, as well as mammary gland epithelium and hepatocytes of homozygous NOER mice. Also, we find the rescue of the endometrial and all phenotypes in heterozygous NOER mice. We thus propose that the presumed persistence of membrane ERα in the endometrium of the C451A homozygous mice (Adlanmerini et al, 2014) may account for the differences described.
Recently, arcuate nucleus kisspeptin/neurokininB/dynorphin neurons were implicated to contribute to estrogen suppressing gonadotropin secretion and abdominal visceral obesity (Mittleman-Smith et al. 2012). In the latter regard, we found that intact homozygous NOER female mice fed normal chow have extensive visceral fat deposition at 10 weeks of age, compared to WT and heterozygote NOER female mice (Figure S4B). Suppression of fat by estrogen is very complicated, involving food intake and energy metabolism differentially mediated through ERα in discrete hypothalamic nuclei (Xu et al, 2011), and direct or indirect actions on adipocyte lipogenesis and differentiation (Pedersen et al., 1992; Heine et al., 2000; Homma et al., 2000). Our findings indicate that membrane ERα plays an important role(s). In summary, we provide clear evidence that the membrane ERα pool is required for normal organ development and function in female mice, mainly acting in conjunction with the nuclear sex steroid receptor pool (Pedram et al., 2009).
Materials and Methods
Mouse construction
A bacterial artificial chromosome with a cysteine 451 to alanine mutation in ERα was created to insert into the esr1 locus in embryonic stem cells. ES cells were then injected into blastocysts from C57BL/6NTac mice, the chimeric male mice positively selected by neo gene expression and chimeric males crossed with Flpe-expressing females to delete the neo cassette. Detailed construction methods are described in SI text. All mouse experiments were approved by the Animal Research and Research and Development Committees at the Department of Veterans Affairs Medical Center, Long Beach.
ERα localization
Hepatocytes and mammary epithelial cells from 8–10 week old wild type, hetero and homozygous NOER female mice were acutely cultured for immuno-fluorescence microscopy and western blot. First antibody to ERα from Santa Cruz Biotechnology (c-terminus, MC-20) and FITC-conjugated second antibody (Vector labs) were used.
Kinase activities
Cell ERK activity was determined at 15 mins as phosphorylation at the active site, tyrosine 202/204 with phospho-antibodies to tyrosine 202/204 (Santa Cruz). AKT activity at 15 mins was determined as Ser473 phosphorylation using phospho (and total) antibodies from Cell Signaling Inc.
Measurement of plasma hormone levels
Blood samples were collected by cardiac puncture and centrifuged. Serum was stored at −80°C until assayed using ELISA or RIA kits (Diagnostic Systems Lab, Webster, TX; Cayman Chemical Company, Ann Arbor, MI).
mRNA, epigenetic, and ChIP assays
mRNA expression by RT-PCR, epigenetic studies, and chromatin immuno-precipitation assays are described in SI text.
Fertility studies
12–18 week male and female WT, heterozygous and homozygous NOER mice were used for multiple matings, and success rates and periods were determined.
Histology, histochemistry and serum hormone measurements
Whole organs were obtained under anesthesia/euthanasia for mounts and thin tissue sections (Pedram et al., 2009). Hematoxylin and eosin staining (reproductive tract tissues) and carmine alum staining for mammary glands were done. Ki67 staining of mouse uteri served as a proliferation marker using ab15580 antibody (Abcam) with formaldehyde-fixed, paraffin-embedded tissue sections. Sections were de-paraffinized, rehydrated, then blocked with 4% serum (30 minutes, 25°C); antigen retrieval by heat mediation was done in citrate buffer (pH 6.0). Incubation with primary antibody (5 μg/ml in blocking buffer,16 hours at 4°C) was followed by FITC-conjugated, goat/anti-rabbit IgG polyclonal (1/100), secondary antibody (Vector Labs) incubation overnight. Fluorescent microscopy ensued.
Uterine response to E2
10–12 wk old female mice were ovariectomized and recovered for 1 week, estrogen (E2) pellets then inserted under the skin for 12 or 21 days, producing physiological serum E2 levels (Pedram et al., 2008). Uterine epithelial thickness was measured. For mRNA, mice were exposed to E2 from pellet for 12 days, the uteri removed and processed for RT-PCR. Uterine weight studies are described in SI text.
E-mem-ECFP expression studies
Hepatocytes from homozygous NOER mice were transfected with a plasmid expressing only the E domain (ligand binding domain) of ERα, targeted exclusively to the membrane by paired myristylation sequences (E-mem ECFP) (Pedram et al., 2009). As control, some cells were transfected with empty vector (ECFP) and studies were performed.
Epigenetic modulation studies
Mammary gland epithelial cells from the three mice types were isolated and cultured. Precipitated histone proteins were collected and resolved by SDS-PAGE on a 10–20% Tris-tricine gel (Bio-Rad Laboratories), and changes in tri-methylation of histone 3 at lysine 27 relative to histone 3 at the PR promoter were investigated (antibodies from Abcam Inc). Total EZH2, and histone 3 proteins were loading controls. PRB expression was determined by RT-PCR at 24 hours of E2 treatment.
Statistical analysis
Mean±SEM were calculated for ANOVA + Schefe’s test. p<0.05 was considered significantly different.
Supplementary Material
Highlights.
Membrane-localized ERα is required for organ development and function
Membrane and nuclear ERα collaborate to regulate key developmental genes
Acknowledgments
The studies were supported by a Merit Review Award from the Department of Veterans Affairs and grant 2RO1CA100366 from NIH to ERL. AP and MR performed the research, ML, SH, and EL designed the research and analyzed the data.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia F, Ascenzi P, Bacedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor a membrane localization regulation by 17-β-estradiol. Mol Biol Cell. 2004;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessiéres E, Kim SH, et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111:E283–E290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorgenesis in estrogen receptor knockout mice. J Mammary Gland Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Springs Harbor Perspect Biol. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BR, Sadler RK, Rochelle DB, Stalmach MA, MacDonald PC, Simpson ER. Plasma lipoprotein regulation of progesterone biosynthesis by human corpus luteum tissue in organ culture. J Clin Endocrinol Metab. 1981;52:875–881. doi: 10.1210/jcem-52-5-875. [DOI] [PubMed] [Google Scholar]
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Ottmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, et al. Non-nuclear estrogen receptorα signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchereau A, Savouret JF, Milgrom E. Control of biosynthesis and post-transcriptional modification of the progesterone receptor. Biol Reprod. 1992;46:174–177. doi: 10.1095/biolreprod46.2.174. [DOI] [PubMed] [Google Scholar]
- Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Fleming JG, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 And dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grümmer R, Traub O, Winterhager E. Gap junction connexin genes cs26 and cx43 are differentially regulated by ovarian steroid hormones in rat endometrium. Endocrinology. 1999;140:2509–2516. doi: 10.1210/endo.140.6.6640. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, Morishige K, Ohmichi M, Matsuzawa Y, Murata Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem. 2000;275:11404–11411. doi: 10.1074/jbc.275.15.11404. [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with Gαi and Gβγ mediate nongenomic signaling by estrogen receptor β. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol Endocrinol. 2012;26:762–774. doi: 10.1210/me.2011-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Nacerddine K, Pietersen A, Beuger V, Pawlitzky I, Cornelissen-Steijger P, Wientjens E, Tauger E, Seibler J, van Lohuizen M, et al. Polycomb group gene Ezh2 regulates mammary gland morphogenesis and maintains the luminal progenitor pool. Stem Cells. 2013;31:1910–20. doi: 10.1002/stem.1437. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate Kisspeptin/Neurokinin B/Dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SB, Børglum JD, Møller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol. 1992;85:13–19. doi: 10.1016/0303-7207(92)90120-u. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: Role of estrogen receptor beta to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O’Mahony F, Lee E, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Deschenes R, Levin ER. DHHC 7 and 21 are palmitoylacyltranferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, O’Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Science Signaling. 2013;6:RA36. doi: 10.1126/scisignal.2004013. [DOI] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng CT. Regulation of lactoferrin gene expression by estrogen and epidermal growth factor: molecular mechanism. Cell Biochem Biophys. 1999;31:49–64. doi: 10.1007/BF02738154. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol. 2000;14:1872–1881. doi: 10.1210/mend.14.11.0551. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor a is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JK, Chodankar R, Purcell DJ, Bittencourt D, Stallcup MR. Gene-specific patterns of coregulator requirements by estrogen receptor-α in breast cancer cells. Mol Endocrinol. 2012;26:955–966. doi: 10.1210/me.2012-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.