Abstract
Purpose
Survey data indicate that continuous EEG (CEEG) monitoring is used with increasing frequency to identify electrographic seizures in critically ill children, but studies of current CEEG practice have not been conducted. We aimed to describe the clinical utilization of CEEG in critically ill children at tertiary care hospitals with a particular focus on variables essential for designing feasible prospective multi-center studies evaluating the impact of electrographic seizures on outcome.
Methods
Eleven North American centers retrospectively enrolled 550 consecutive critically ill children who underwent CEEG. We collected data regarding subject characteristics, CEEG indications, and CEEG findings.
Key Findings
CEEG indications were encephalopathy with possible seizures in 67% of subjects, event characterization in 38% of subjects, and management of refractory status epilepticus in 11% of subjects. CEEG was initiated outside routine work hours in 47% of subjects. CEEG duration was <12 hours in 16%, 12-24 hours in 34%, and >24 hours in 48%. Substantial variability existed among sites in CEEG indications and neurologic diagnoses, yet within each acute neurologic diagnosis category a similar proportion of subjects at each site had electrographic seizures. Electrographic seizure characteristics including distribution and duration varied across sites and neurologic diagnoses.
Significance
These data provide a systematic assessment of recent CEEG use in critically ill children and indicate variability in practice. The results suggest that multi-center studies are feasible if CEEG monitoring pathways can be standardized. However, the data also indicate that electrographic seizure variability must be considered when designing studies addressing the impact of electrographic seizures on outcome.
Keywords: EEG Monitoring, Seizure, Status Epilepticus, Pediatric, Non-Convulsive Seizure
Introduction
Electrographic seizures have been reported in 10-40% of critically ill children who undergo continuous electroencephalographic (CEEG) monitoring in pediatric intensive care units (ICU) or emergency departments.(Alehan et al., 2001, Hosain et al., 2005, Jette et al., 2006, Tay et al., 2006, Saengpattrachai et al., 2006, Abend and Dlugos, 2007, Abend et al., 2009, Shahwan et al., Williams et al., 2011, Greiner et al., 2012, Kirkham et al., 2012, Abend et al., 2011a) Electrographic seizures may be subdivided into electroclinical seizures (also referred to as convulsive seizures or clinically evident seizures) or non-convulsive seizures (also referred to as EEG-only seizures). Electroclinical seizures refer to seizures with a clinical correlate while non-convulsive seizures refer to seizures without any clinical correlate identified by bedside caregivers or video review by encephalographers.(Abend et al., 2013, Tsuchida et al., 2013) Most electrographic seizures in critically ill children are non-convulsive seizures.(Jette et al., 2006, Shahwan et al., 2010, Williams et al., 2011, McCoy et al., 2011, Greiner et al., 2012, Kirkham et al., 2012, Schreiber et al., 2012, Abend et al., 2011a)
In the context of these data, surveyed physicians report increasing use of CEEG for critically ill children. In a 2009 survey of 330 adult and pediatric neurologists regarding CEEG use, 83% of physicians reported using CEEG in the ICU at least once per month and 86% of physicians managed non-convulsive seizures in critically ill patients at least five times per year. The majority of respondents reported that CEEG was indicated to identify electrographic seizures in patients with altered mental status with or without recent convulsions. (Abend et al., 2010) A 2011 survey addressing CEEG use at 61 large North American pediatric hospitals reported a 30% increase in the number of critically ill patients undergoing CEEG from 2010 to 2011.(Sanchez et al., 2013)
Using a large multi-center retrospective study of consecutive patients, we aimed to describe current pediatric critical care CEEG utilization. Further, we aimed to assess practice variability among centers and subject variability across underlying diagnoses. These data are needed to design feasible prospective multi-center studies of electrographic seizure management and outcome.
Methods
This retrospective study was carried out by 11 pediatric institutions within the Pediatric Critical Care EEG Group. The study was approved by the Institutional Review Board at each site. Each of the 11 sites provided data for 50 consecutive children aged 1 month to 21 years who underwent CEEG in the pediatric ICU. All CEEG practices and clinical management followed standards of care at each individual institution. Children admitted to the pediatric ICU for planned epilepsy related management, such as epilepsy surgery or epilepsia partialis continua management, were excluded. If there were multiple CEEG sessions during the same admission, then only data from the first session was included. CEEG was considered to be a single session if interruptions lasted less than 12 hours.
We determined the number of days required by each site to record 50 consecutive patients who underwent CEEG in the pediatric ICU. We collected information on the following variables: age, gender, acute neurologic diagnosis, mental status at CEEG onset, prior developmental delay/intellectual disability diagnoses, prior epilepsy diagnosis, and mortality. Specific acute neurologic diagnoses were grouped into three general categories: (1) epilepsy-related, (2) acute structural (stroke, central nervous system inflammation or autoimmune disorder, traumatic brain injury, central nervous system infection, brain malformation, tumor/oncologic, and hypoxic-ischemic encephalopathy), and (3) acute non-structural (sepsis, metabolic, pharmacologic sedation, toxin, paralytic administration). CEEG variables included: electrographic seizure occurrence, electrographic seizure characteristics, and inter-ictal epileptiform discharge occurrence. As recently reviewed (Abend et al., 2013) and consistent with prior studies of seizures in critically ill children, (Jette et al., 2006, Abend et al., 2009, McCoy et al., 2011, Abend et al., 2011a, Williams et al., 2011, Schreiber et al., 2012) electrographic seizures were defined as abnormal, paroxysmal electroencephalographic events that were different from the background, lasted longer than ten seconds (or shorter if associated with a clinical seizure), had a plausible electrographic field, and evolved in morphology and spatial distribution. For the main analysis, electrographic seizures were classified as electrographic status epilepticus if any single seizure lasted longer than 30 minutes or if recurrent seizures together lasted for more than 30 minutes in any one hour epoch (50% seizure burden), a definition which is consistent with prior studies.(Abend et al., 2009, Abend et al., 2011a, Greiner et al., 2012, Topjian et al., 2013, Abend et al., 2013) In a separate summary, we determined how many subjects would meet criteria for electrographic status epilepticus with three different definitions: (1) any seizure lasting longer than 30 minutes, (2) seizures each lasting less than 30 minutes but totaling 30 minutes during a one hour epoch (50% seizure burden), or (3) typical seizures lasting longer than five minutes. The five minute definition of status epilepticus originated in the recent Neurocritical Care Society guideline which defines status epilepticus as “five minutes or more of continuous clinical and/or electrographic seizure activity.”(Brophy et al., 2012) Per clinical practice at the study sites, almost all CEEG was performed with time-locked video and notations regarding events from bedside caregivers. These data were used to classify the proportion of electrographic seizures that had a clinical correlate as all (100%, all electrographic seizures were electroclinical), most (50-99%), some (1-49%), and none (0%, all electrographic seizures were non-convulsive). The electrographic seizure onset distribution was classified as focal onset, multi-focal onset, or generalized onset. At the time that the electrographic seizure involved the highest number of electrodes, the electrographic seizure maximal extent distribution was classified as focal-unilateral or bilateral.
Standardized data collection as performed at each site using a web-based form with 37 multiple-choice questions using REDCap (Research Electronic Data Capture) electronic data capture tools (Wechsler et al., 2013) hosted at the Children's Hospital of Philadelphia Research Institute. Descriptive statistics are presented as medians and interquartile ranges (IQR) for continuous variables and as counts and percentages for categorical variables. The chi-square test was employed to determine whether the proportion of subjects with various characteristics differed across sites. The Kruskal-Wallis test was used to test the median differences across sites and across the three acute neurologic diagnosis groups. Fisher's exact test was used to detect acute neurologic diagnosis group differences for subject and seizure characteristics (count data). All statistics were calculated using STATA/SE (Version 12.0, STATA Corp, Texas, USA).
Results
550 subjects were included. The median age was 36.5 months (IQR 9 months - 10.2 years). Two-hundred and ninety five subjects (54%) were male. CEEG was initiated outside routine work hours (17:00-08:00) in 164 of 347 (47%) subjects with CEEG onset time available.
Subject and CEEG characteristics by center are listed in Table 1. To obtain 50 consecutive subjects, sites collected data on monitored patients over a median of 13.7 months (IQR 6.4 – 21.9 months). The number of subjects in each acute neurologic diagnosis category, (classified as epilepsy-related, acute structural, or acute non-structural) was significantly different across centers (p<0.001). Mortality was significantly different across centers (p<0.001). The number of subjects in each CEEG indications category (encephalopathy with possible nonconvulsive seizures, events of unclear etiology requiring EEG monitoring for diagnostic clarification, or refractory status epilepticus management) varied significantly across centers (p<0.001). CEEG duration was <12 hours in 88 subjects (16%), 12-24 hours in 187 subjects (34%), 24-48 hours in 129 subjects (23%), 48-72 hours in 44 subjects (8%), >72 hours in 94 subjects (17%) and unknown in 8 subjects (1%). The number of subjects with short (<24 hours) or long (≥24 hours) CEEG duration was significantly different across centers (p<0.001). The mean (±standard deviation) CEEG duration was longer in children who were comatose (41 ± 24 hours) than those who were obtunded (32 ± 22 hours) or had normal mental status (25 ± 21 hours) (p<0.001). The mean CEEG duration was longer in children with inter-ictal epileptiform discharges (40 ± 25 hours) versus those without inter-ictal epileptiform discharges (28 ± 20 hours) (p<0.001).
Table 1.
Subject and CEEG monitoring characteristics by center (N=550).
| Center | Days for 50 Subjects |
Age in months median (IQR) |
Sex (male) N (%) |
Clinical seizure prior to CEEG N (%) |
Prior developmental delay or intellectual disability N (%) |
Prior Epilepsy N (%) |
CEEG >24 hours N (%) |
Seizure Occurrence by Acute Neurologic Diagnosis Category N with seizures / N in category (%) |
CEEG Monitoring Indication ** N (%) |
Mortality N (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute Structural |
Acute Non- Structural |
Epilepsy- Related |
Encephalopathy | Event | RSE Management |
|||||||||
| 1 | 920 | 44.5 (15,134) | 27 (54%)* | 41 (82%) | 35 (70%) | 30 (60%) | 25 (50%) | 1/5 (20%) | 3/12 (25%) | 17/33 (52%) | 11 (22%) | 38 (76%) | 13 (26%) | 2 (4%) |
| 2 | 52 | 24 (3, 144) | 28 (56%) | 23 (46%) | 19 (38%) | 9 (18%) | 5 (10%) | 1/20 (5%) | 4/22 (18%) | 3/8 (38%) | 18 (36%) | 18 (36%) | 6 (12%) | 6 (12%) |
| 3 | 496 | 36 (11,113) | 29 (58%) | 33 (66%) | 30 (60%) | 19 (38%) | 25 (50%) | 7/23 (30%) | 0/10 (0%) | 6/17 (35% | 39 (78%) | 21 (42%) | 6 (12%) | 3 (6%) |
| 4 | 416 | 28 (6,91) | 30 (60%) | 26 (52%) | 24 (48%) | 15 (30%) | 18 (38%) | 7/21 (33%) | 3/17 (18%) | 7/12 (58%) | 41 (82%) | 17 (34%) | 1(2%) | 13 (26%) |
| 5 | 769 | 56.2 (9,147) | 24 (48%) | 32 (64%) | 25 (50%) | 20 (40%) | 34 (68%) | 4/25 (16%) | 3/13 (23%) | 9/12 (75%) | 47 (94%) | 13 (26%) | 2 (4%) | 10 (20%) |
| 6 | 148 | 57.5 (11.5,138.5) | 33 (66%) | 20 (40%) | 14 (28%) | 15 (30%) | 19 (38%) | 3/28 (11%) | 2/10 (20%) | 8/12 (67%) | 30 (60%) | 16 (32%) | 10 (20%) | 2 (4%) |
| 7 | 293 | 31 (9.5,82) | 25 (50%) | 26 (52%) | 15 (30%) | 12 (24%) | 36 (72%) | 10/30 (33%) | 3/8 (38%) | 3/12 (25%) | 50 (100%) | 17 (34%) | 1 (2%) | 12 (24%) |
| 8 | 146 | 23 (5,110) | 29 (58%) | 19 (38%) | 12 (24%) | 11 (22%) | 36 (72%) | 9/32 (28%) | 0/3 (0%) | 7/15 (47%) | 38 (76%) | 7 (14%) | 4 (8%) | 10 (20%) |
| 9 | 239 | 61.7 (13.8,149.6) | 17 (34%) | 38 (76%) | 15 (30%) | 11 (22%) | 30 (60%) | 5/26 (19%) | 2/13 (15%) | 5/11 (45%) | 41 (82%) | 27 (54%) | 7 (14%) | 10 (20%) |
| 10 | 694 | 30.5 (11,102) | 30 (60%) | 27 (54%) | 16 (32%) | 14 (28%) | 21 (42%) | 4/16 (25%) | 3/16 (19%) | 8/18 (44%) | 39 (78%) | 9 (18%) | 2 (4%) | 3 (6%) |
| 11 | 635 | 25.5 (9,109) | 23 (46%) | 20(40%) | 22 (44%) | 18 (38%) | 18 (38%) | 7/19 (37%) | 0/17 (0%) | 8/14 (57%) | 14 (28%) | 26 (52%) | 10 (20%) | 2 (4%) |
| Overall N (%) |
Median (IQR) 416 (194-665) |
Median (IQR) 36.5 (9-122) |
295 (54%) | 305 (55%) | 227 (41%) | 174 (32%) | 267 (49%) | 58/245 (24%) | 23/141 (16%) | 81/164 (49%) | 386 (67%) | 209 (38%) | 62 (11%) | 72 (13%) |
| p-value | 0.41 | 0.1 | <0.001 | <0.001 | 0.005 | <0.001 | <0.001*** | <0.001 | <0.001 | <0.001 | <0.001 | |||
| 0.22 | 0.46 | 0.41 | ||||||||||||
Notes:
Cells values represent the number of subjects. For example, 27 of 50 subjects (54%) from Center 1 were male.
Subjects could have multiple CEEG monitoring indications.
There was a significant difference in seizure occurrence by acute neurologic diagnosis category (p<0.001) but within each acute neurologic diagnosis category there was no significant difference in seizure occurrence by center (Acute Structural p=0.22, Acute Non-Structural p=0.46, Epilepsy-Related p=0.41).
Electrographic seizures occurred in 162 of 550 subjects (29%). Electrographic seizure characteristics by site are listed in Table 2 and Figure 1. Despite the differences in patient characteristics shown in Table 1, the proportions of subjects with electrographic seizures or subjects with a sufficiently high seizure burden to be classified as electrographic status epilepticus did not differ across sites (p=0.41 and p=0.60, respectively). Further, as shown in Table 1, the proportion of subjects with electrographic seizures within each acute neurologic diagnosis category did not differ across sites (p=0.22 for acute structural diagnoses, p=0.46 for acute non-structural diagnoses, and p=0.41 for epilepsy-related diagnoses). Table 2 illustrates that the electrographic seizures observed in critically ill children have substantial variability in their characteristics, including the proportion with a clinical correlate and the electrographic seizure maximal distribution.
Table 2.
Electrographic seizure characteristics by center (N=550).
| Center | Subjects with Electrographic Seizures (including electrographic status epilepticus) |
Subjects with Electrographic Status Epilepticus | Electrographic Seizure Characteristics by Subject | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Correlate (N=157) | Duration (N=158) | Seizure Maximal Distribution (N=161) | ||||||||||
| All (100%) | Most (50-99%) | Some (1-49%) | None (0%) | <1min | 1-5mins | 6-30mins | >30mins | Focal-Unilateral | Bilateral | |||
| 1 | 21* | 6 | 9 | 3 | 1 | 8 | 12 | 5 | 3 | 1 | 5 | 15 |
| 2 | 8 | 3 | 1 | 3 | 0 | 3 | 0 | 4 | 2 | 0 | 7 | 0 |
| 3 | 13 | 7 | 1 | 3 | 5 | 4 | 7 | 5 | 1 | 0 | 2 | 11 |
| 4 | 17 | 5 | 6 | 5 | 1 | 5 | 9 | 4 | 2 | 2 | 8 | 9 |
| 5 | 16 | 3 | 1 | 2 | 7 | 6 | 3 | 10 | 3 | 0 | 11 | 5 |
| 6 | 13 | 7 | 7 | 2 | 2 | 1 | 6 | 3 | 1 | 2 | 4 | 9 |
| 7 | 16 | 10 | 2 | 0 | 6 | 8 | 3 | 6 | 6 | 1 | 11 | 5 |
| 8 | 16 | 5 | 2 | 2 | 2 | 9 | 5 | 5 | 2 | 3 | 10 | 6 |
| 9 | 12 | 6 | 3 | 0 | 6 | 3 | 3 | 8 | 1 | 0 | 10 | 6 |
| 10 | 15 | 5 | 5 | 1 | 1 | 8 | 4 | 7 | 3 | 1 | 11 | 4 |
| 11 | 15 | 4 | 6 | 1 | 2 | 4 | 8 | 6 | 1 | 0 | 6 | 6 |
| Overall N (%) |
162 (29%) | 61 (11%) | 43 (27%) | 22 (14%) | 33 (21%) | 59 (38%) | 60 (38%) | 63 (40%) | 25 (16%) | 10 (6%) | 85 (53%) | 76 (47%) |
| p-value | 0.41 | 0.6 | 0.003 | 0.095 | 0.086 | 0.002 | ||||||
Note: Cells values represent the number of subjects. For example, 21 of 50 subjects (42%) from Center 1 had electrographic seizures.
Figure 1.
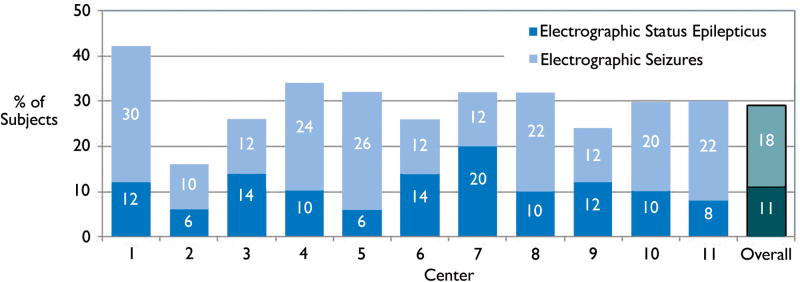
Percentage of subjects with electrographic seizures and electrographic status epilepticus by center.
The proportion of subjects classified as electrographic status epilepticus varied by the status epilepticus definition. Thirty-five subjects (6%) had typical electrographic seizures lasting longer than five minutes, 28 subjects (5%) had any seizure lasting longer than thirty minutes, and 31 subjects (6%) had recurrent electrographic seizures which were each less than 30 minutes but together totaled more than 30 minutes within an hour. The proportions of subjects with varying definitions of electrographic status epilepticus were similar across neurologic diagnosis categories (Table 3).
Table 3.
Subjects with status epilepticus depending on definition.
| Status Epilepticus Definition | Neurologic Diagnosis Category N (%) | |||
|---|---|---|---|---|
| Total (N=550) | Acute Structural (N=245) | Acute Non-Structural (N=141) | Epilepsy- Related (N=164) | |
| Typical seizure longer than 5 minutes | 35 (6%) | 20 (8%) | 4 (3%) | 11 (7%) |
| Any seizure longer than 30 minutes | 28 (5%) | 14 (6%) | 6 (4%) | 8 (5%) |
| Recurrent Seizures which were each less than 30 minutes but totaled 30 minutes in a 1 hour epoch | 31 (6%) | 12 (5%) | 6 (4%) | 13 (8%) |
Table 4 provides data regarding subject and electrographic seizure characteristics for subjects with traumatic brain injury, stroke, and hypoxic ischemic encephalopathy. These are three common neurologic conditions requiring critical care for which CEEG is performed. Although the electrographic seizure incidence was similar, these neurologic diagnoses differed in age, prior developmental delay or intellectual disability, mental status at CEEG onset, initial CEEG background category, electrographic seizure onset distribution, electrographic seizure maximal extent distribution, and mortality.
Table 4.
Subject and seizure characteristics by acute neurologic diagnosis.
| Variable | All Diagnoses (N=550) |
Specific Acute Neurologic Diagnosis N (%) |
|||
|---|---|---|---|---|---|
| Traumatic Brain Injury (N=61) |
Hypoxic Ischemic Encephalopathy (N=71) |
Stroke (N=33) |
p-value | ||
| Age (median and IQR) | 36.5 (9-122) | 11.5 (4-80.5) | 24 (5-132) | 50 (4.3-157) | 0.2601 |
| Age Groups | 0.029 | ||||
| <2 months | 21 (4%) | 1 (2%) | 5 (7%) | 4 (12%) | |
| 2 months-1 year | 133 (24%) | 29 (48%) | 20 (28%) | 6 (18%) | |
| 1 year - 10 years | 253 (46%) | 17 (28%) | 27 (38%) | 10 (30%) | |
| >10 years | 143 (26%) | 14 (23%) | 19 (27%) | 13 (28%) | |
| Sex (male) | 295 (54%) | 36 (59%) | 38 (54%) | 14 (42%) | 0.306 |
| Prior Developmental Delay / Intellectual Disability | 227 (41%) | 3 (5%) | 20 (28%) | 6 (18%) | 0.001 |
| Prior Epilepsy | 174 (32%) | 4 (7%) | 6 (8%) | \ | 0.306 |
| Seizure Prior to CEEG | 162 (29%) | 17 (28%) | 27 (38%) | 13 (39%) | 0.377 |
| Mental Status at CEEG | <0.001 | ||||
| Normal | 71 (13%) | 7 (14%) | 1 (1%) | 2 (6%) | |
| Lethargic/Obtunded | 297 (56%) | 22 (44%) | 25 (36%) | 16 (50%) | |
| Comatose | 158 (30%) | 21 (42%) | 43 (62%) | 14 (44%) | |
| Initial CEEG Background | 0.003 | ||||
| Normal | 94 (17%) | 17 (28%) | 6 (8%) | 4 (12%) | |
| Slow/Disorganized | 337 (61%) | 30 (49%) | 26 (37%) | 21 (64%) | |
| Discontinuous | 38 (7%) | 5 (8%) | 11 (15%) | 4 (12%) | |
| Burst-Suppression | 29 (5%) | 3 (5%) | 11 (15%) | 2 (6%) | |
| Attenuated/Featureless | 52 (9%) | 6 (10%) | 17 (24%) | 2 (6%) | |
| Mortality (died) | 73 (13%) | 11 (18%) | 26 (37%) | 4 (12%) | 0.009 |
| CEEG Monitoring Duration | 0.183 | ||||
| <12 hours | 88 (16%) | 6 (10%) | 8 (11%) | 4 (13%) | |
| 12-24 hours | 187 (35%) | 16 (26%) | 14 (20%) | 10 (31%) | |
| 25-48 hours | 129 (24%) | 12 (20%) | 18 (26%) | 13 (41%) | |
| 49-72 hours | 44 (8%) | 11 (18%) | 13 (19%) | 1 (3%) | |
| >72 hours | 94 (17%) | 16 (26%) | 17 (24%) | 4 (13%) | |
| Electrographic Seizures | 162 (29%) | 18 (30%) | 12 (17%) | 10 (30%) | 0.16 |
| Electrographic Status Epilepticus | 61 (38%) | 10 (16%) | 5 (7%) | 3 (9%) | 0.227 |
| Seizure Clinical Correlate | 0.434 | ||||
| All (100%) | 43 (27%) | 3 (17%) | 1 (8%) | \ | |
| Most (50-99%) | 22 (14%) | 4 (22%) | \ | 1 (11%) | |
| Some (1-49%) | 33 (21%) | 4 (22%) | 3 (25%) | 2 (22%) | |
| None (0%) | 59 (38%) | 7 (39%) | 8 (67%) | 6 (67%) | |
| Seizure Duration | 0.879 | ||||
| <1 minute | 60 (38%) | 5 (28%) | 4 (33%) | 2 (20%) | |
| 1-5 minutes | 63 (40%) | 6 (33%) | 6 (50%) | 4 (40%) | |
| 6-30 minutes | 25 (16%) | 6 (33%) | 2 (17%) | 3 (30%) | |
| >30 minutes | 10 (6%) | 1 (6%) | \ | 1 (10%) | |
| Seizure Onset Distribution | |||||
| Focal | 97 (57%) | 12 (67%) | 5 (42%) | 9 (90%) | 0.013 |
| Multi-Focal | 34 (20%) | 4 (22%) | 3 (25%) | 1 (10%) | 0.805 |
| Generalized | 39 (23%) | 2 (11%) | 4 (33%) | \ | 0.427 |
| Seizure Maximal Distribution | |||||
| Focal-Unilateral | 85 (53%) | 13 (72%) | 6 (46%) | 9 (90%) | 0.031 |
| Bilateral | 76 (47%) | 5 (28%) | 7 (52%) | 1 (10%) | 0.53 |
Legend: \ = no data
Discussion
Electrographic seizures and electrographic status epilepticus are common in critically ill children(Alehan et al., 2001, Hosain et al., 2005, Jette et al., 2006, Tay et al., 2006, Saengpattrachai et al., 2006, Abend and Dlugos, 2007, Abend et al., 2009, Shahwan et al., Williams et al., 2011, Abend et al., 2011a, Greiner et al., 2012, Kirkham et al., 2012) and recent studies have begun to explore the impact of these seizures on outcome.(Lambrechtsen and Buchhalter, 2008, Greiner et al., 2012, Kirkham et al., 2012, Schreiber et al., 2012, Gwer et al., 2012, Topjian et al., 2013) Most of these studies involved critically ill children with a variety of underlying acute neurologic diagnoses and have grouped together all types of electrographic seizures. The current data obtained from consecutive children who underwent CEEG at 11 large pediatric centers illustrate that children undergoing CEEG are quite heterogeneous in terms of clinical and seizure characteristics. This clinical and seizure heterogeneity is important to understand in developing future studies which are feasible and conducted in appropriate cohorts. Variability in care may also provide important information regarding different outcomes based on comparative effectiveness analyses.
Management and outcome studies need to address seizure burden, which is often categorized as electrographic seizures or electrographic status epilepticus. Our data indicate that differing definitions of electrographic status epilepticus may have a substantial impact on subject classification. This variability is consistent with a prior study which reported that 51% of seizures lasted longer than 5 minutes while 27% lasted longer than 30 minutes.(Williams et al., 2011) Some prior studies of electrographic seizure epidemiology in critically ill children have defined status epilepticus as a single electrographic seizure lasting more than 30 minutes or recurrent electrographic seizures totaling 30 minutes within one hour. (Abend et al., 2009, Abend et al., 2011a, Greiner et al., 2012, Topjian et al., 2013, Abend et al., 2013) A prior study utilizing this definition reported that electrographic status epilepticus, but not electrographic seizures, was associated with worse short-term outcome and mortality.(Topjian et al., 2013) Only 11% of children in our study would meet this definition of status epilepticus. Within this group, about 5% of subjects had a single 30 minute seizure and 6% of subjects had recurrent seizures, indicating that this composite definition of electrographic status epilepticus may actually be composed of two subgroups which might require different management and have different outcomes. A recent Neurocritical Care Society guideline makes similar recommendations for convulsive and non-convulsive status epilepticus and defines status epilepticus as “5 minutes or more of (i) continuous clinical and/or electrographic seizure activity or (ii) recurrent seizure activity without recovery (returning to baseline) between seizures.”(Brophy et al., 2012) The second component may be difficult to quantify in critically ill encephalopathic children, but our data indicate that about 6% would meet the five-minute criterion. Studies will need to carefully define seizure burden criteria, and the current data regarding the number of subjects available for varying electrographic status epilepticus definitions will help establish enrollment needs for future studies.
Design decisions for prospective CEEG studies will need to consider several aspects of clinical practice. First, consistent with the around-the-clock nature of critical care, nearly half of CEEG studies were initiated outside routine work hours. If future studies require rapid enrollment or consent immediately upon CEEG initiation then a system will be required for off-hours enrollment or nearly half of potentially eligible subjects could not be enrolled. Second, future studies may need to determine what portions of CEEG are clinically indicated and which portions should be considered study components. Since CEEG is costly, these decisions may have a substantial impact on study finances. In our observational study, only 16% of subjects underwent CEEG for less than 12 hours which suggests that 1-2 days of CEEG could be considered a component of clinical care for most patients at most institutions.
Nearly every subject characteristic varied across centers, including whether clinical seizures occurred prior to monitoring, prior epilepsy diagnosis, prior intellectual disability diagnosis, acute neurologic diagnosis category, mortality, and CEEG indications. This is consistent with survey data indicating that there is substantial practice variability.(Abend et al., 2010, Sanchez et al., 2013) This variability likely reflects that CEEG resources differ across institutions and that few data are available to implement fully evidence-based CEEG pathways and algorithms. A survey of pediatric centers in the United States and Canada reported that only 34% had any type of critical care EEG pathway.(Sanchez et al., 2013) Such inter-center variability could limit the generalizability of prospective studies focused on clinically indicated CEEG. However, if most components of care could be standardized across centers and adequate number of subjects was available, then some variability in care could provide an opportunity to conduct comparative effectiveness studies of outcome stratified by the remaining differences between centers. Interestingly, our observational data indicate that although there were significant differences in the acute neurologic categories (epilepsy-related, acute structural, acute non-structural) monitored across institutions, within each acute neurologic diagnosis category there was no significant difference in electrographic seizure occurrence. This indicates that if centers chose to standardize their CEEG monitoring indicates then homogeneity of seizure occurrence could be achieved.
These data may assist in designing multi-center studies by providing information regarding patient variability needed for sample size calculations. For example, these data help to establish how many subjects would be available given specific inclusion or exclusion criteria, and how many subjects would have electrographic seizures with specific characteristics. For example, a prior study of consecutive children with hypoxic ischemic injury due to cardiac arrest identified electrographic seizures in about half of children who underwent CEEG (Abend et al., 2009) but it is unknown whether identifying and managing these electrographic seizures improves outcome. Suppose a study aimed to determine whether electrographic seizures in children after cardiac arrest were associated with a 15% increase in the incidence of unfavorable outcome (either mortality or unfavorable outcome on a neuropsychological outcome measure). Based on Table 4, the seizure incidence in this multi-center cohort was about 17%, which is lower than reported by the prior single center study.(Abend et al., 2009) This indicates we would enroll about one seizure subject for every four non-seizure subjects. With a cohort of 79 subjects with electrographic seizures and 315 subjects without electrographic seizures and an alpha of 0.05, we would have 80% power to detect a 15% difference in unfavorable outcome. Without standardization efforts, our data indicate that 11 sites could enroll 71 subjects in about 1-2 years, meaning this study might require 5 years if additional sites were not included. Our data also warn of several other issues which will be essential to consider. First, about 30% of subjects had pre-existing neurodevelopmental abnormalities, warning that studies will either need to exclude patients who are neurodevelopmentally abnormal (slowing enrollment) or represent outcome as an interval change in neurodevelopmental status rather than an absolute value. Second, the subjects with electrographic seizures will have substantial variability in seizure burden. Our data indicate about one-third will only experience brief electrographic seizures lasting less than one minute. Further, about half of subjects with have focal-unilateral electrographic seizures while half will have bilateral seizures. Thus, sub-analyses within the electrographic seizure subject group may be required. Very importantly, the wide variation across centers indicates that the numbers used in these calculations may vary depending on which centers participate in the study. Preliminary feasibility studies focused on CEEG standardization and better understanding inter-center variability may help ensure large resource-intense studies are optimally designed.
Study findings need to be interpreted in the setting of data acquisition. First, this was a retrospective study of clinically indicated CEEG so the number of subjects and subject characteristics could be different in prospective studies involving screening of all eligible critically ill children. Second, as CEEG use increases, it is possible that centers are adopting broader CEEG indications to identify children with electrographic seizures. If broader indications are used, this may lower the seizure incidence rates. Small variations in CEEG pathways may have a substantial impact on resource utilization, indicating that CEEG criteria must be carefully considered.(Gutierrez-Colina et al., 2012) Third, EEG and seizure characteristics were reported by encephalographers at each center and not by a central EEG interpretation core, potentially leading to bias from inter-rater agreement limitations.(Abend et al., 2011b)
Despite these limitations, data regarding current practice of CEEG, inter-center variability, and subject and EEG characteristics will be useful in designing future trials. Attending to these data will increase the likelihood that future multi-center studies are designed appropriately to answer important questions regarding the impact on outcome of electrographic seizure identification and management.
Acknowledgments
This study was performed by the Pediatric Critical Care EEG Group (PCCEG) which is the pediatric subgroup of the Critical Care EEG Monitoring Research Consortium (CCEMRC). Nicholas Abend is funded by NIH K23NS076550 and the Children's Hospital of Philadelphia Department of Pediatrics. Dennis Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276. Christopher Giza receives research support from the NINDS/NIH, University of California, Thrasher Research Foundation, Today's and Tomorrow's Children Fund and the Child Neurology Foundation/Winokur Family Foundation. Cecil Hahn is funded by the Canadian Institutes of Health Research, the SickKids Foundation and the PSI Foundation. Tobias Loddenkemper receives support from the National Institutes of Health/NINDS, a Career Development Fellowship Award from Harvard Medical School and Boston Children's Hospital, the Program for Quality and Safety at Boston Children's Hospital, the Payer Provider Quality Initiative, The Epilepsy Foundation of America (EF-213583 and EF-213882), the Center for Integration of Medicine and Innovative Technology, the Epilepsy Therapy Project, the Pediatric Epilepsy Research Foundation, and from investigator initiated research grants from Lundbeck and Eisai. Kristin McBain is funded by the Canadian Institutes of Health Research, the SickKids Foundation and the PSI Foundation. Eric Payne is funded by grants from Alberta Innovates Health Solutions and the Canadian League Against Epilepsy. Iván Sánchez Fernández is funded by a grant for the study of Epileptic Encephalopathies from “Fundación Alfonso Martín Escudero”.
Footnotes
- This study was approved by the institutional review board at each institution.
- We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest Disclosures: Dr. Abend has given expert testimony in medico-legal cases and receives royalties from Demos Medical Publishing.
Dr. Dlugos has given expert testimony in medico-legal cases.
Dr. Giza is a commissioner on the California State Athletic Commission, a member of the steering committee for the Sarah Jane Brain Project, a consultant for the National Hockey League Players' Association, a member of the concussion committee for Major League Soccer, a member of the Advisory Board for the American Association for Multi-Sensory Environments (AAMSE), and a subcommittee chair for the CDC Pediatric TBI guideline workgroup; has received honoraria and funding for travel for invited lectures on traumatic brain injury/concussion; has received royalties from Blackwell Publishing for “Neurological Differential Diagnosis.” He has given expert testimony in medico-legal cases.
Dr. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an Associate Editor for Seizure, and performs video electroencephalogram long-term monitoring, electroencephalograms, and other electrophysiological studies at Boston Children's Hospital and bills for these procedures.
References
- Abend NS, Chapman KE, Gallentine WB, Goldstein J, Hyslop AE, Loddenkemper T, Nash KB, Riviello JJ, Jr, Hahn CD. Electroencephalographic monitoring in the pediatric intensive care unit. Curr Neurol Neurosci Rep. 2013;13:330. doi: 10.1007/s11910-012-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–170. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382–389. doi: 10.1007/s12028-010-9337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Gutierrez-Colina AM, Topjian AA, Zhao H, Guo R, Donnelly M, Clancy RR, Dlugos DJ. Nonconvulsive seizures are common in critically ill children. Neurology. 2011a;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Gutierrez-Colina AM, Zhao H, Guo R, Marsh E, Clancy RR, Dlugos DJ. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. Journal of Clinical Neurophysiology. 2011b;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Topjian A, Ichord R, Herman ST, Helfaer M, Donnelly M, Nadkarni V, Dlugos DJ, Clancy RR. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol. 2001;16:484–487. doi: 10.1177/088307380101600704. [DOI] [PubMed] [Google Scholar]
- Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ, Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 2012 doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. EEG Monitoring in Critically Ill Children: Indications and Strategies. Pediatric Neurology. 2012;46:158–161. doi: 10.1016/j.pediatrneurol.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwer S, Idro R, Fegan G, Chengo E, Garrashi H, White S, Kirkham FJ, Newton CR. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- Kirkham FJ, Wade AM, Mcelduff F, Boyd SG, Tasker RC, Edwards M, Neville BG, Peshu N, Newton CR. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49:615–625. doi: 10.1111/j.1528-1167.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- Mccoy B, Sharma R, Ochi A, Go C, Otsubo H, Hutchison JS, Atenafu EG, Hahn CD. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- Saengpattrachai M, Sharma R, Hunjan A, Shroff M, Ochi A, Otsubo H, Cortez MA, Carter Snead O., 3rd Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–1518. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- Sanchez SM, Carpenter J, Chapman KE, Dlugos DJ, Gallentine W, Giza CC, Goldstein JL, Hahn CD, Kessler SK, Loddenkemper T, Riviello JJ, Abend NS. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. Journal of Clinical Neurophysiology. 2013;30:156–160. doi: 10.1097/WNP.0b013e31827eda27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31–38. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504–1509. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- Topjian AA, Gutierrez-Colina AM, Sanchez SM, Berg RA, Friess SH, Dlugos DJ, Abend NS. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, Nguyen S, Weinstein S, Scher MS, Riviello JJ, Clancy RR. American Clinical Neurophysiology Society Standardized EEG Terminology and Categorization for the Description of Continuous EEG Monitoring in Neonates: Report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- Wechsler LR, Tsao JW, Levine SR, Swain-Eng RJ, Adams RJ, Demaerschalk BM, Hess DC, Moro E, Schwamm LH, Steffensen S, Stern BJ, Zuckerman SJ, Bhattacharya P, Davis LE, Yurkiewicz IR, Alphonso AL. Teleneurology applications: Report of the Telemedicine Work Group of the American Academy of Neurology. Neurology. 2013;80:670–676. doi: 10.1212/WNL.0b013e3182823361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]


