Abstract
The widespread use of endoscopic ultrasound has facilitated the evaluation of subepithelial and surrounding lesions of the gastrointestinal tract. Deep pelvic endometriosis, with or without infiltration of the intestinal wall, is a frequent disease that can be observed in women in their fertile age. Patients of this disease may present nonspecific signs and symptoms or be completely asymptomatic. Laparoscopic surgical resection of endometriotic lesions is the treatment of choice in symptomatic patients. An accurate preoperative evaluation is indispensable for therapeutic decisions mainly in the suspicion of intestinal wall and/or urinary tract infiltration, and also in cases where we need to establish histological diagnosis or to rule out malignant disease. Diagnostic tools, including transrectal ultrasound, magnetic resonance image, transvaginal ultrasound, barium enema, and colonoscopy, play significant roles in determining the presence, depth, histology, and other relevant data about the extension of the disease. Diagnostic algorithm depends on the clinical presentation, the expertise of the medical team, and the technology available at each institution. This article reviews and discusses relevant clinical points in endometriosis, including techniques and outcomes of the study of the disease through transrectal ultrasound and fine-needle aspiration.
Keywords: endometriosis, endoscopic ultrasonography, fine-needle aspiration
Introduction
The concept of endometriosis is based on histological confirmation of ectopic implants of glands and/or endometrial stroma sensitive to hormones but not including those located in the myometrium.1,2,3
The pathogenesis of the disease is probably multifactorial. Retrograde menstruation is the most widespread theory that explains the implants.4 The development of endometriosis from the metaplasia of the pluripotential coelomic epithelium may also serve as an explanation.
The disease affects between 8% and 15% of women in childbearing age.5,6 Epidemiological data are conflicting, and clinical manifestations of this illness are usually nonspecific, making the profiles of high-risk patients difficult to establish.2,7
Implants can be unique or may occur in various parts of the body. They are commonly found in the ovaries, fallopian tubes, pouch of Douglas, uterosacral ligaments, pelvic peritoneum, uterus, sigmoid colon, rectum, ileum, appendix, bladder, ureter, cecum, rectovaginal septum, and vagina.8,9,10,11,12 The presence of endometriosis in lymph nodes and other sites are less frequent.8,9,13,14,15,16,17,18,19,20,21 A lesion infiltrating 5 mm or more beyond the peritoneal epithelium is considered deep pelvic endometriosis.22
Intestinal endometriosis is the most common extra genital disease that affects between 3% and 37% of women with endometriosis.8,11 Up to 95% of intestinal endometriosis is found in the rectum and sigmoid colon. In addition, it may be present in more than one intestinal segment in 39.1% of patients or be found isolated, without being present in other pelvic sites in 20.6% of cases.8,10,12,23,24,25,26 Deep invasion of the intestinal wall is frequent, with infiltration of the muscularis propria or even of the submucosa. The mucosa is infiltrated in less than 5% of intestinal lesions.9,22,27
Intestinal endometriosis is difficult to diagnose and should be considered a severe disease. An adequate diagnosis of deep endometriotic lesions remains a challenge28 and usually occurs only years after the onset of symptoms. The time elapsed from the first complaints until the diagnosis of endometriosis is 7.0 (range 3.5-12.1) years.29 The lack of specific signs and symptoms can lead to errors in diagnosis and treatment.7,30,31 Even for cases showing signs, symptoms, and/or tests suggestive of endometriosis, other intestinal diseases, such as intestinal malignant neoplasm, should be ruled out to avoid delay or wrong medical treatment.17,18,32
The main gynecological clinical manifestations include chronic pelvic pain, back pain, menstrual disorders, and infertility.6,79,18,27,33 Among women with endometriosis, up to 60% present chronic, not necessarily cyclic, intestinal symptoms. Diarrhea, constipation, tenesmus, nausea, vomiting, fever, anorexia, weight loss, and hematochezia may be present at different intensities.3,34 Even without parietal invasion, an endometriotic lesion adjacent to any intestinal segment may cause digestive symptoms.25
Gynecological pelvic exam is considered essential for evaluating the extent of pelvic lesions.35 Through vaginal and rectal touch examination, thickening or nodularity in the pouch of Douglas, uterosacral ligaments, and/ or the rectovaginal septum is the most significant data.33,36 However, the absence of positive signs does not rule out the disease.37,38
In the presence of intestinal infiltration, clinical treatment is not effective, and the chronic use of systemic therapy can lead to side effects.7,25,31,39
For surgical treatment of symptomatic pelvic endometriosis, laparoscopic surgical resection of all identified lesions is currently the best method of choice.25,33,40 A preoperative map is crucial for the management of the disease.10 At the presence of lesions in intestinal or urinary organs, a gastrointestinal or urologic surgeon is respectively recommended. Different surgical approaches are available for intestinal lesions: superficial skinning, partial longitudinal resection (with linear stapler), nodulectomy (with an endorectal circular stapler or with partial surgical resection of the wall), or segmentectomy (Figure 1). The proper choice of surgical technique depends on the extension, position, and depth of the lesion, which must be previously well defined through imaging methods.28,33,40,41,42
Figure 1.

Macroscopic aspect of intestinal endometriosis (segmentectomy of the sigmoid).
Diagnostic tools, including transrectal ultrasound (TRUS) (Figure 2), magnetic resonance imaging (MRI) (Figure 3), transvaginal ultrasound (TVUS) (Figure 4), barium enema (Figure 5), and colonoscopy (Figure 6), play significant roles in determining a precise preoperative diagnosis of the disease.18,40,43,44,45,46,47,48,49,50 These data are useful in avoiding unnecessary laparoscopic approaches. Moreover, they are used for preoperative prediction and discussion of surgical plan, as well as possible complications with the patient (Table 1).51
Figure 2.
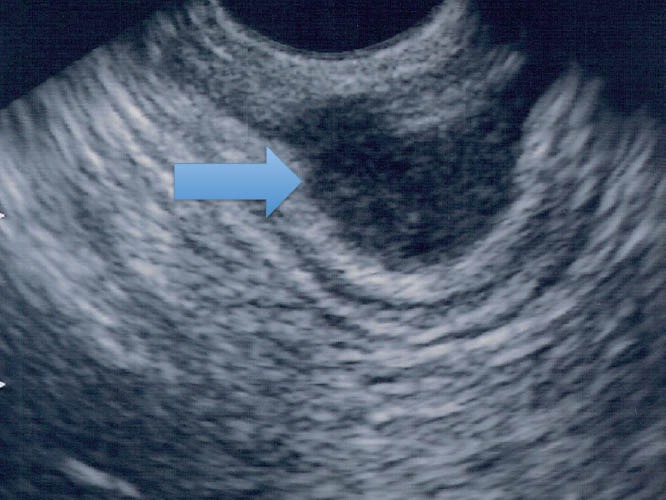
Endometriotic infiltrating lesion in the intestinal wall (TRUS). TRUS: transrectal ultrasound.
Figure 3.

Endometriotic infiltrating lesion in the intestinal wall (MRI). MRI: magnetic resonance imaging.
Figure 4.
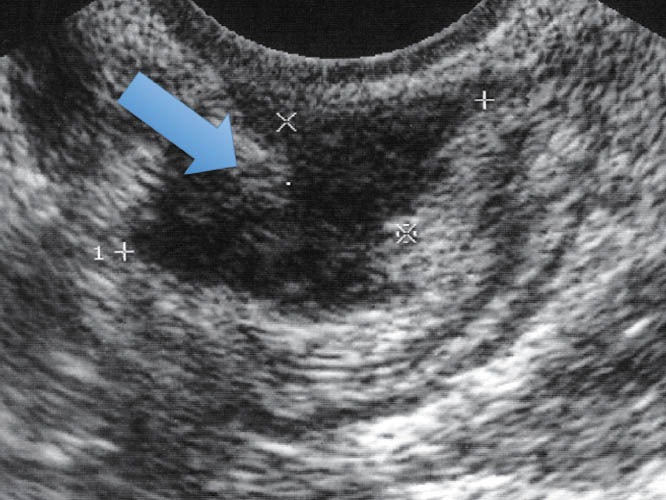
Endometriotic infiltrating lesion in the intestinal wall (TVUS). TVUS: transvaginal ultrasound.
Figure 5.
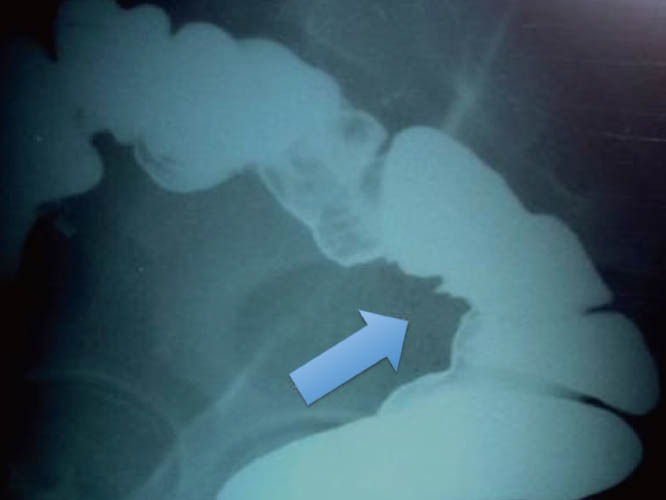
Endometriotic infiltrating lesion in the intestinal wall (Barium enema).
Figure 6.
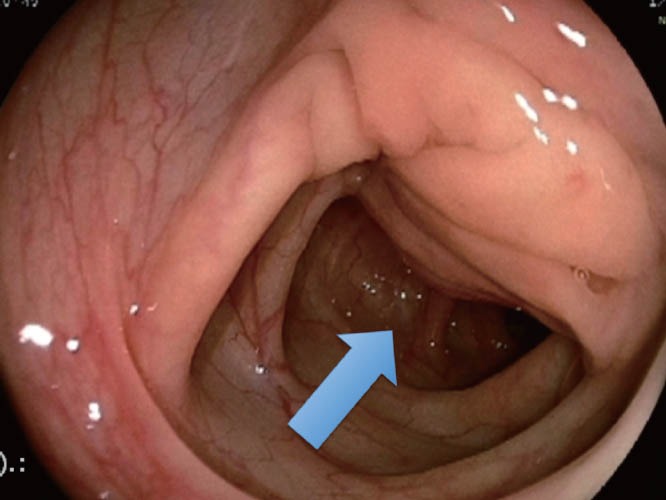
Endometriotic infiltrating lesion in the intestinal wall (colonoscopy).
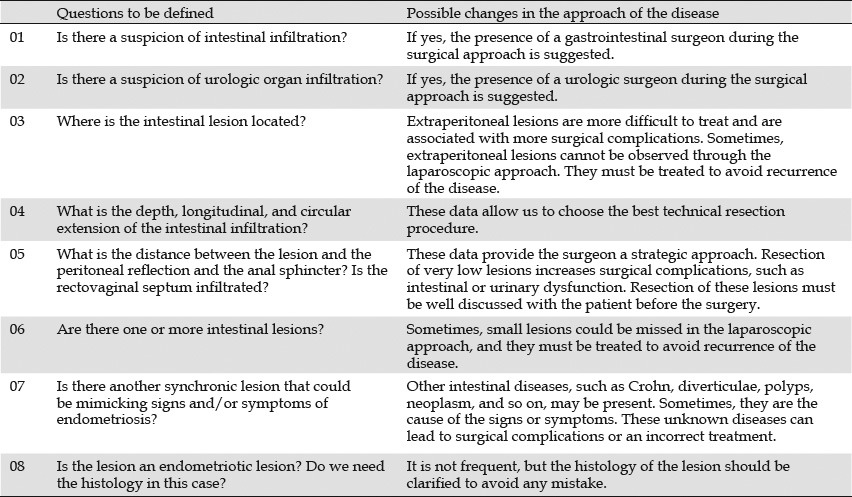
Table 1.
Main questions to be defined preoperatively for a better treatment (surgical or clinical) plan51
MRI is very useful in the complete evaluation of the pelvis (pelvic floor, bladder, ureter, and muscles). It is the best option for the evaluation of ovarian endometriosis and for the accurate diagnosis of deep implants in the intestinal wall or rectovaginal septum.43,44 Due to low cost and easy access, several authors pointed out that TVUS should be the first examination for the diagnosis of various gynecological diseases, including intestinal endometriosis.46,48,52
Barium enema presents 88% of sensitivity in the detection of deep intestinal lesions; however, its specificity is very low (54%).35,45
Colonoscopy provides specific signs of endometriosis in only 50% of deep intestinal lesions, such as subephitelial lesions that promote deformation and reduction of the lumen.53 Sometimes, the mucosa that covers the subepithelial lesion presents edema, enanthema, friability, irregularity of surface, and/or vascular patterns.50,51
Given the high sensitivity and specificity obtained from using different preoperative diagnostic tools, the laparoscopic approach of endometriosis should be reserved for surgical treatment of the disease.54
In gastrointestinal practice, TRUS is considered the test choice to assess lesions infiltrating the intestinal wall with high accuracy in the determination of depth and histology.25,26,28,46,53,55,56,57,58,59,60,61,62 However, TRUS is not widely used in the management of intestinal endometriosis. This review aims to provide knowledge and references to endoscopic ultrasonographers for the improvement of TRUS and fine-needle aspiration (FNA) in the algorithm of the diagnosis of endometriosis.
Methods
Articles in PubMed were searched in English and in French. For relevant clinical points, gynecological and gastrointestinal reference books in English and in Portuguese were consulted. For technical review, all the references above were consulted. The literature search was conducted prior to March 1 2012, not limited to publication year.
The keywords used in the PubMed search include the following: endometriosis with transrectal ultrasonography, endoscopic ultrasonography, and endorectal ultrasonography. A total of 137 articles were identified. Majority of the reports are case series. This review focuses on the accuracy of TRUS and its comparison with other diagnostic tools for intestinal endometriosis.
Results
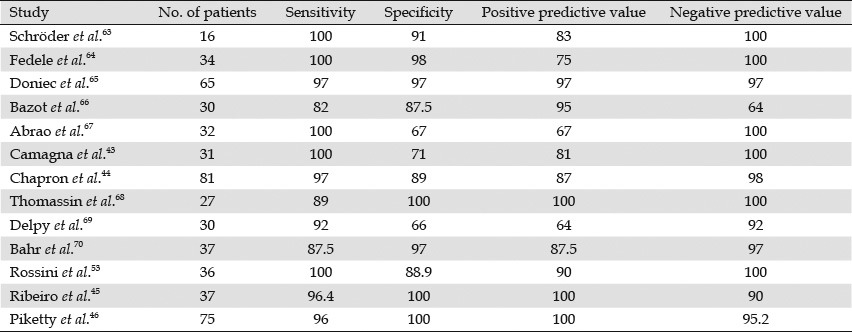
TRUS was considered a diagnostic method for detecting the presence of deep rectal endometriosis. Initially, we compared preoperative TRUS with the histology of the specimens obtained during open or laparoscopic surgery. Table 2 shows the effectiveness of TRUS in predicting intestinal infiltration.43,44,45,46,53,63,64,65,66,67,68,69,70
Table 2.
Studies that evaluated the endometriosis use of endorectal ultrasonography for predicting rectal infiltration of deep pelvic endometriosis
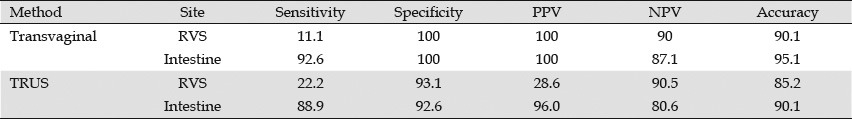
In 2007, Bazot et al. compared TRUS with TVUS for the infiltration of the rectovaginal septum and intestinal wall in 81 patients (Table 3).48 TVUS was performed without bowel preparation using a 5 to 9 MHz rigid probe, whereas TRUS was performed using a 7.5 to 12 MHz flexible echoendoscope.
Table 3.
Transvaginal ultrasonography vs. TRUS for the diagnosis of deep endometriosis[48]
Bergamini compared TRUS with TVUS through water contrast in the rectum using a 6.5 MHz curvilinear rigid probe in both examinations. The results include sensitivities of 88.2% and 96%, specificities of 80% and 80%, positive predictive values of 95.7% and 98%, and negative predictive values of 57.1% and 81.8% for TRUS and TVUS, respectively.71
The introduction of MRI in the evaluation of patients with endometriosis led to the comparison of this technique with TRUS. The studies that compared both methods are listed in Table 4.44,72
Table 4.
Comparison between MRI and TRUS for the diagnosis of deep endometriosis

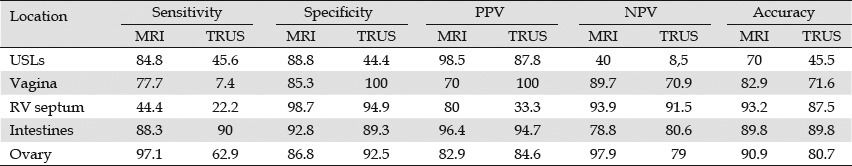
In 2007, Bazot compared MRI with endoscopic ultrasound (EUS) for the diagnosis of deep infiltrating endometriosis in different locations for 88 patients. MRI performed better than EUS, except for the diagnosis of intestinal endometriosis (Table 5).73
Table 5.
Comparison between MRI and TRUS performance for the diagnosis of deep infiltrating endometriosis in different locations73
Only one article compared the performance of MRI, EUS, and TVUS for the diagnosis of deep infiltrating endometriosis of 92 patients. The results are shown in Table 6.74
Table 6.
Comparison among MRI, TRUS, and TVS performance for the diagnosis of deep infiltrating endometriosis in different locations74
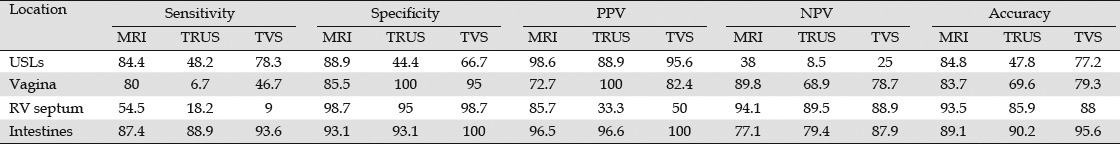
In terms of technical review, few studies described in detail any special TRUS technical procedure. TRUS is mainly performed with sedation. Most studies performed TRUS using a 7.5 MHz to 12 MHz radial flexible echoendoscope without special tricks for the procedure. Few studies used rigid probes through different techniques and equipment, and only one study used miniprobes. Only one detailed description was available for linear rigid probes, and it is summarized below.63,64,65,66,67,68,69,70,71,72,73,74
Basic techniques with rigid probes
The patient should be positioned in the left lateral decubitus with flexion of the thighs and legs. First, a deep rectal touch examination should be performed to check for anorectal stenosis and/or nodules in the regions of the anus, rectum, rectovaginal septum, pouch of Douglas, cervix, and paracervical regions. Subsequently, the rigid probe (Figure 7) should be introduced through the anus and immediately pointed to the back of the patient. The probe should then be slid over the sacrum for up to approximately 7 cm to 10 cm in the rectal lumen. At this point, a balloon coupled over the probe is filled with water (at least 40 mL). The probe is then pushed up gently with short up and down movements until the distal sigmoid colon. In this position, the right and left iliac vessels (Figure 8) and sometimes the bifurcation of abdominal aorta can be observed (Figure 9). Evaluation of the intestinal wall and surrounding tissues, including pelvic organs and iliac vessels, is performed using movements of introduction, traction, and rotation of the probe on its longitudinal axis (clockwise and counterclockwise), as well as by compression or decompression of the transducer against the wall.
Figure 7.

Hitachi Rigid Linear Probe (EUP U 33) used in the study for intestinal endosonography.
Figure 8.
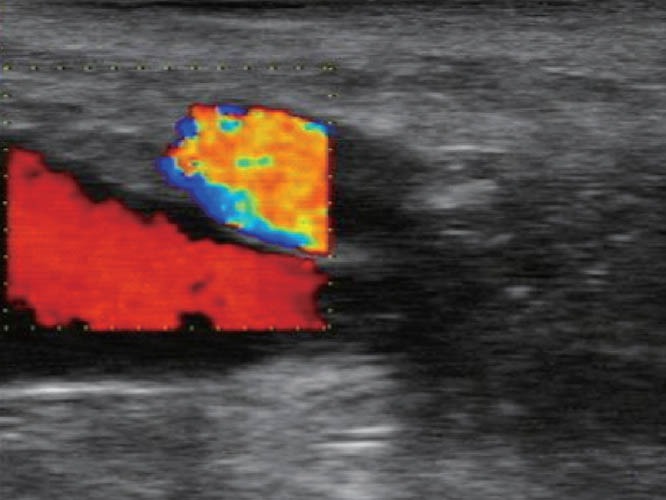
TRUS – right iliac vessels. TRUS: transrectal ultrasound.
Figure 9.
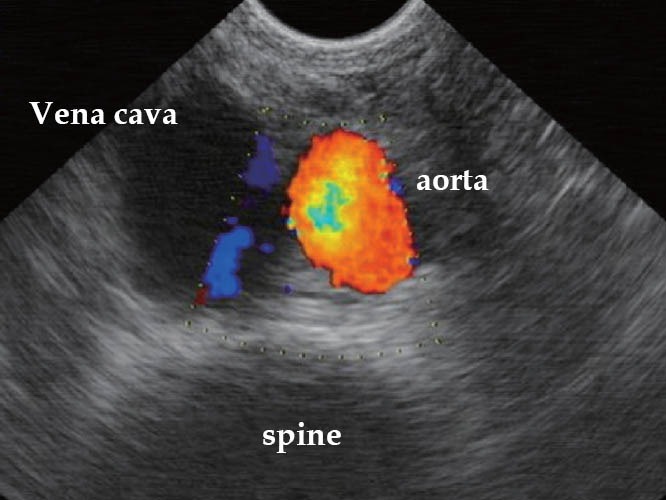
TRUS – vena cava, aorta, and spine (Hitachi biplane probe). TRUS: transrectal ultrasound.
To determine the depth of the endometriotic lesions, the presence of hypoechogenic, irregular, homogeneous or heterogeneous lesions, around or infiltrating pelvic structures or the intestinal wall are considered suspect of endometriosis (Figure 10). As shown in Table 7, only one study proposed a standard classification for the determination of the depth (Figure 11) and location (Figure 12) of intestinal parietal invasion.75,76
Figure 10.
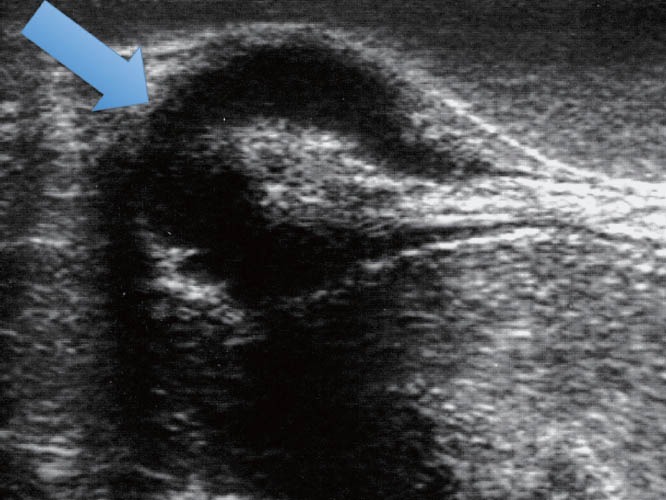
TRUS – hypoechogenic and heterogeneous lesions infiltrating the intestinal wall. TRUS: transrectal ultrasound.
Table 7.
Echo-logic classification of intestinal endometriosis75

Figure 11.
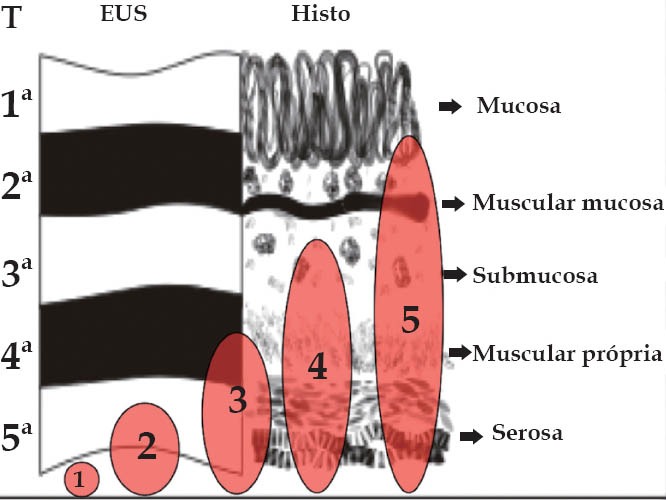
Echo-logic schematic classification of the depth of intestinal infiltration (ueT1-T5).75,76
Figure 12.
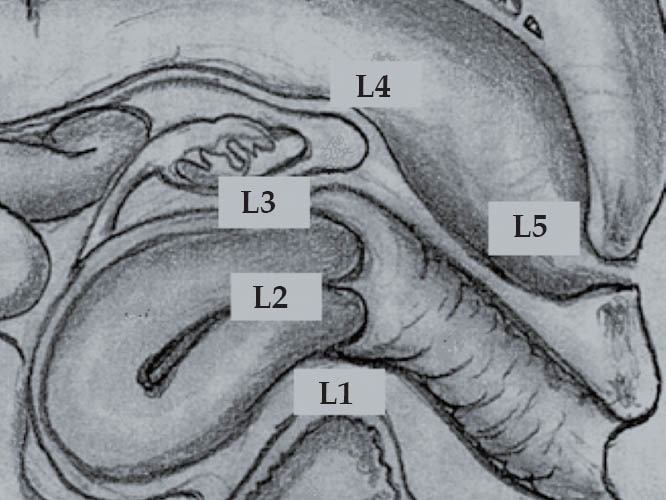
Echo-logic schematic classification according to pelvic site (ueL1-L5).75,76
Figure 13.
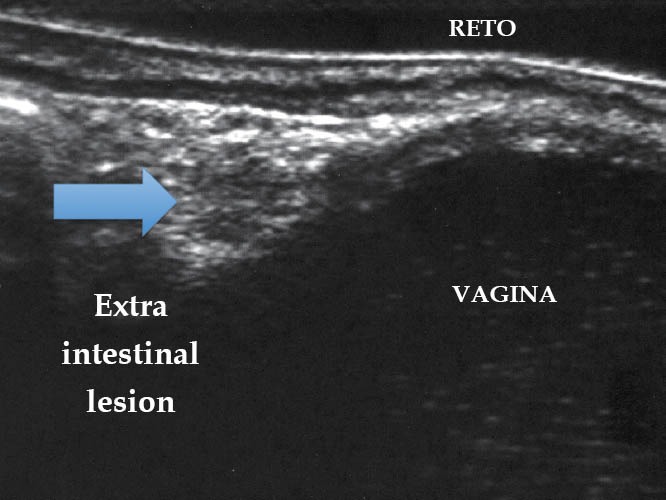
Endometriotic lesion ueT1 (TRUS). TRUS: transrectal ultrasound.
Figure 14.
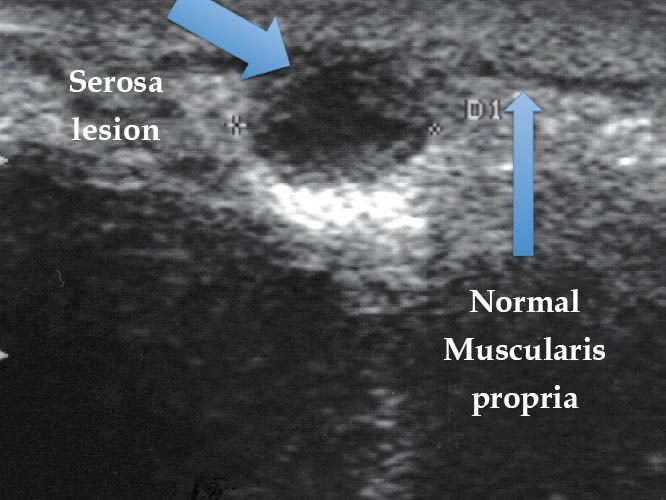
Endometriotic lesion ueT2 (TRUS). TRUS: transrectal ultrasound.
Figure 15.
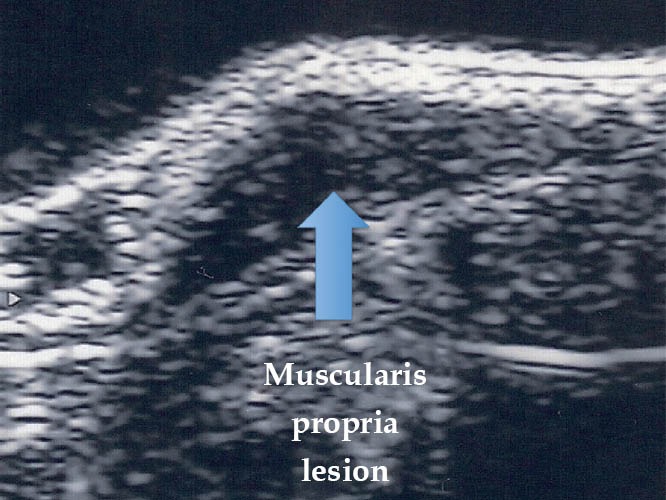
Endometriotic lesion ueT3 (TRUS). TRUS: transrectal ultrasound.
Figure 16.
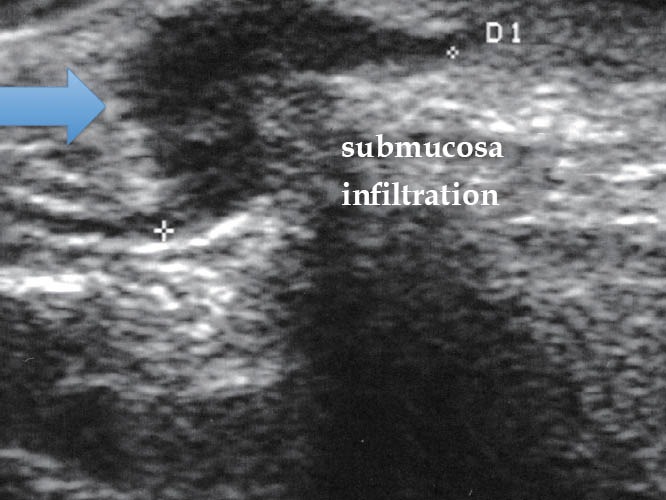
Endometriotic lesion ueT4 (TRUS). TRUS: transrectal ultrasound.
Figure 17.
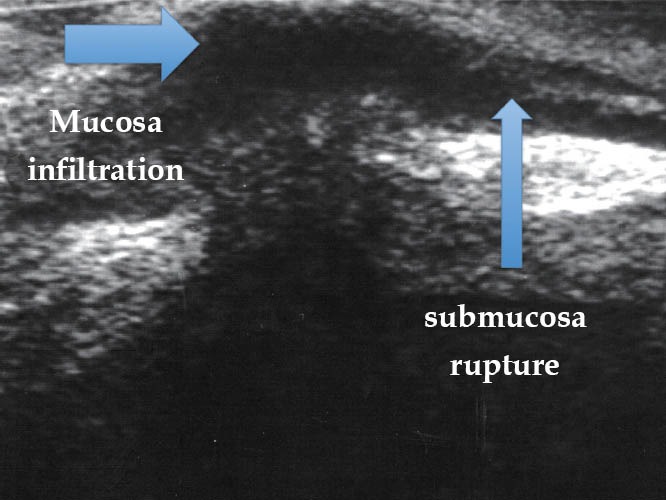
Endometriotic lesion ueT5 (TRUS). TRUS: transrectal ultrasound.
Only five studies employed FNA: four retrospectives studies with few cases and one prospective with 97 patients.77,78,79,80,81 All except one used flexible EUS probes for FNA without any comment about special techniques. Rossini employed the rigid TRUS-FNA technique using a transvaginal probe, with a guide to perform FNA (Figure 18).81 According to the author, TRUS-FNA must be performed only in intestinal lesions, which infiltrate, at least, the deep muscular layer (ueT3), avoiding seeding implants in the path of the needle (Figure 19). In addition, the author stated that prophylaxis antibiotic is not necessary because the needle does not pass beyond the thickness of the affected intestinal wall. If the lesion is adherent to a cystic ovarian lesion, FNA with cystic penetration must be avoided, and prophylaxis antibiotic is recommended. During the puncture, the lesions are hard, and a grating sensation is usually felt. The author standardized five punctures to obtain materials for histological analysis. Chiba needles (19 or 22 Gauge), measuring at least 20 cm in length, or flexible standard needles for EUS (19 or 22 Gauge) can be used with the rigid transducer. No special stains were described in any of the papers (Figures 20–22).
Figure 18.
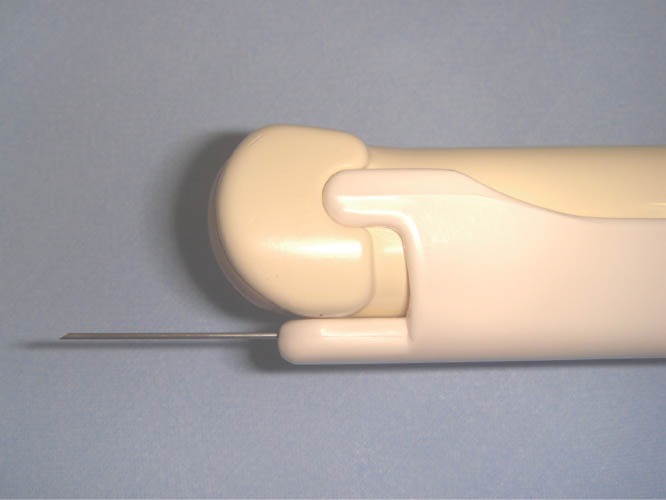
Rigid Probe Hitachi EUP V-33 and DCHN-22-20 needle used in the study for TRUS-FNA. FNA: fine-needle aspiration.
Figure 19.
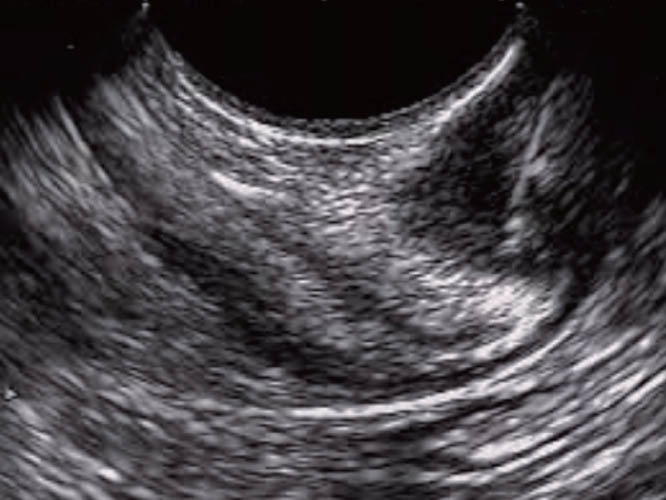
Intestinal endosonography showing the needle introduced within the intestinal lesion.
Figure 20.
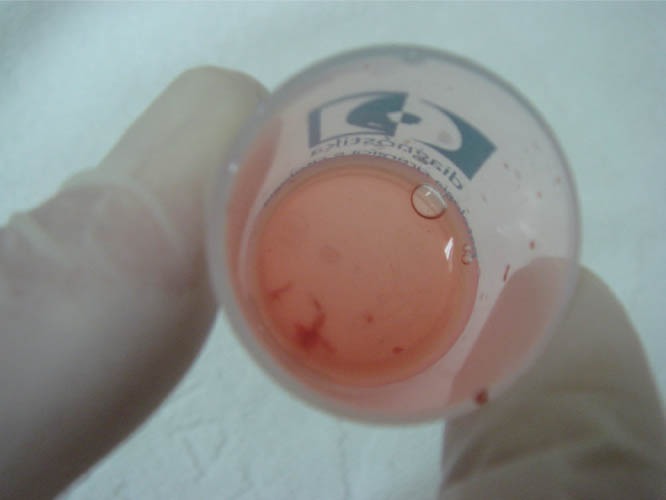
Material collected via FNA into the vial of 10% formaldehyde. FNA: fine-needle aspiration.
Figure 22.
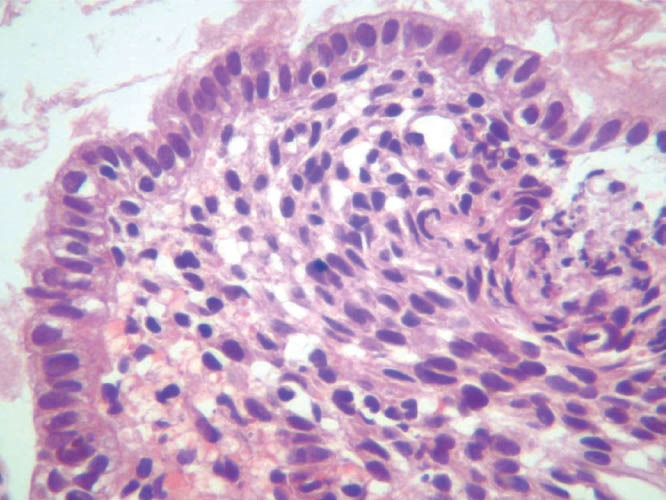
Histological image of the material obtained via FNA highlighting stromal pattern. FNA: fine-needle aspiration.
Figure 21.
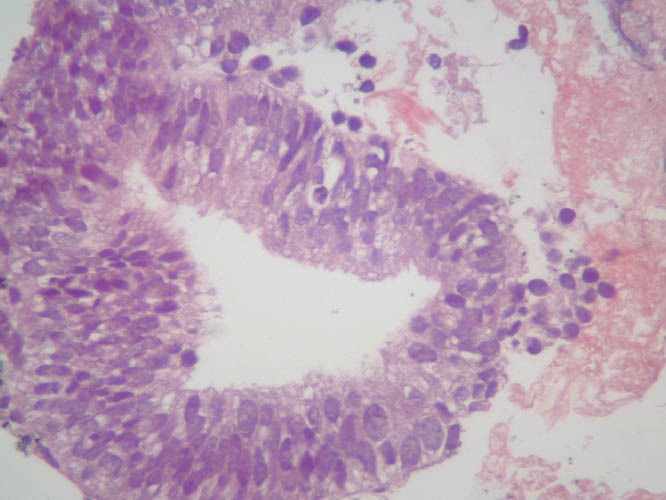
Histological image of the material obtained via FNA highlighting glandular pattern. FNA: fine-needle aspiration.
Discussion
Endometriosis is a disease affecting women's health with high prevalence between menarche and menopause.5,8,40 Intestinal endometriosis occurs in 3% to 37% of women with endometriosis.8 An appropriate treatment for the disease could be selected by considering essential parameters, such as staging and histological confirmation.1,28
Until recently, the absence of a correct preoperative diagnosis frequently leads to unnecessary surgeries.9 Current available imaging examinations provide an accurate preoperative idea of the presence and level of lesions that infiltrate the intestinal wall and other pelvic structures. The most used methods are MRI, TVUS, colonoscopy, barium enema, and TRUS.25,28,32,41,44
Comparative studies showed better results by using either TURS or TVUS in the evaluation of lesions that infiltrate the intestinal wall (Table 3).48,52 However, these data should be analyzed with caution because published studies evaluated only selected patients with high probability of deep endometriosis, and sonographers were not blinded about the clinical data of the patients. In addition, most studies did not compare all the different diagnostic methods in the same study. Moreover, the type of transducer and the generation of the technology used for TRUS and other imaging methods vary from one publication to another. The terminology used in most studies does not adopt a standard classification system concerning the depth of intestinal infiltration in layers and topographical distribution in the pelvis. Finally, the median size of the lesion is different, and percentage of the circumference of the intestinal infiltration was not mentioned in any paper.26,54,82,83 Information and uniformity are lacked in the studies; hence, comparison of data among different diagnostic tools is difficult.
The signs and symptoms of endometriosis are non -specific and may even be absent. Therefore, during initial medical investigation, patients following different algorithms according to the main clinical manifestation have three types. Patients with predominant gastrointestinal symptoms will naturally follow gastrointestinal algorithms, those with predominant gynecological symptoms will probably follow gynecological algorithms, and those with occasional incidentalomas may follow other algorithms depending on the suspicion on the images.
MRI has lower sensitivity and specificity than TRUS and TVUS in determining the extent of infiltration on the intestinal wall and does not allow histological diagnosis (Tables 4–6).43,44,84,85 MRI has the capacity to evaluate all pelvic organs and has high specificity for differentiating endometriosis from other ovarian cystic lesions. Thus, this method is usually performed in all cases of suspected deep pelvic endometriosis, allowing complete mapping of pelvic lesions. In addition, MRI may be used in re-evaluating images after the test. Hence, we suggest that MRI should be the first test in the diagnosis algorithm of deep endometriosis. For cases when MRI results show specific sites of endometriosis or are negative, more specific tests focusing on the clinical and MRI results (e.g., TRUS for intestinal infiltration) should be done to obtain adequate data about the lesions.
TVUS is useful in the diagnosis of uterosacral ligaments and other gynecologic structures. It may also be used in determining the level of infiltration of the rectum, distal sigmoid colon, and rectovaginal septum in patients with deep endometriosis85,86,87 (Table 6).74 In most cases, transvaginal transducers could be placed at an appropriate focal length from the lesion because intestinal endometriotic lesions are usually located near the posterior fornix of the vagina. However, histological diagnosis using TVUS-FNA still presents limitations, i.e., the risk of peritoneal and/or vaginal implants in the path of the needle. Standard TVUS is less expensive and more available. However, techniques for the evaluation of deep pelvic lesions are not widely used because they require special training, learning curve, and dedicated group of interest. These facts are well exposed in data from clinical practice and literatures. Before a correct diagnosis can be achieved, patients have already spent many years (time elapsed) and have undergone various TVUS.29
Barium enema has a low specificity in the diagnosis of infiltration of the intestinal wall and does not allow for histological diagnosis.45,88
Colonoscopy has a low sensitivity in providing the depth of infiltration of the intestinal wall and can only permit histological diagnosis in 5% of cases.7,25,30,31,89,90 Nevertheless, up to 60% of patients with deep endometriosis present nonspecific chronic intestinal signs and symptoms.2 Thus, in all these cases, colonoscopy is an essential test for ruling out the suspicion of inflammatory and malignant epithelial diseases of the colon and rectum.2,7,9,40,50,89,90,91,92,93,94 Once symptoms indicate colonoscopy, performing colonoscopy and TRUS in the same procedure with a unique intestinal cleansing and sedation seems to be a better technical and cost effective approach than performing the two methods separately.
In gastrointestinal practice, TRUS is the standard test in determining the depth of invasion of intestinal wall lesions.56 The results obtained in determining the presence, depth, and other data regarding endometriotic lesions of the sigmoid, rectum, and rectovaginal septum justify its clinical application.44,53,72
Although less frequent, inflammatory diseases, epithelial and subepithelial neoplasm, or invading extrinsic tumors of the intestinal wall can mimic the sonographic features of endometriosis. Although some of these features are nonspecific, they help differentiate the lesions.95,96,97 Endometriotic lesions are greater in depth and do not infiltrate the mucosal layer. The “C” format often found in advanced stages of intestinal endometriosis due to fibrosis retraction normally does not occur in malignant diseases.53,75 Other subepithelial benign lesions usually do not infiltrate more than one intestinal layer. Sonographic signs of serosa infiltration are strongly suggestive of malignant lesions. However, they are also present in almost all intestinal endometriotic lesions. Therefore, histological diagnosis cannot be determined only using data from imaging examinations.
TRUS is the standard technology for FNA in subepithelial and surrounding intestinal lesions.56,57,58 In 2010, Rossini performed TRUS-FNA in 85 patients with suspected endometriotic lesions, characterizing the histological findings of endometriosis in 97%.98 This number established better results than the results of Pishvaian that, in a retrospective study, evaluated five patients and obtained histological results of endometriosis in only one case.60 However, in that study, only three patients were operated. Therefore, comparison of the results between FNA and surgical specimens is impossible. The results of the first study are also higher than those obtained generally in subepithelial lesions of the intestinal wall.56,57,58 The author may have gotten better results because FNA was performed with five punctures in each lesion.
The hypothetical risk of seeding of these structures was avoided because TRUS FNA was performed without the penetration of the peritoneal cavity, and no other organs were transfixed.
In asymptomatic or oligosymptomatic patients, histological confirmation of intestinal endometriosis using TRUS-FNA, a minimally invasive procedure, rules out the diagnosis of neoplasm. Although rare, if not diagnosed early, neoplasm can bring about disastrous consequences to the patient. With a correct histological diagnosis, the asymptomatic or oligosymptomatic disease could be safely controlled through clinical and imaging examinations. The same approach is valid in symptomatic patients who do not agree with intestinal resection.
After surgical intestinal resections, staplers can lead to an inflammatory reaction and produce an infiltrative process. TRUS-FNA can be useful in post surgical intestinal resections for differentiating the inflammatory process from a recurrence of the disease in the anastomosis region, thereby helping in the therapeutic decision.
The possibility of obtaining the histological pattern (stromal, glandular, and mixed) and the degree of histological differentiation of the disease (non-differentiated, well differentiated, and mixed) before beginning the treatment should stimulate the development of more precise medical therapeutics focusing on this information.99,100 In addition, intestinal lesion samples may be used for research on alternative treatments based on immunohistochemical or other available biological markers.
TRUS-FNA is considered a safe procedure. Therefore, special interest should be placed on this technology as a possible tool to precisely place microparticles of slow-release drugs, such as hormones and/or anti-inflammatory agents, directly at the site of the lesion. The inoculation of small doses may have their systemic side effects reduced or eliminated. The use of stem cells could also be a potential method for conservative treatment at the lesion site of the intestinal disease through fine-needle inoculation.
Conflicts of interest
The authors disclosed no financial relationships relevant to this publication.
References
- 1.Rock JA, Markham SM. Pathogenesis of endometriosis. Lancet. 1992;340:1264–7. doi: 10.1016/0140-6736(92)92959-j. [DOI] [PubMed] [Google Scholar]
- 2.Abrão MS, Dias Júnior JA, Podgaec S. Histórico e aspectos epidemiológicos da endometriose: uma doença prevalente e de conhecimento antigo. In: Abrão MS, editor. Endometriose: uma visão contemporânea. Rio de Janeiro: Revinter; 2000. pp. 1–11. [Google Scholar]
- 3.Rochet Y, Lansac J, Drogou F. Les manifestations digestives de l’endométriose. Soc Chir Lyon. 1975;71:247–52. [Google Scholar]
- 4.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–69. [Google Scholar]
- 5.Jenkinson EL, Brown WH. Endometriosis: a study of 117 cases with special reference to constricting lesions of the rectum and sigmoid colon. JAMA. 1943;122:349–54. [Google Scholar]
- 6.Samper ER, Slagle GW, Hand AM. Colonic endometriosis: its clinical spectrum. South Med J. 1984;77:912–4. doi: 10.1097/00007611-198407000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Meyers WC, Kelvin FM, Jones RS. Diagnosis and surgical treatment of colonic endometriosis. Arch Surg. 1979;114:169–75. doi: 10.1001/archsurg.1979.01370260059009. [DOI] [PubMed] [Google Scholar]
- 8.Williams TJ, Pratt JH. Endometriosis in 1,000 consecutive celiotomies: incidence and management. Am J Obstet Gynecol. 1977;129:245–50. doi: 10.1016/0002-9378(77)90773-6. [DOI] [PubMed] [Google Scholar]
- 9.Lansac J, Pierre F, Letessier E. Digestive endometriosis: results of a multicenter investigation. Contrib Gynecol Obstet. 1987;16:192–204. [PubMed] [Google Scholar]
- 10.Chapron C, Fauconnier A, Vieira M, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. 2003;18:157–61. doi: 10.1093/humrep/deg009. [DOI] [PubMed] [Google Scholar]
- 11.Kratzer GL, Salvati EP. Collective review of endometriosis of the colon. Am J Surg. 1955;90:866–9. doi: 10.1016/0002-9610(55)90713-3. [DOI] [PubMed] [Google Scholar]
- 12.Vercellini P, Vendola N, Presti M, et al. Multifocal endometriosis. A case report. J Reprod Med. 1993;38:815–9. [PubMed] [Google Scholar]
- 13.Jimenez M, Miles RM. Inguinal endometriosis. Ann Surg. 1960;51:903–11. doi: 10.1097/00000658-196006000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattes R, Shepard F, Tovell H, et al. A clinical and pathologic study of endometriosis of the lung. Surg Gynecol Obstet. 1956;103:552–8. [PubMed] [Google Scholar]
- 15.Charles D. Endometriosis and hemorrhagic pleural effusion. Obstet Gynecol. 1957;10:309–12. [PubMed] [Google Scholar]
- 16.Heneghan MA, Teixidor HS. Pleuroperitoneal endometriosis. AJR Am J Roentgenol. 1979;133:727–30. doi: 10.2214/ajr.133.4.727. [DOI] [PubMed] [Google Scholar]
- 17.Aronchick CA, Brooks FP, Dyson WL, et al. Ileocecal endometriosis presenting with abdominal pain and gastrointestinal bleeding. Dig Dis Sci. 1983;28:566–72. doi: 10.1007/BF01308161. [DOI] [PubMed] [Google Scholar]
- 18.Craninx M, D’Haens G, Cokelaere K, et al. Crohn´s disease and intestinal endometriosis: an intriguing co-existence. Eur J Gastroenterol Hepatol. 2000;12:217–21. doi: 10.1097/00042737-200012020-00014. [DOI] [PubMed] [Google Scholar]
- 19.Javert CT. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis. Including five case reports of endometrial tissue in pelvic lymph nodes. Cancer. 1949;2:399–410. doi: 10.1002/1097-0142(194905)2:3<399::aid-cncr2820020304>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Lombardo L, Mateos JH, Barroeta FF. Subarachnoid hemorrhage due to endometriosis of the spinal canal. Neurology. 1968;18:423–6. doi: 10.1212/wnl.18.5.423. [DOI] [PubMed] [Google Scholar]
- 21.Mizrahi S, Mayzler O, Goldstein D, et al. Endometriosis simulating a colonic obstructive neoplasm. Eur J Surg Oncol. 2003;29:766–7. doi: 10.1016/s0748-7983(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 22.Cornillie FJ, Oosterlynck D, Lauweryns JM, et al. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53:978–83. doi: 10.1016/s0015-0282(16)53570-5. [DOI] [PubMed] [Google Scholar]
- 23.Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549–52. doi: 10.2214/ajr.138.3.549. [DOI] [PubMed] [Google Scholar]
- 24.Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193–219. [PubMed] [Google Scholar]
- 25.Canis M, Botchorishvili R, Slim K, et al. Endométriose digestive. A propos de huit cas de résection colorectale. J Gynecol Obstet Biol Reprod (Paris) 1996;25:699–709. [PubMed] [Google Scholar]
- 26.Roseau G, Dumontier I, Palazzo L, et al. Rectosigmoid endometriosis: endoscopic ultrasound features and clinical implications. Endoscopy. 2000;32:525–30. doi: 10.1055/s-2000-9008. [DOI] [PubMed] [Google Scholar]
- 27.Chapron C, Fauconnier A, Dubuisson JB, et al. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod. 2003;18:760–6. doi: 10.1093/humrep/deg152. [DOI] [PubMed] [Google Scholar]
- 28.Tardif D, Poncelet C, Bénifla JL, et al. Exploration paraclinique des endométrioses. Rev Prat. 1999;49:263–8. [PubMed] [Google Scholar]
- 29.Arruda MS, Petta CA, Abrão MS, et al. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18:756–9. doi: 10.1093/humrep/deg136. [DOI] [PubMed] [Google Scholar]
- 30.Caccese WJ, McKinley MJ, Bronzo RL, et al. Endoscopic confirmation of colonic endometriosis. Gastrointest Endosc. 1984;30:191–3. doi: 10.1016/s0016-5107(84)72366-2. [DOI] [PubMed] [Google Scholar]
- 31.Abbo L, Segre D, Liberatore E, et al. Endometriosi del sigma perforata. A proposito di un caso. Minerva Chir. 1995;50:393–7. [PubMed] [Google Scholar]
- 32.Rankin GB, Sivak MV., Jr . Indications, contraindications and complications of colonoscopy. In: Sivak MV Jr, editor. Gastroenterology endoscopy. 2nd ed. Philadelphia: W.B. Saunders; 2000. p. 1226. [Google Scholar]
- 33.Duepree HJ, Senagore AJ, Delaney CP, et al. Laparoscopic resection of deep pelvic endometriosis with rectosigmoid involvement. J Am Coll Surg. 2002;195:754–8. doi: 10.1016/s1072-7515(02)01341-8. [DOI] [PubMed] [Google Scholar]
- 34.Neme RM, Abrão MS. Fisiopatologia e quadro clínico da endometriose. In: Abrão MS, editor. Endometriose: uma visão contemporânea. Rio de Janeiro: Revinter; 2002. pp. 55–65. [Google Scholar]
- 35.Koninckx PR, Meuleman C, Oosterlynck D, et al. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;65:280–7. [PubMed] [Google Scholar]
- 36.Fernandez H, Harmas A. Présentations cliniques et histoire naturelle des endométrioses. Rev Prat. 1999;49:258–62. [PubMed] [Google Scholar]
- 37.Kinkel K, Chapron C, Balleyguier C, et al. Magnetic resonance imaging characteristics of deep endometriosis. Hum Reprod. 1999;14:1080–6. doi: 10.1093/humrep/14.4.1080. [DOI] [PubMed] [Google Scholar]
- 38.Dragisic KG, Padilla LA, Milad MP. The accuracy of the rectovaginal examination in detecting cul-de-sac disease in patients under general anaesthesia. Hum Reprod. 2003;18:1712–5. doi: 10.1093/humrep/deg350. [DOI] [PubMed] [Google Scholar]
- 39.Fedele L, Bianchi S, Zanconato G, et al. Gonadotropin-releasing hormone agonist treatment for endometriosis of the rectovaginal septum. Am J Obstet Gynecol. 2000;183:1462–7. doi: 10.1067/mob.2000.108021. [DOI] [PubMed] [Google Scholar]
- 40.Régenet N, Métairie S, Cousin GM, et al. Colorectal endometriosis. Diagnosis and management. Ann Chir. 2001;126:734–42. doi: 10.1016/s0003-3944(01)00614-9. [DOI] [PubMed] [Google Scholar]
- 41.Badawy SZ, Freedman L, Numann P, et al. Diagnosis and management of intestinal endometriosis. A report of five cases. J Reprod Med. 1988;33:851–5. [PubMed] [Google Scholar]
- 42.Parc Y, Mowlin Y, Loc’h P, et al. Pseudotumoral rectal endometriosis. Ann Chir. 1995;49:435–9. [PubMed] [Google Scholar]
- 43.Camagna O, Dhainaut C, Dupuis O, et al. Surgical management of rectovaginal septum endometriosis from a continuous series of 50 cases. Gynecol Obstet Fertil. 2004;32:199–209. doi: 10.1016/j.gyobfe.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Chapron C, Vieira M, Chopin N, et al. Accuracy of rectal endoscopic ultrasonography and magnetic resonance imaging in the diagnosis of rectal involvement for patients presenting with deeply infiltrating endometriosis. Ultrasound Obstet Gynecol. 2004;24:175–9. doi: 10.1002/uog.1107. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro HS, Ribeiro PA, Rossini L, et al. Double-contrast barium enema and transrectal endoscopic ultrasonography in the diagnosis of deeply infiltrating endometriosis. J Minim Invasive Gynaecol. 2008;15:315–20. doi: 10.1016/j.jmig.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Piketty M, Chopin N, Dousset B, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009;24:602–7. doi: 10.1093/humrep/den405. [DOI] [PubMed] [Google Scholar]
- 47.Goncalves MO, Podgaec S, Dias JA, Jr, et al. Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod. 2010;25:665–71. doi: 10.1093/humrep/dep433. [DOI] [PubMed] [Google Scholar]
- 48.Bazot M, Malzy P, Cortez A, et al. Accuracy of transvaginal sonography and rectal endoscopy sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2007;30:994–1001. doi: 10.1002/uog.4070. [DOI] [PubMed] [Google Scholar]
- 49.Faccioli N, Manfredi R, Mainardi P, et al. Barium enema evaluation of colonic involvement in endometriosis. AJR Am J Roentgenol. 2008;190:1050–4. doi: 10.2214/AJR.07.3062. [DOI] [PubMed] [Google Scholar]
- 50.Colaiacovo R. Tese (Mestrado) São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2008. Importância do exame proctológico e da colonoscopia total em pacientes portadoras de endometriose pélvica profunda. [Google Scholar]
- 51.Averbach M, Rossini L. Endometriose intestinal. In: Averbach M, Correa P, editors. Colonoscopia. São Paulo: Santos; 2010. pp. 299–309. [Google Scholar]
- 52.Bazot M, Detchev R, Cortez A, et al. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod. 2003;18:1686–92. doi: 10.1093/humrep/deg314. [DOI] [PubMed] [Google Scholar]
- 53.Rossini L. Tese (Mestrado) São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2005. Valor da Endossonografia Intestinal na propedêutica da endometriose do reto e sigmóide. [Google Scholar]
- 54.Darai E, Thomassin I, Barranger E, et al. Feasibility and clinical outcome of laparoscopic colorectal resection for endometriosis. Am J Obstet Gynecol. 2005;192:394–400. doi: 10.1016/j.ajog.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 55.Van Dam J, Sivak MV., Jr . Foreword. In: van Dam J, Sivak MV Jr, editors. Gastrointestinal endosonography. Philadelphia: W.B. Saunders; 1999. p. Ix. [Google Scholar]
- 56.Giovannini M, Seitz JF, Monges G, et al. Ponction-cytologie guidée sous échoendoscopie sectorielle électronique. Résultats chez 26 malades. Gastroenterol Clin Biol. 1993;17:465–70. [PubMed] [Google Scholar]
- 57.Kameyama H, Niwa Y, Arisawa T, et al. Endoscopic ultrasonography in the diagnosis of submucosal lesions of the large intestine. Gastrointest Endosc. 1997;46:406–11. doi: 10.1016/s0016-5107(97)70032-4. [DOI] [PubMed] [Google Scholar]
- 58.Hara K, Yamao K, Ohashi K, et al. Endoscopic ultrasonography and endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of lower digestive tract disease. Endoscopy. 2003;35:966–9. doi: 10.1055/s-2003-43473. [DOI] [PubMed] [Google Scholar]
- 59.Dagher C, Palazzo L, Guillermand-Gerard H, et al. Endoscopic ultrasonography-guided fine needle aspiration of a nodule in the rectovaginal septum. Cytopathology. 2007;18:255–9. doi: 10.1111/j.1365-2303.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 60.Pishvaian AC, Ahlawat SK, Garvin D, et al. Role of EUS and EUS-guided FNA in the diagnosis of symptomatic rectosigmoid endometriosis. Gastrointest Endosc. 2006;63:331–5. doi: 10.1016/j.gie.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Rossini LGB, Ribeiro PAG, Colaiacovo R, et al. Role of EUS-FNA in the diagnosis of intestinal endometriosis. Gastrointest Endosc. 2009;69:S256. [Google Scholar]
- 62.Artifon EL, Furuya CK, Sakai P, et al. Diagnosis of endometriosis with EUS. Gastrointest Endosc. 2009;69:AB334. doi: 10.1016/j.gie.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 63.Schröder J, Lohnert M, Doniec JM, et al. Endoluminal ultrasound diagnosis and operative management of rectal endometriosis. Dis Colon Rectum. 1997;40:614–7. doi: 10.1007/BF02055389. [DOI] [PubMed] [Google Scholar]
- 64.Fedele L, Bianchi S, Portuese A, et al. Transrectal ultrasonography in the assessment of rectovaginal endometriosis. Obstet Gynecol. 1998;91:444–8. doi: 10.1016/s0029-7844(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 65.Doniec JM, Kahlke V, Peetz F, et al. Rectal endometriosis: high sensitivity and specificity of endorectal ultrasound with an impact for the operative management. Dis Colon Rectum. 2003;46:1667–73. doi: 10.1007/BF02660773. [DOI] [PubMed] [Google Scholar]
- 66.Bazot M, Detchev R, Cortez A, et al. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod. 2003;18:1686–92. doi: 10.1093/humrep/deg314. [DOI] [PubMed] [Google Scholar]
- 67.Abrão MS, Neme RM, Averbach M, et al. Rectal endoscopic ultrasound with a radial probe in the assessment of rectovaginal endometriosis. J Am Assoc Gynecol Laparosc. 2004;11:50–4. doi: 10.1016/s1074-3804(05)60010-2. [DOI] [PubMed] [Google Scholar]
- 68.Thomassin I, Bazot M, Detchev R, et al. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004;190:1264–71. doi: 10.1016/j.ajog.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Delpy R, Barthet M, Gasmi M, et al. Value of endorectal ultrasonography for diagnosing rectovaginal septal endometriosis infiltrating the rectum. Endoscopy. 2005;37:357–61. doi: 10.1055/s-2005-861115. [DOI] [PubMed] [Google Scholar]
- 70.Bahr A, de Parades V, Gadonneix P, et al. Endorectal ultrasonography in predicting rectal wall infiltration in patients with deep pelvic endometriosis: a modern tool for an ancient disease. Dis Colon Rectum. 2006;49:869–75. doi: 10.1007/s10350-006-0501-x. [DOI] [PubMed] [Google Scholar]
- 71.Bergamini V, Ghezzi F, Scarperi S, et al. Preoperative assessment of intestinal endometriosis: A comparison of transvaginal sonography with water-contrast in the rectum, transrectal sonography, and barium enema. Abdom Imaging. 2010;35:732–6. doi: 10.1007/s00261-010-9610-z. [DOI] [PubMed] [Google Scholar]
- 72.Dumontier I, Roseau G, Vincent B, et al. Apport comparé de l’enchoendoscopie et l’imagerie par résonance magnétique dans le bilan de l’endometriose pelvienne profonde. Gastroenterol Clin Biol. 2000;24:1197–204. [PubMed] [Google Scholar]
- 73.Bazot M, Bornier C, Dubernard G, et al. Accuracy of magnetic resonance imaging and rectal endoscopic sonography for the prediction of location of deep pelvic endometriosis. Hum Reprod. 2007;22:1457–63. doi: 10.1093/humrep/dem008. [DOI] [PubMed] [Google Scholar]
- 74.Bazot M, Lafont C, Rouzier R, et al. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825–33. doi: 10.1016/j.fertnstert.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Rossini L, Ribeiro PAG, Aoki T, et al. The echo-logic classification for deep pelvic endometriosis. Gastrointest Endosc. 2002;56:S133. [Google Scholar]
- 76.Ribeiro PAG, Rossini L, Almeida-Prado RA, et al. The echo-logic for deep pelvic endometriosis. J Am Assoc Gynecol Laparosc. 2002;9:S74. [Google Scholar]
- 77.Leyden J, Winter DC, Clarke E, et al. Endoscopic ultrasound and EUS-guided FNA in the diagnosis of rectal endometriosis. Ir Med J. 2009;102:301. [PubMed] [Google Scholar]
- 78.Artifon EL, Franzini TA, Kumar A, et al. EUS-guided FNA facilitates the diagnosis of retroperitoneal endometriosis. Gastrointest Endosc. 2007;66:620–2. doi: 10.1016/j.gie.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 79.Pishvaian AC, Ahlawat SK, Garvin D, et al. Role of EUS and EUS-guided FNA in the diagnosis of symptomatic rectosigmoid endometriosis. Gastrointest Endosc. 2006;63:331–5. doi: 10.1016/j.gie.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Artifon EL, Furuya CK, Sakai P, et al. Diagnosis of endometriosis with EUS. Gastrointest Endosc. 2009;69:AB334. doi: 10.1016/j.gie.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 81.Rossini LG, Assef MS, Schneider NC, et al. EUS/Trus-FNA for preoperative histological diagnosis of deep intestinal endometriosis. Gastrointest Endosc. 2011;73:AB170–171. [Google Scholar]
- 82.Roseau G, Palazzo L, Cornier E, et al. Endométriose recto-sigmoidienne: diagnostic par écho-endoscopie. Méd Chir Dig. 1993;22:20–1. [Google Scholar]
- 83.Thomassin I, Bazot M, Detchev R, et al. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004;190:1264–71. doi: 10.1016/j.ajog.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Abrão MS, Gonçalves MO, Gonzales M, et al. It is possible to evaluate deeply infiltrating endometriosis with transvaginal ultrasound. Eur J Obstet Gyn R B. 2005;123:S1–S66. [Google Scholar]
- 85.Abrão MS, Gonçalves MOC, Dias JA, et al. Comparison between clinical examination, transvaginal sonography and magnetic imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22:3092–7. doi: 10.1093/humrep/dem187. [DOI] [PubMed] [Google Scholar]
- 86.Dessole S, Farina M, Rubattu G, et al. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil Steril. 2003;79:1023–7. doi: 10.1016/s0015-0282(02)04952-x. [DOI] [PubMed] [Google Scholar]
- 87.Bazot M, Thomassin I, Hourani R, et al. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol. 2004;24:180–5. doi: 10.1002/uog.1108. [DOI] [PubMed] [Google Scholar]
- 88.Landi S, Barbieri F, Fiaccavento A, et al. Preoperative double-contrast barium enema in patients with suspected intestinal endometriosis. J Am Assoc Gynecol Laparosc. 2004;11:223–8. doi: 10.1016/s1074-3804(05)60203-4. [DOI] [PubMed] [Google Scholar]
- 89.Graham B, Mazier WP. Diagnosis and management of endometriosis of the colon and rectum. Dis Colon Rectum. 1988;31:952–6. doi: 10.1007/BF02554893. [DOI] [PubMed] [Google Scholar]
- 90.Midorikawa Y, Kubota K, Kubota K, et al. Endometriosis of the rectum causing bowel obstruction: a case report. Hepatogastroenterology. 1997;44:706–9. [PubMed] [Google Scholar]
- 91.Farinon AM, Vadora E. Endometriosis of the colon and rectum: an indication for peroperative coloscopy. Endoscopy. 1980;12:136–9. doi: 10.1055/s-2007-1021730. [DOI] [PubMed] [Google Scholar]
- 92.Pierre F, Letessier E, Body G, et al. Léndometriose recto-sigmoidienne. Resultates dúne enquete multicentrique collectant 69 dossiers. Lyon Chir. 1990;86:293–7. [Google Scholar]
- 93.Bozdech JM. Endoscopic diagnosis of colonic endometriosis. Gastrointest Endosc. 1992;38:568–70. doi: 10.1016/s0016-5107(92)70518-5. [DOI] [PubMed] [Google Scholar]
- 94.Martin DC, Batt RE. Retrocervical, retrovaginal pouch, and rectovaginal septum endometriosis. J Am Assoc Gynecol Laparosc. 2001;8:12–7. doi: 10.1016/s1074-3804(05)60543-9. [DOI] [PubMed] [Google Scholar]
- 95.Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc. 1990;36:342–50. doi: 10.1016/s0016-5107(90)71061-9. [DOI] [PubMed] [Google Scholar]
- 96.Catalano MF. Endoscopic ultrasonography in the diagnosis of submucosal tumor: need for biopsy. Endoscopy. 1994;36:788–91. doi: 10.1055/s-2007-1009107. [DOI] [PubMed] [Google Scholar]
- 97.Boyce GA, Sivak MV, Rösh T, et al. Evaluation of submucosa upper gastrointestinal tract lesions by endoscopic ultrasound. Gastrointest Endosc. 1991;37:449–54. doi: 10.1016/s0016-5107(91)70778-5. [DOI] [PubMed] [Google Scholar]
- 98.Rossini L. Tese (Doutorado) São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2010. Sensisibilidade e aspectos técnicos da punção guiada por endossonografia intestinal no diagnóstico histológico da endometriose profunda do reto e do sigmoide distal. [Google Scholar]
- 99.Schweppe KW, Wynn RM. Ultrastructural changes in endometriotic implants during the menstrual cycle. Obstet Gynecol. 1981;58:465–73. [PubMed] [Google Scholar]
- 100.Schweppe KW, Wynn RM. Endocrine dependency of endometriosis: an ultrastructural study. Eur J Obstet Gynecol Reprod Biol. 1984;17:193–208. doi: 10.1016/0028-2243(84)90143-6. [DOI] [PubMed] [Google Scholar]


