Abstract
For the management of pancreatic abscess, endoscopic ultrasound (EUS)-guided puncture and drainage has become recognized as a safer and more effective alternative to surgery. Typically, a double-pigtail plastic stent is placed for drainage. When an abscess is complicated by infected necrosis, endoscopic evacuation is essential. However, endoscopic evacuation carries a high risk of hemorrhage and needs to be performed daily to be effective. We describe EUS-guided endoscopic evacuation and placement of a fully covered metal stent following two failed evacuations. Patient recovery time was excellent, and no complications occurred.
Keywords: pancreatic abscess, endoscopic ultrasound, metal stent, balloon dilation, endoscopic necrosectomy
INTRODUCTION
Pancreatic abscess (PA) is a common complication of acute pancreatitis or pancreatic pseudocyst infection that is associated with a high mortality. PA is defined as a circumscribed intra-abdominal collection of pus that is typically in proximity to the pancreas and contains little or no pancreatic necrosis. Treatment for PA is complicated.1 Surgery, while recommended, carries a significant morbidity (13%-53%) and mortality (6.2%–25%).2,3,4,5
Endoscopic ultrasound (EUS)-guided puncture and drainage is a widely accepted non-surgical intervention for pancreatic pseudocyst6,7,8,9,10,11 that also can be used in PA treatment. Endoscopic interventions include endoscopic cystogastrostomy, balloon dilation of the fistula, endoscopic necrosectomy, and placement of double-pigtail stents for drainage.1
Here, we report a case of large PA that was not resolved by an initial trial involving the placement of multiple double-pigtail stents, the placement of a nasogastric decompression tube for lavage, or repeated debridement. It ultimately was resolved by debridement and placement of a fully covered metal stent for drainage.
CASE REPORT
A 59-year-old woman with severe epigastric pain and fever lasting 3 days was referred to our hospital. At admission, she complained of abdominal pain and fever. Computed tomography (CT) indicated pancreatic necrosis and a large pseudocyst involving the head, body, and tail of the pancreas. The presence of fever suggested that the pseudocyst was infected. The cyst location and clinical symptoms supported EUS-guided pseudocyst drainage. Informed consent for the procedure was signed by the patient.
A longitudinal echoendoscope (Pentax EG-3830-UT, Pentax Corporation, Japan) with a working channel of 3.8 mm was introduced to scan for the pseudocyst and mark the puncture point. The contact zone (i.e., the closest approximation of the region between the gastric wall and the cyst wall) was identified. Color Doppler then was applied to identify interposing vessels and thus avoid them during puncture. An EchoTip Ultra endoscopic needle (19-G, Willson-CooK Medical Inc, USA) was then introduced via the working channel of the echoendoscope, and the cyst was punctured under EUS guidance. A sample of the cyst was aspirated for biochemical, cytological, and tumor marker analysis. The needle path was then dilated by the cystotome (10-Fr, Wilson Cook Medic) and a balloon dilator (10 mm, Changzhou Jiuhong Medical instrument Co., Ltd, China) to form a fistula. A double pigtail stent then was introduced for the drainage of pus. Following this procedure, the patient's fever was not resolved (Fig. 1).
Figure 1.
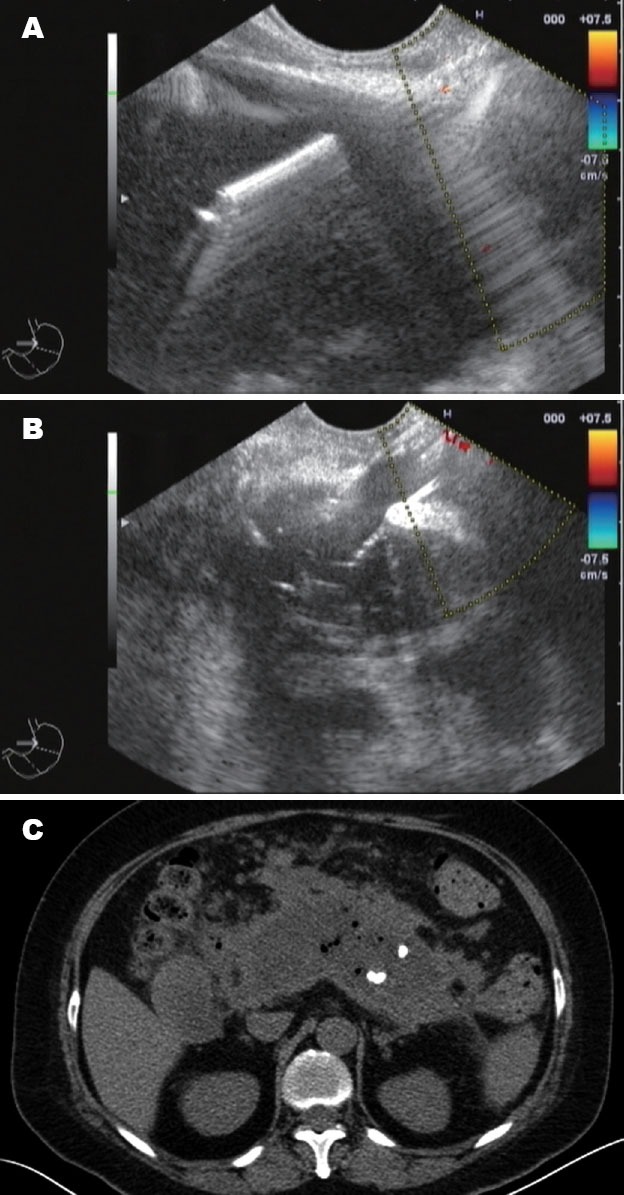
A: Endoscopic ultrasound (EUS) showed a large cyst involving the head, body, and tail of the pancreas, and was punctured under EUS guidance; B: The needle path was dilated by the cystotome and a balloon dilator to form a fistula; C: Computed tomography scan showed no reduction of the cyst1 week later.
Endoscopic necrosectomy was subsequently performed via basket 2 weeks later (Fig. 2). The majority of the necrotic and purulent material was evacuated, and a double pigtail stent and depressing tube were introduced for drainage. Lavage was performed daily with saline solution. Necrosectomy was repeated after 2 weeks. The patient's fever resolved only transiently after necrosectomy.
Figure 2.
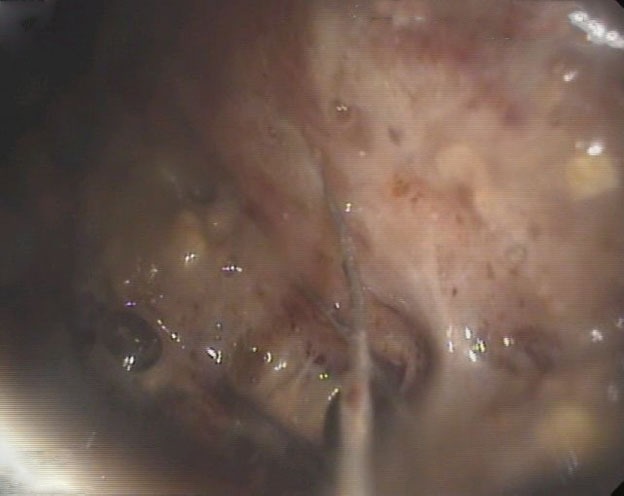
Endoscopic necrosectomy was subsequently performed via basket 2 weeks later.
A large diameter stent was placed to facilitate adequate drainage during a third endoscopic intervention. Following this necrosectomy, a guide wire (Tracer metro direct wire guide, 0.035 inch /480 mm, Wilson Cook Medical Inc, USA) was kept in the abscess to guide the placement of a covered metal stent (14 mm/40 mm, Micro-Tech/Nan Jing Co., Ltd.). A double pigtail stent (PE double-pigtail 10 Fr/7 cm, ENDO-FLEX GmbH, Germany) was introduced through the metal stent for auxiliary drainage (Fig. 3). The patient's temperature returned to normal after the procedure. CT scan showed a more than 70% reduction of the abscess 1 week later (Fig. 4). CT scan indicated substantial reduction of the pseudocyst 1 month later (Fig. 5). The metal stent was expelled from the fistula on its own without notice. The double pigtail stent was removed during a follow-up EUS examination.
Figure 3.
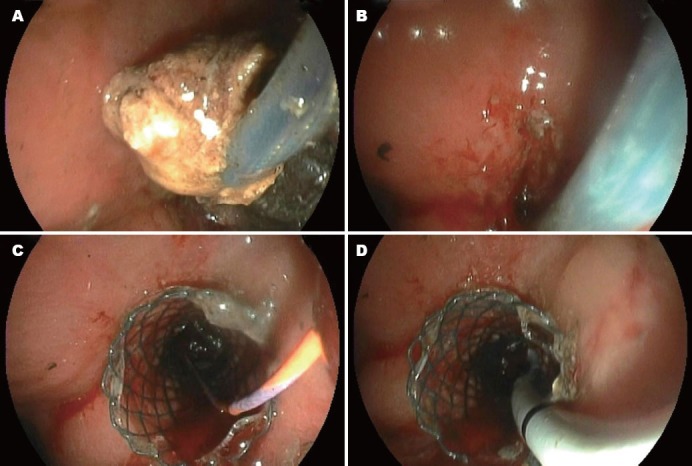
A: The third time necrosectomy. The majority of the necrotic and purulent material was evacuated; B: The placement of a covered metal stent; C: A guide wire was kept in the metal stent to guide the placement of a double pigtail stent for auxiliary drainage.
Figure 4.
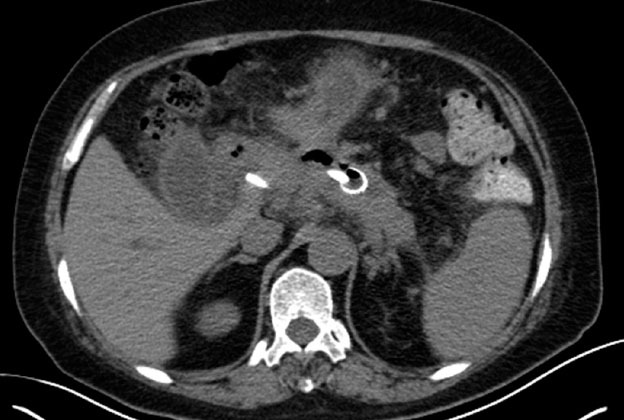
Computed tomography scan showed a more than 70% reduction of the abscess 1 week after the metal stent placement.
Figure 5.
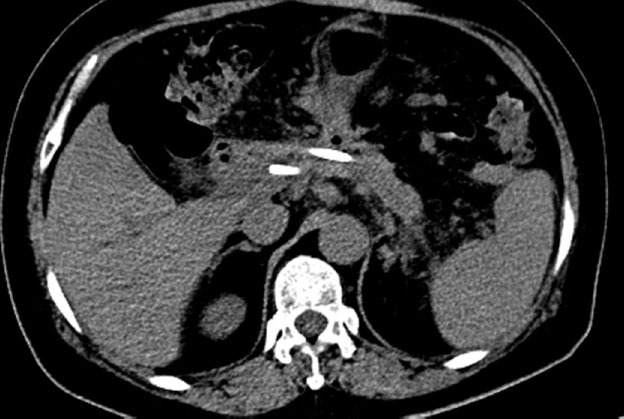
Computed tomography scan indicated substantial reduction of the pseudocyst 1 month later.
DISCUSSION
We describe a case in which an infected pseudocyst developed into a PA. Management approaches for PA include endoscopic, radiologic (percutaneous), and surgical procedures (e.g., open or laparoscopic drainage). Pancreatic pseudocyst traditionally is treated surgically, but this approach is accompanied by a high morbidity (13%–53%) and mortality (6.2%–25%).2,3,4,5 An alternative nonsurgical treatment involves percutaneous drainage with a long catheter; however, the effectiveness of this approach is inconsistent even for the drainage of a non-contagious pancreatic pseudocyst.12,13
During the past decade, endoscopic treatment emerged as a preferred approach in the management of pancreatic pseudocyst and abscess. Long-term placement of a stent in the pancreatic duct is likely to induce morphological changes in the duct and the surrounding tissues. Therefore, EUS-guided pancreatic pseudocyst drainage via cystoenterostomy is considered an effective therapy.14,15 When PAs are complicated by infected necrosis, plastic stents and daily lavage are inadequate.
Evacuation of necrotic materials also is effective in the treatment of complicated PA. Prior to this report, no data was available regarding the optimal frequency of necrosectomy, but it was recommended that necrosectomy be performed daily until the necrotic material had been completely removed.1 Fever is one of the most sensitive indicators in patients with PA. If the endoscopic intervention succeeds, fever will resolve quickly. In our case, the patient's temperature was reduced rapidly only upon repeated necrosectomy. Daily necrosectomy is time-consuming and induces physical and mental suffering in patients. Necrotic debridement also carries a substantial risk of bleeding. If the hemorrhage is not self-limited, it can be difficult to manage endoscopically. Thus, following initial debridement, a large lumen stent to facilitate adequate drainage should be considered.
To provide adequate drainage while avoiding the need for repeated evacuation, we placed a covered metal stent after the third necrosectomy. The patient recovered quickly following this procedure. Transluminal drainage via metal stents has been performed in numerous cases since 2008.16,17 Covered metal stents are retrievable, which is essential for benign lesions. In theory, the covered metal stent can easily be removed within 1 month. In our case, when the patient returned for 1-month follow-up, CT scan indicated an almost complete collapse of the abscess cavity with the metal stent expelled. These results support the safety of stent placement, but also highlight the limitation of early stent migration. Novel metal stent designs involving large lumens for the collection of extra-intestinal fluid are warranted.
To our knowledge, this report describes the first case of EUS-guided metal sent placement to treat PA after repeated endoscopic necrosectomy failed. Additional studies are needed to validate this method, determine the optimal timing of metal stent intervention, and prevent premature stent expulsion.
REFERENCES
- 1.Seewald S, Groth S, Omar S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm. Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-del Castillo C, Rattner DW, Makary MA, et al. Debridement and closed packing for thetreatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–84. doi: 10.1097/00000658-199811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartwig W, Werner J, Muller CA, et al. Surgical management of severe pancreatitis including sterile necrosis. J Hepatobil Pancreat Surg. 2002;9:429–35. doi: 10.1007/s005340200053. [DOI] [PubMed] [Google Scholar]
- 4.Branum G, Galloway J, Hirchowitz W, et al. Pancreatic necrosis: results of necrosectomy, packing, and ultimate closure over drains. Ann Surg. 1998;227:870–7. doi: 10.1097/00000658-199806000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsiotos GG, Luque-de Leon E, Soreide JA, et al. Management of necrotizing pancreatitis by repeated operative necrosectomy using a zipper technique. Am J Surg. 1998;175:91–8. doi: 10.1016/s0002-9610(97)00277-8. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M. EUS-guided pancreatic pseudocyst drainage. Gastrointest Endosc. 2007;9:32–8. doi: 10.1016/j.giec.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Seifert H, Dietrich C, Schmitt T, et al. Endoscopic ultrasound-guided one step transmural drainage of cystic abdominal lesions with a large-channel echo endoscope. Endoscopy. 2000;32:255–9. doi: 10.1055/s-2000-93. [DOI] [PubMed] [Google Scholar]
- 8.Vosoghi M, Sial S, Garret B, et al. EUS-guided pancreatic pseudocyst drainage: review and experience at Harbor-UCLA Medical Center. Med Gen Med. 2002;4:2. [PubMed] [Google Scholar]
- 9.Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223–7. doi: 10.1016/j.gie.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Wenchman L, Kylanpaa ML, Puolakkainen P, et al. Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20:603–7. doi: 10.1007/s00464-005-0201-y. [DOI] [PubMed] [Google Scholar]
- 11.Cahen D, Rauws E, Fockens P, et al. Endoscopic drainage of pancreatic pseudocysts: long term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–83. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 12.Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest endosc. 2006;63:635–43. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Bradley EL, Howard TJ, van Sonnenberg E, et al. Intervention in necrotizing pancreatitis: an evidence based review of surgical and percutaneous alternatives. J Gastrointest Surg. 2008;12:634–9. doi: 10.1007/s11605-007-0445-z. [DOI] [PubMed] [Google Scholar]
- 14.Barkay O, Sherman S, McHenry L, Yoo BM, et al. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duc cannulation at ERCP. Gastrointest Endosc. 2010;71:1166–73. doi: 10.1016/j.gie.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Krüger M, Schneider AS, Manns MP, et al. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–16. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Talreja JP, Shami VM, Ku J, et al. Transenteric drainage of pancreatic fluid collections with fully covere self-expanding metallic stents (with video) Gastrointestinal Endosc. 2008;68:1199–203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Belle S, Collet P, Post S, et al. Temporary cystogastrostomy with self-expending metallic stents for pancreatic necrosis. Endoscopy. 2010;42:493–5. doi: 10.1055/s-0029-1244021. [DOI] [PubMed] [Google Scholar]


