Abstract
Since the introduction of endoscopic ultrasonography (EUS), many centers have utilized this imaging modality for transmural pancreatic fluid collection (PFC) drainage. The expanded use of EUS has resulted in increased safety and efficacy of endoscopic PFC drainage. The major procedural steps include EUS-guided transgastric or transduodenal fistula creation into the PFC, and stent placement or nasocystic drain deployment to decompress the collection. In this and other applications, EUS has become a major therapeutic advancement in the field of endoscopy and has figured in myriad diagnostic applications. Recent research indicates a number of situations in which EUS-guided PFC drainage is appropriate. These include unusual location of the collection, small window of entry, non-bulging collections, coagulopathy, intervening varices, or failed conventional transmural drainage. In this study, we discuss the EUS-guided technique and review current literatures.
Keywords: endoscopic ultrasound, pancreatic fluid collection, pancreatic pseudocyst, non-bulging pancreatic fluid collection
INTRODUCTION
Pancreatic fluid collections (PFCs) can develop secondarily to fluid leakage or liquefaction of pancreatic necrosis.1 PFCs are also observed in association with acute and chronic pancreatitis, abdominal trauma, and surgery.2,3,4,5 The Atlanta Classification has been accepted as the current standard classification system.6,7 Although the above situations may precipitate PFC formation, other factors are known to influence their creation further, including underlying pancreatic ductal damage, severity of acute pancreatitis, and maturation of the collection with respect to the onset of acute pancreatitis.7,8,9,10,11 Simple PFCs do not necessarily need endoscopic ultrasonography (EUS)-guided drainage. Abdominal pain, infected collection, gastric outlet or biliary obstruction, fluid leakage, fistulization, and PFC enlargement are all indications for drainage.7,8,9,10,11 Although we are presently reviewing the endoscopic drainage of PFCs, other modalities are also viable options. Some of these alternative therapies include direct surgical drainage and percutaneous drainage. However, morbidity rates of 7%–37% have been reported for surgical drainage.7,12,13,14 Radiologically guided percutaneous drainage has been shown to be an effective treatment modality for all types of PFCs,15 but can also produce adverse events. An in-dwelling catheter is required for the percutaneous technique, which may lead to a new possible nidus for infection and percutaneous fistula formation.16,17,18 Many tertiary care centers have adopted the endoscopic approach for PFC drainage.7,19,20 Endoscopic drainage clinical success rates of 70%–87% have been reported, with complication rates of 11%–34%. The endoscopic drainage of PFC can be accomplished with the transmural or transpapillary placement of plastic endoprostheses, an option that has fallen out of favor in very recent years.7,22
Conventional transmural drainage without EUS carries a high risk of perforation in the absence of an obvious bulge.23,24 Whereas bulging collections provide an easier target for the operator, non-bulging PFCs are present in 42%–48% of cases.25,26 With EUS guidance, entry into cysts has been shown to be safer.23 EUS has also provided an advantage in the drainage of pancreatic abscesses and organized liquefied necrotic collections in addition to the above described non-bulging PFCs.1,7,27
MATERIALS AND METHODS
Appropriate candidates
PFC development in acute or chronic pancreatitis dictates different indications for drainage.23 We recommend cross-sectional computed tomography (CT) or magnetic resonance imaging (MRI) prior to drainage. This imaging is essential in defining the anatomy of the patient and determining an appropriate target window for intervention. It also permits the exclusion of a cystic neoplasm in patients without a history of acute or chronic pancreatitis. When the fluid collection does not acutely respond to conservative management, drainage is indicated especially if the patient has signs of sepsis. In chronic pancreatitis, drainage should be performed for symptomatic management including pain, gastric outlet obstruction, or biliary compression resulting in jaundice. Size alone has not yet been described as a sole indicator for PFC drainage.
Non-bulging fluid collections, known portal hypertension/ high pretest probability of bleeding, prior failed traditional transmural drainage, or the need to exclude cystic neoplasm are all indications for considering EUS-guided drainage.29,30,31,32 Determining if the collection primarily comprises liquid contents or if there is a component of solid debris is important. We recommend assessing the main pancreatic duct at the time of PFC drainage with endoscopic retrograde cholangiopancreatography. Patients with major main pancreatic duct leaks may require stent placement to bridge the leak.
Appropriate endoscopists
EUS for PFC drainage should only be performed by physicians trained in both EUS and endoscopic retrograde cholangiopancreatography (ERCP) skill sets. This procedure should only be performed in centers with available pancreatico-biliary surgeons and interventional radiologists in the event of complications.
Patient preparation
All patients should receive periprocedural antibiotic therapy. Because EUS-guided PFC drainage is technically challenging and time consuming, we recommend the procedure be performed under general anesthesia.
Instrumentation
Linear array echo-endoscopes with channel sizes of at least 3.4 mm should be used. This scope size allows the placement of larger 10 French (Fr) stents.23,32 The GF-UCT 140-180 (Olympus America, PA, USA) has a working channel of 3.7 mm and the EG 38UT (Pentax, Japan) has a working channel of 3.8 mm. For pseudocyst puncture, using a 19-G FNA needle is preferable (Wilson-Cook, Winston-Salem, NC, USA) so that a larger 0.035 inch guide wire can be inserted through the needle for pseudocyst drainage. Dilation of the fistula created can be performed using a wire-guided balloon or cystenterostome.26
The single-step approach led to the development of instruments that utilize a 19-G stainless steel puncture needle (Grosse, Daldorf, Germany) loaded with a modified 7- or 10-Fr stent and a Teflon pusher catheter (Wilson-Cook).34,35 A needle-wire device introduced by Giovannini et al.36 consists of a 0.035 inch in needle wire suitable for cutting current, a 5.5-Fr dilator, and an 8.5-Fr stent (6 cm long) with a pusher pre-assembled on the same catheter (Giovannini Needle Wire Oasis, Cook Endoscopy, Winston-Salem, NC, USA). However, these products are not currently available in the USA.
Pre-drainage evaluation
As described above, abdominal CT or MRI (with contrast) should be obtained to describe the patient anatomy and aid in describing the collection contents. Imaging also helps describe the relationship of the PFC with the surrounding lumen and vascular structures, and discount any other underlying etiologies of PFC for which treatment may differ.23,29 Given that this is an invasive procedure, the patient should have a complete blood count to assess for thrombocytopenia and coagulopathy.
Procedure description
Using EUS, the PFC is first located (Fig. 1). Color Doppler ultrasound is then used to identify regional and surrounding vasculature. A fistula between the pseudocyst and the stomach or duodenum is created by introducing a 19-G needle directly into the PFC (Fig. 2). A sample of cyst contents is aspirated and submitted for biochemical analysis. If infection is suspected, a sample should be sent for Gram staining and culturing. Contrast filling of the pseudocyst can be conducted under direct fluoroscopy to assess and document the size and boundaries, as well as determine if communication with the pancreatic duct is apparent. Drainage can be achieved using either the needle-knife or Seldinger techniques. In the latter, a 0.035 inch guide wire is introduced through the needle and coiled within the pseudocyst (Fig. 3). The fistula created is then dilated with either a 6- or 8-mm balloon over the guide wire coiled into the pseudocyst (Fig. 4). The balloon is exchanged off the guide wire and one or two 10-Fr double-pigtail endoprostheses are placed (Fig. 5). At some institutions, a nasocystic drain may be placed to flush the fluid collection.37 An alternative to the balloon dilation technique involves using a cystenterostome over the guide wire to enlarge the fistula by cautery.26 If the pancreatic duct is disrupted or a dominant stricture is present, pancreatic duct stenting should also be performed.27
Figure 1.
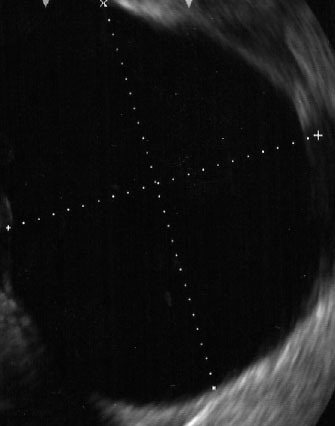
Endoscopic ultrasound demonstrating liquid pancreatic fluid collection (pseudocyst).
Figure 2.
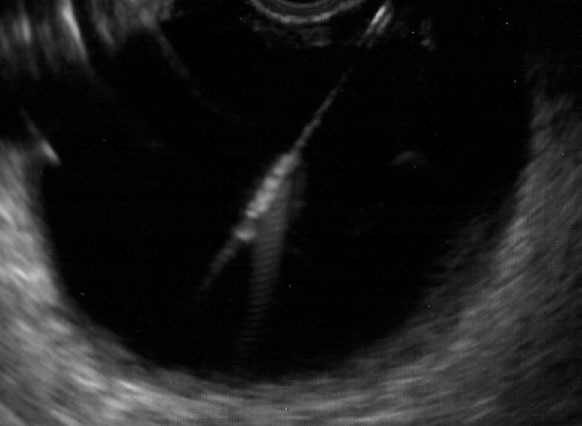
Endoscopic ultrasound showing needle puncturing targeted fluid collection.
Figure 3.
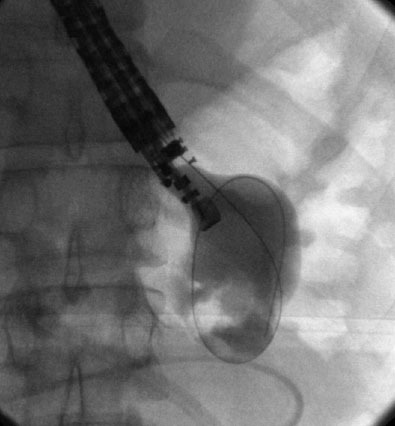
Fluoroscopic image of a wire coiled into a pancreatic fluid collection.
Figure 4.
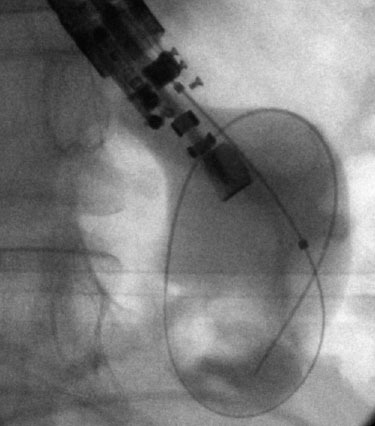
Fluoroscopic image of a wire-guided balloon placed at the level of the fistula created to drain the pancreatic fluid collection.
Figure 5.
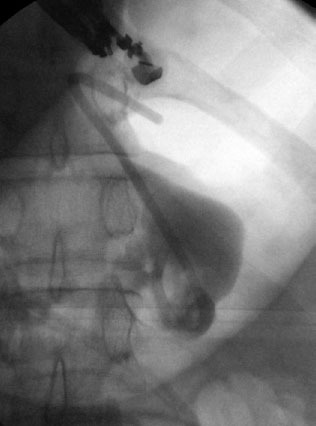
Fluoroscopic image of a double pigtail stent deployed into the pancreatic fluid collection.
Literature review
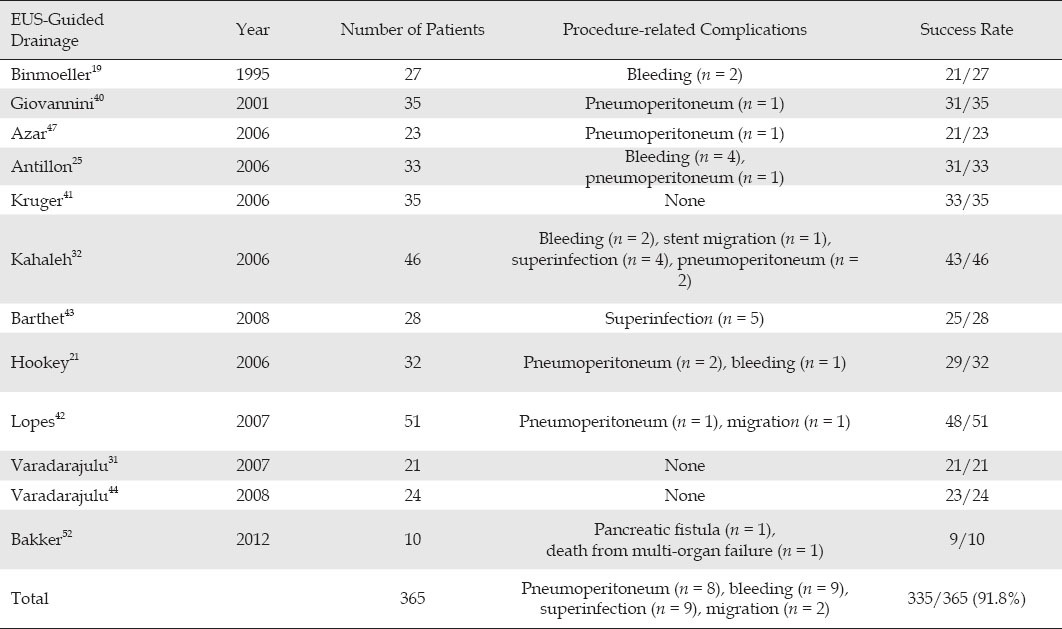
Wiersema et al.38 reported the first EUS-guided drainage of a PFC in 1992. The method was performed using an interventional (large-channel) EUS endoscope. Shortly after, Binmoeller et al.39 reported an overall initial success rate of 78% with EUS-guided pseudocyst drainage in 1995. Giovannini et al.40 reported an 88.5% success rate (n = 35) for the same procedure for either pseudocyst drainage or pancreatic abscess drainage; four of the patients went on to require surgery. A 2006 prospective cohort study of Antillon et al.25 revealed that 82% of patients who underwent EUS-guided drainage achieved complete pseudocyst resolution, with only 2 out of 33 enrollees experiencing major complications and only 1 experiencing recurrence over 46 weeks. A prospective case series by Kruger et al.41 described 36 patients who underwent EUS-guided drainage with a single-step needle-wire device and 8.5-Fr stents. They found a resolution rate of 88% with a 12% recurrence rate over a 24-month period. PFC resolution was achieved by additional endoscopic cyst irrigation in 10 patients (30%). This finding may be related to the use of smaller diameter plastic stents. Hookey et al.21 studied 116 patients with fluid collections (acute, pseudocysts, necrosis, and abscess) who underwent EUS-guided drainage. They employed a transmural drainage technique with EUS guidance in 32 patients, and EUS guidance was used in 19 out of 41 patients who had a combination of transpapillary and transmural drainage with EUS. EUS was used in 44% (51/116) of all cases. Success was achieved in those who underwent EUS-guided transmural drainage in 90.6% (29/32) of patients. The recurrence rate was 12.5% (4/32) with three complications (9.4%) noted. In this group, 37.5% (12/32) patients had bulging fluid collections. A 2007 study by Lopes et al.42 covered 51 patients who underwent EUS-guided transmural drainage of PFCs in a retrospective review. This review described a 94% (48/51) success rate. There was a recurrence rate of 17.7% over 39 weeks. The complication rate for abscesses was decreased by the placement of two stents, but not by the placement of a nasocystic drain.
Kahaleh et al.32 reported a prospective study comparing 99 patients who underwent pseudocyst drainage using either conventional transmural drainage or EUS-guided drainage. There were 53 patients with a visible bulge and no obvious portal hypertension who underwent conventional drainage, and 46 patients underwent EUS-guided drainage. A comparable number of patients in each group underwent transpapillary stent placement for pancreatic duct disruption or stricture. The success rates at 1 month (93% vs. 94%) and 6 months (84% vs. 91%) were comparable. Complications occurred in 19% of EUS-guided drainage vs. 18% of conventional transmural drainage, including bleeding (3), infection (8), stent migration (3), and pneumoperitoneum (5). There was no clear difference between the efficacy or safety of the two techniques. The study concluded that the choice of technique is likely best predicted by individual patient presentation and local expertise, and recommended EUS for non-bulging collections and pseudocysts at risk for bleeding (i.e., intervening vessels or coagulopathy).
Barthet et al.43 published a similar concept using EUS-guided drainage, which was performed on 28 patients (56%). About 90% of these patients achieved sustained response over 12 months.
A study from Varadarajulu in 2008 compared the success rates of EUS and EGD for the transmural drainage of pancreatic pseudocysts. In this prospective randomized trial, successful drainages were realized in all patients in the EUS group, but in only 33% (5/15) of the patients in the conventional EGD group.44 Major procedure-related bleeding occurred in 2 patients in the EGD group, and one of them died. Given these results, the authors concluded that EUS should be considered as a first-line therapy for pseudocyst drainage. However, a higher than average failure rate in the conventional group was noted compared with a previous study.26 A summary of published data is presented in Table 1.
Table 1.
Outcomes in patients who underwent EUS-guided pancreatic fluid collection drainage
To date, there is no large, randomized control study comparing convention transmural and EUS-guided drainage in similar cohorts. The type and number of appropriate stents following pancreatic pseudocyst drainage also remains equivocal. However, many authors recommend large plastic double pigtailed stents.20,32 Talreja et al.45 reported successes with metal stents. In their prospective case series of 18 patients who underwent drainage of PFCs using covered self-expandable metallic stents (VIABIL; Conmed, Utica, NY, USA), all but two patients underwent drainage with EUS guidance. In total, 95% (17/18) of the patients successfully responded, with 78% of the patients achieving complete resolution of their fluid collection. Another group has reported their experience with the use of metal stents for PFC drainage and facilitating necrosectomy.46
Metal stents may provide some advantages over plastic ones. The provided radial force can tamponade bleeding vessels within the PFC wall (Fig. 6–9). A novel stent with a “dog bone” shape that can be deployed under EUS control may offer the possibility of apposing the PFC wall better to the stomach wall (Figure 10) and thereby provide better drainage. Large, fully covered metal stents (esophageal stents) can offer a safer pathway for draining pancreatic necrosis (Fig. 11–13).
Figure 6.
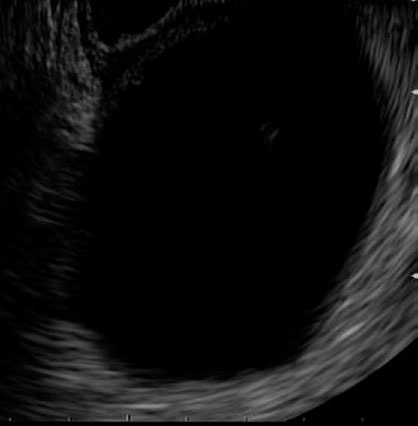
Endoscopic ultrasound of a PFC prior to puncture and bleeding (patient A).
Figure 9.
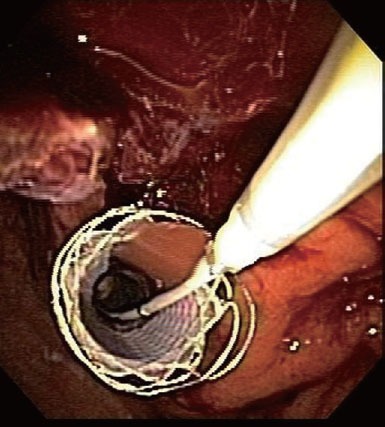
Endoscopic image of a fully covered metal stent deployed to tamponade the bleeding successfully (patient A).
Figure 10.
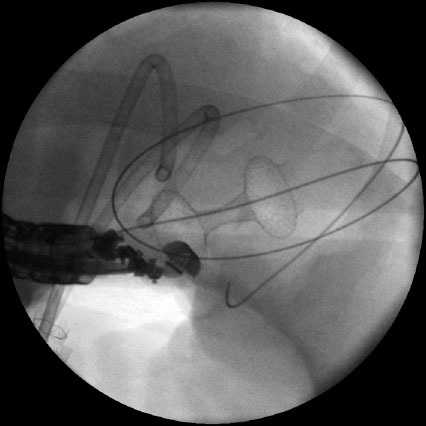
Fluoroscopic image of a “dog bone”-shaped stent deployed to drain a pseudocyst.
Figure 11.
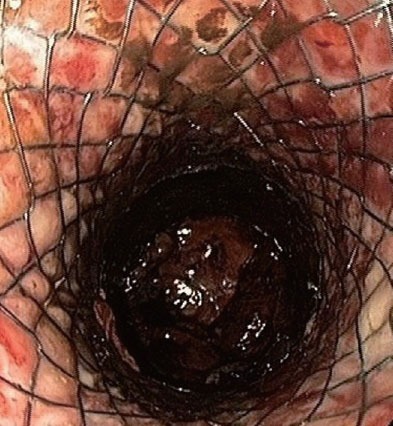
Endoscopic image of an esophageal stent deployed through the stomach wall into a pancreatic necrosis (patient B).
Figure 13.
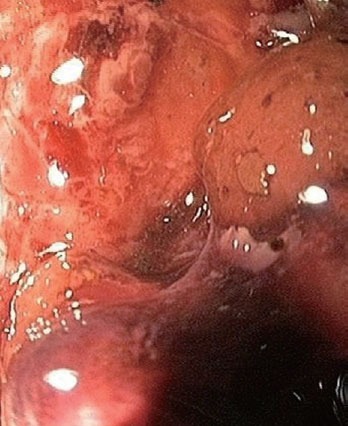
Endoscopic image of the interior of a pancreatic necrosis (patient B).
Figure 7.
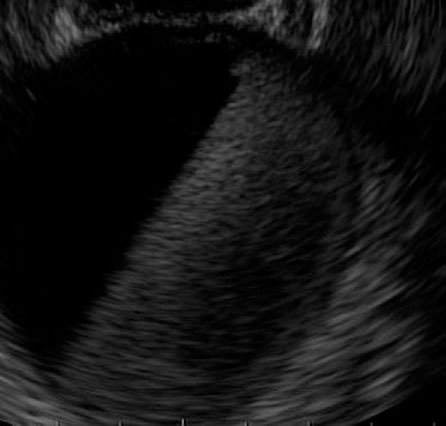
Endoscopic ultrasound of a PFC filled with blood after puncture (patient A).
Figure 8.
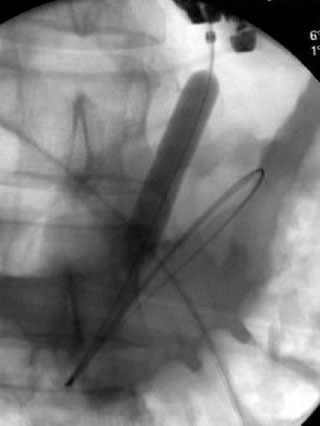
Fluoroscopic images of a balloon dilation of the fistula tract to tamponade the bleeding (patient A).
Figure 12.
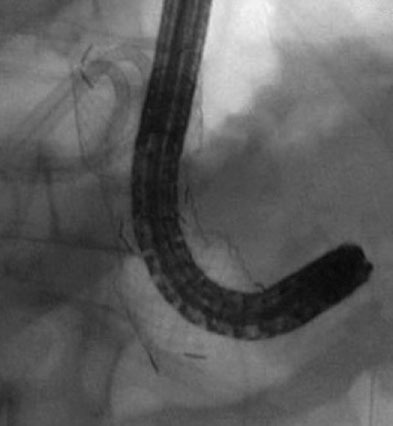
Fluoroscopic image of an upper endoscope advanced through the metal stent into the pancreatic necrosis (patient B).
Recently, Puri et al.48 have published new outcome data on EUS-guided pseudocyst drainage using combined endoprosthesis and nasocystic drainage. A total of 40 patients resistant to conservative treatment and having no bulge seen on endoscopy were subjected to EUS-guided symptomatic pseudocyst drainage. Successful drainage using EUS was achieved in all subjects. One patient required surgical resection of an infected pseudocyst because of bleeding inside the cyst. All patients had the double pigtail stents removed within 10 weeks.
Zheng et al.49 examined the efficacy and safety of 21 EUS-guided transgastric stentings of PFCs specifically resulting from trauma. They were able to stent 90.5% (19/21) of these patients successfully, but the other two patients required surgery for pseudocyst drainage. Their complications included two each of infected pseudocysts and stent obstructions. No PFC recurrence was observed over 29 months.
In 2011, Varadarajulu et al.50 analyzed complications due to EUS-guided PFC drainage in a large study group of 148 patients. Two patients (1.3%) experienced perforation at the site of transmural stenting, which both occurred with the PFC located in the uncinate region of the pancreas. No perforation occurred elsewhere. One patient experienced bleeding and another had migration of the PFC stent. Four patients experienced infection as a complication. The authors concluded EUS-guided PFC drainage as a safe and effective procedure, and also noted that a majority of the few complications seen were managed endoscopically.
Will et al.51 studied 147 patients prospectively treated over a five-year period. They enrolled patients presented with pseudocysts, abscesses, and necrosis. Within a follow-up period of between 19.4 and 20.9 months, they documented definitive therapeutic success in 96.9% of patients with pseudocysts, 97.5% of those with pancreatic abscesses, and 94.1% of those with necrosis. The overall average recurrence rate among the three different diagnoses was 15.4%.51
Evidently, although more studies are needed, there is a growing body of research supporting EUS-guided transmural PFC drainage as a safe and effective alternative approach.
CONCLUSION
The application frequency of EUS-guided PFC drainage has dramatically increased over the last decade. Consequently, numerous studies focused on the safety and efficacy of evolving techniques. Many of these studies examined whether EUS guidance is more beneficial than conventional transmural pancreatic pseudocyst drainage. Nevertheless, there is still no reported large, prospective multicenter and randomized, controlled trial that compares the two approaches to date. EUS guidance offers clear advantages over conventional drainage, including defining the characteristics of a particular PFC, ruling out alternative diagnoses such as malignancy, and assessing for intervening vasculature that can be important procedurally. EUS guidance was also shown to be advantageous in accessing non-bulging PFCs, or in high-risk clinical scenarios such as coagulopathy, intervening varices, and failed conventional transmural drainage. The challenges of accessibility to EUS guidance and available endoscopists trained in the modality remain. Continued advances in instrumentation, expansion to more tertiary care centers, and further training of personnel can help make EUS techniques safer and more efficacious. Future large studies are also necessary for the continued evaluation of these techniques.
REFERENCES
- 1.Baron TH, Thaggard WG, Morgan DE, et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–64. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 2.Baillie J. Pancreatic pseudocysts (part I) Gastrointest Endosc. 2004;59:873–9. doi: 10.1016/s0016-5107(04)00354-2. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CL, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–60. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvanitakis M, Delhaye M, Chamlou R, et al. Endoscopic therapy for main pancreatic duct rupture after silastic-ring vertical gastroplasty. Gastrointest Endosc. 2005;62:143–51. doi: 10.1016/s0016-5107(05)01627-5. [DOI] [PubMed] [Google Scholar]
- 5.Kloppel G. Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 2000;17:7–15. [PubMed] [Google Scholar]
- 6.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13 1992. Arch Surg. 1993;128:586–90. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 7.Baron TH, Harewood GC, Morgan DE, et al. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- 8.Baron TH, Morgan DE. The diagnosis and management of fluid collections associated with pancreatitis. Am J Med. 1997;102:555–63. doi: 10.1016/s0002-9343(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 9.Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gyn Obstet. 1990;170:411–7. [PubMed] [Google Scholar]
- 10.Bradley EL, Clements JL, Jr, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg. 1979;137:135–41. doi: 10.1016/0002-9610(79)90024-2. [DOI] [PubMed] [Google Scholar]
- 11.Gouyon B, Levy P, Ruszniewski P, et al. Predictive factors in the outcome of pseudocysts complicating alcoholic chronic pancreatitis. Gut. 1997;41:821–5. doi: 10.1136/gut.41.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warsaw AL, Rattner DW. Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg. 1985;202:720–4. doi: 10.1097/00000658-198512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley EL., III A fifteen year experience with open drainage for infected pancreatic necrosis. Surg Gyn Obstet. 1993;177:215–22. [PubMed] [Google Scholar]
- 14.Boerma D, van Gulik TM, Obertop H, et al. Internal drainage of infected pancreatic pseudocysts: safe or sorry? Dig Surg. 1999;16:501–6. doi: 10.1159/000018776. [DOI] [PubMed] [Google Scholar]
- 15.van Sonnenberg E, Wittich GR, Casola G, et al. Percutaneous drainage of infected and noninfected pancreatic pseudocysts: experience in 101 cases. Radiology. 1989;170:757–61. doi: 10.1148/radiology.170.3.2644662. [DOI] [PubMed] [Google Scholar]
- 16.Ahearne PM, Baillie JM, Cotton PB, et al. An endoscopic retrograde cholangiopancreatography (ERCP)-based algorithm for the management of pancreatic pseudocysts. Am J Surg. 1992;163:111–5. doi: 10.1016/0002-9610(92)90262-p. [DOI] [PubMed] [Google Scholar]
- 17.Adams DB, Harvey TS, Anderson MC. Percutaneous catheter drainage of infected pancreatic and peripancreatic fluid collections. Arch Surg. 1990;125:1554–7. doi: 10.1001/archsurg.1990.01410240032006. [DOI] [PubMed] [Google Scholar]
- 18.Neff R. Pancreatic pseudocyst and fluid collections: percutaneous approaches. Surg Clin North Am. 2001;81:399–403. doi: 10.1016/s0039-6109(05)70127-4. [DOI] [PubMed] [Google Scholar]
- 19.Binmoeller KF, Seifart H, Walter A, et al. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–24. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 20.Cahen D, Rauws E, Fockens P, et al. Endoscopic drainage of pancreatic pseudocysts; long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–83. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 21.Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–43. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Delhaye M, Matos C, Deviere J. Endoscopic management of chronic pancreatitis. Gastrointest Endosc Clin N Am. 2003;13:717–42. doi: 10.1016/s1052-5157(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 23.Giovannini M. EUS-Guided Pancreatic Pseudocyst Drainage. Techniques in Gastrointest Endosc. 2007;9:32–8. [Google Scholar]
- 24.Howell DA, Holbrook RF, Bosco JJ, et al. Endoscopic needle localization of pancreatic pseudocysts before transmural drainage. Gastrointest Endosc. 1993;39:693–8. doi: 10.1016/s0016-5107(93)70225-4. [DOI] [PubMed] [Google Scholar]
- 25.Antillon MR, Shah RJ, Stiegmann G, et al. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez Cortes E, Maalak A, Le Moine O, et al. Endoscopic cystenterostomy of nonbulging pancreatic fluid collections. Gastrointest Endosc. 2002;56:380–6. doi: 10.1016/s0016-5107(02)70042-4. [DOI] [PubMed] [Google Scholar]
- 27.Arvanitakis M, Delhaye M, Bali MA, et al. Pancreatic fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609–19. doi: 10.1016/j.gie.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 28.Baron TH. Endoscopic Drainage of Pancreatic Pseudocysts. J Gastrointest Surg. 2008;12:369–72. doi: 10.1007/s11605-007-0334-5. [DOI] [PubMed] [Google Scholar]
- 29.Andren-Sandberg A, Dervenis C. Pancreatic Pseudocysts in the 21st century. Part II. Natural History. JOP. 2004;5:64–70. [PubMed] [Google Scholar]
- 30.Jacobson B, Baron T, Adler DG, et al. ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363–70. doi: 10.1016/s0016-5107(04)02779-8. [DOI] [PubMed] [Google Scholar]
- 31.Varadarajulu S, Wilcox CM, Tamhane A, et al. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107–19. doi: 10.1016/j.gie.2007.03.1027. [DOI] [PubMed] [Google Scholar]
- 32.Kahaleh M, Shami VM, Conway MR, et al. Comparison of EUS and Conventional Endoscopic Drainage of Pancreatic Pseudocyst. Endoscopy. 2006;38:355–9. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 33.Varadarajulu S, Bang JY, Phadnis MA, et al. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–8. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 34.Seifert H, Dietrich C, Schmitt T, et al. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echoendoscope. Endoscopy. 2000;32:255–9. doi: 10.1055/s-2000-93. [DOI] [PubMed] [Google Scholar]
- 35.Seifert H, Faust D, Schmitt T, et al. Transmural drainage of cystic peripancreatic lesions with a new large-channel echoendoscope. Endoscopy. 2001;33:1022–6. doi: 10.1055/s-2001-18927. [DOI] [PubMed] [Google Scholar]
- 36.Giovannini M, Bernardini D, Seitz JF. Cystogastrostomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200–3. doi: 10.1016/s0016-5107(98)70165-8. [DOI] [PubMed] [Google Scholar]
- 37.Baron TH. Endoscopic Drainage of Pancreatic Pseudocysts, Abscesses and Organized (Walled-Off) Necrosis. In: Baron TH, Kozarek R, Carr-Locke DL, editors. ERCP. Chapter 45. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 38.Wiersema MJ. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44:614–17. doi: 10.1016/s0016-5107(96)70022-6. [DOI] [PubMed] [Google Scholar]
- 39.Binmoeller KF, Soehendra N. Endoscopic ultrasonography in the diagnosis and treatment of pancreatic pseudocysts. Gastrointest Endosc Clin N Am. 1995;5:805–16. [PubMed] [Google Scholar]
- 40.Giovannini M, Pesenti CH, Rolland AL, et al. Endoscopic ultrasound guided drainage of pancreatic pseudocyst and pancreatic abscess using a therapeutic echoendoscope. Endoscopy. 2001;33:473–7. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 41.Kruger M, Schneider AS, Manns MP, et al. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–16. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 42.Lopes CV, Pesenti C, Bories E, et al. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudoscysts and abscesses. Scan J Gastroenterol. 2007;42:524–9. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 43.Barthet M, Lamblin G, Gasmi M, et al. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245–52. doi: 10.1016/j.gie.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Varadarajulu S, Christein JD, Tamhane A, et al. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–11. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Talreja JP, Shami VM, Ku J, et al. Transenteric drainage of pancreatic fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Antillon MR, Bechtold ML, Bartalos CR, et al. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis. Gastrointest Endosc. 2009;69:178–80. doi: 10.1016/j.gie.2008.03.1066. [DOI] [PubMed] [Google Scholar]
- 47.Azar RR, Oh YS, Janec EM, et al. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688–92. doi: 10.1016/j.gie.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Puri R, Mishra S, Thandassery R, et al. Outcome and Complications of EUS Guided Pancreatic Pseudocyst Drainage Utilizing Combined Endoprosthesis and Naso-cystic Drain. J Gastroenterol Hepatol. 2012 doi: 10.1111/j.1440-1746.2012.07089.x. doi:10.1111/j.1440 y1746.2012.07089.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Zheng M, Qin M. Endoscopic ultrasound guided transgastric stenting for the treatment of traumatic pancreatic pseudocyst. Hepatogastroenterology. 2011;58:1106–9. doi: 10.5754/hge11059. [DOI] [PubMed] [Google Scholar]
- 50.Varadarajulu S, Christein JD, Wilcox CM. Frequency of complications during EUS-guided drainage of pancreatic fluid collections in 148 consecutive patients. J Gastroenterol Hepatol. 2011;26:1504–8. doi: 10.1111/j.1440-1746.2011.06771.x. [DOI] [PubMed] [Google Scholar]
- 51.Will U, Wanzar C, Gerlach R, et al. Interventional ultrasound-guided procedures in pancreatic pseudocysts, abscesses and infected necroses – treatment algorithm in a large single-center study. Ultraschall Med. 2011;32:176–83. doi: 10.1055/s-0029-1245949. [DOI] [PubMed] [Google Scholar]
- 52.Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053–61. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]


