Abstract
Pancreatic cystic lesions are being detected with an increasing frequency. Endoscopic ultrasound (EUS) provides both diagnostic and therapeutic means for pancreatic cystic lesions. Detailed imaging and EUS-guided fine-needle aspiration provide additional information on pancreatic cystic lesions. EUS-guided pseudocyst drainage has advantages over conventional drainage modalities. EUS-guided cyst ablation is a promising therapeutic modality.
Keywords: endosonography, pancreatic cyst
INTRODUCTION
Pancreatic cystic lesions (PCLs) are being detected more frequently, at least partly because of the increased use of cross-sectional imaging.1 The reported prevalence of PCLs ranges from 1.2% to 19.6% in image-based studies.2,3,4 In the past, 80%–90% of PCLs were considered to be pseudocysts.5 The current consensus is that pancreatic cystic neoplasms (PCNs) account for up to 60% of all PCLs, followed by injury- and inflammation-related cysts (30%).6 PCNs include intraductal papillary mucinous neoplasms (IPMNs), mucinous cystic neoplasms (MCNs), serous cystic neoplasms (SCNs), solid-pseudopapillary neoplasms (SPNs), cystic neuroendocrine neoplasms, ductal adenocarcinomas with cystic degeneration, and acinar-cell cystic neoplasm.7 SPNs frequently present as cystic lesions, and were once thought to be true cystic lesions. The current belief is that the cavities of SPNs represent a necrotic or degenerative process.6
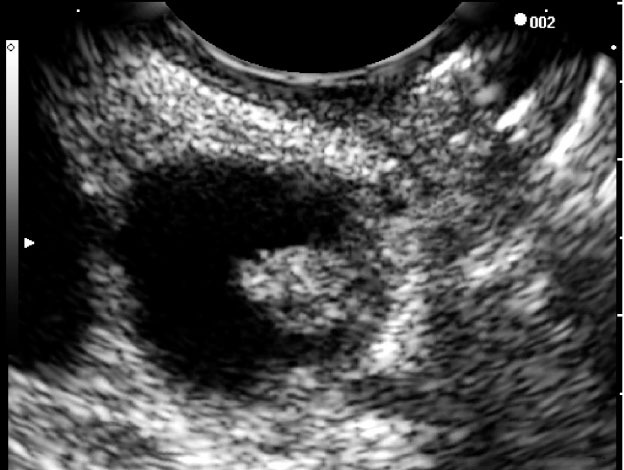
When endoscopic ultrasound (EUS) was first introduced, its primary role was as a diagnostic tool.8 The introduction of curvilinear array endosonoscopes enabled the possibility of EUS-guided fine-needle aspiration (FNA), hereafter denoted as EUS-FNA (Fig. 1). Within a relatively short time, the concept of interventional EUS has been developed, and its clinical applications are being established. This review focuses on the diagnostic and therapeutic applications of EUS to PCLs.
Figure 1.
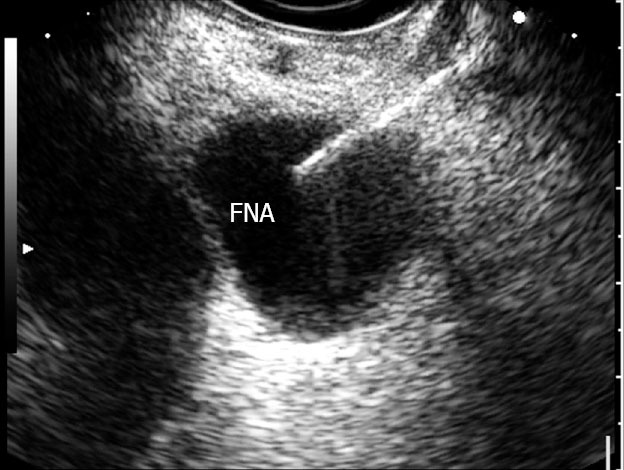
Simple unilocular cyst undergoing fine needle aspiration.
DIAGNOSTIC APPLICATIONS OF EUS TO PCLs
When faced with PCLs, cystic neoplasms must be differentiated from pseudocysts. The diagnosis of a pseudocyst is primarily based on a patient history compatible with pancreatitis, with additional information from laboratory and imaging features.9 However, clinicians should always remember that patients with PCNs may also present with pancreatitis, and that patients with a pseudocyst may have no apparent history suggestive of pancreatitis.9
Sahani et al.10 simply but usefully classified PCLs based on imaging morphological features: (1) unilocular (pseudocysts, IPMNs, unilocular macrocystic serous cystadenoma, and lymphoepithelial cysts); (2) microcystic (SCNs); (3) macrocystic (MCNs and IPMNs); and (4) cysts with a solid component (MCNs, IPMNs, cystic neuroendocrine neoplasms, SPNs, ductal adenocarcinoma with cystic degeneration, and metastasis).
With its widespread availability and ability to detect cystic lesions, computed tomography (CT) is an excellent diagnostic tool for PCLs.11 Magnetic resonance (MR) imaging with MR cholangiopancreatography is widely used given its ability to demonstrate the relationship between a PCL and the pancreatic duct.10 There are some characteristic imaging features of PCNs. For example, one can readily diagnose SCN when a central scar with calcification is observed on a cross-sectional image.12 Other features diagnostic of SCNs are the honey-combed or microcystic appearance of the lesions. MCNs are diagnosed based on their unilocular or macrocystic appearance.13 Peripheral calcification on CT is also specific for MCNs.14 For main-duct IPMNs, the cystic dilation of the main pancreatic duct is a characteristic finding. Filling defects that may represent mucinous or papillary tumors may be noted.15 In branch-duct IPMNs, the branch ducts are cystically dilated and communicate with the main pancreatic duct.15 MR imaging can also be used to differentiate IPMN from chronic pancreatitis. In a report comparing the MR imaging features of IPMN and those of chronic pancreatitis, pancreatic duct dilatation without stricture as well as bulging ampulla, nodule in a duct, grape-like cyst shape, and nodule in a cyst were all specific for IPMNs.16 Image findings of main pancreatic duct involvement, cyst diameter > 3 cm, and the presence of mural nodules are associated with malignant IPMNs.17
In EUS, the findings typical of SCNs are multiple small, anechoic areas and thin septations (Fig. 2).18 MCNs appear as fluid-filled, thin-walled, septated cavities.19 EUS findings of IPMNs include the dilation of the main pancreatic duct or branch duct with or without mural nodules and intraluminal contents.19 In one report that compared EUS findings with the surgical histopathology of IPMNs, the presence of a dilated main pancreatic duct, solid lesions, pancreatic ductal filling defects, or thickened septa within any cyst in EUS was associated with malignancy.20 Kubo et al.21 revealed that for IPMNs, the marked dilatation of the main pancreatic duct (≥10 mm) in main-duct IPMNs and large tumors (>40 mm) with irregular septa in branch-duct IPMNs in EUS were both associated with malignancy. In contrast, mural nodules >10 mm in height in EUS were associated with malignancy in both types of IPMN (Fig. 3–5). A cyst diameter >30 mm is one of the factors that predict malignant branch-duct IPMNs.17 Some EUS-based studies21 reported different cutoff values of cyst diameters that predict malignant branch-duct IPMNs. However, considerable variability among the measurements of the PCL size among EUS, CT, and MR imaging has been reported.22 Zhong et al.23 recently indicated that most echogenic lesions detected during EUS of PCLs are mucus. The authors concluded that mucus is usually hypoechoic compared with adjacent soft tissue, with a smooth edge and a hyperechoic rim.23
Figure 2.
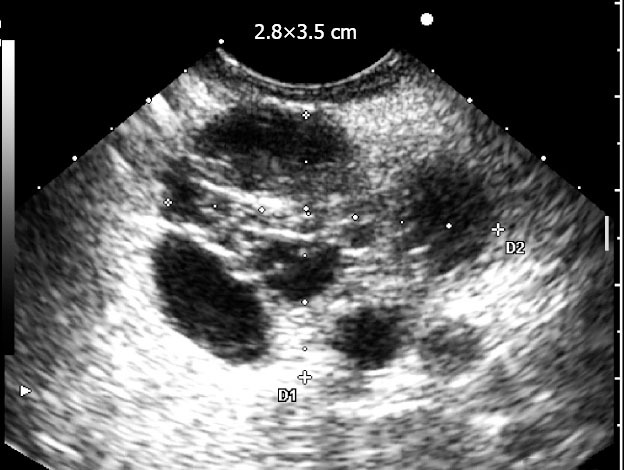
Microcystic serous cystadenoma.
Figure 3.
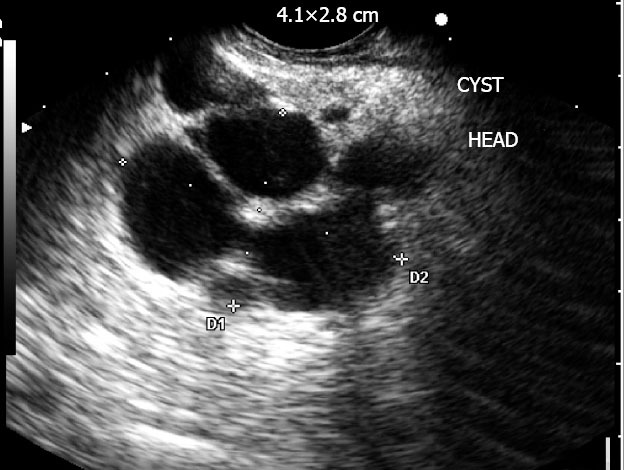
Complex multilocular cyst consistent with a benign intraductal papillary mucinous neoplasm.
Figure 5.
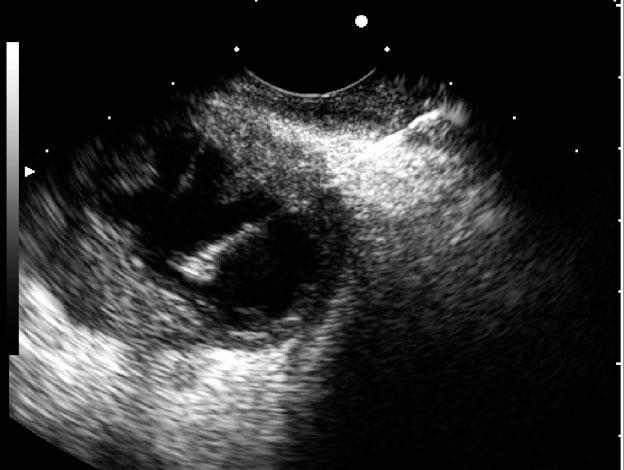
Malignant intraductal papillary mucinous neoplasm with a thickened wall.
Figure 4.
Intraductal papillary mucinous neoplasm with mural nodule (histologically, high-grade dysplasia).
EUS-FNA is another powerful tool in the diagnosis of PCLs. It provides the specimens for cyst fluid analysis and cytology. Pseudocysts are usually high in cyst fluid amylase and low in cyst fluid carcinoembryonic antigen (CEA).7 Cyst fluid CEA is useful for identifying mucinous PCLs.24,25 A cyst fluid CEA level of 192 ng/mL was highly accurate for the diagnosis of mucinous PCLs.24 Increasing the cutoff value of the cyst fluid CEA level increases the diagnostic specificity at the cost of sensitivity.26 In addition to amylase and other tumor markers, the cyst fluid analyses of DNA, intedeukin-1β, and microRNA have been reported.27,28,29
EUS can be used as a platform to deliver high-resolution imaging probes into PCLs. Konda et al. reported the feasibility of needle-based confocal laser endomicroscopy (nCLE) during the EUS-FNA of pancreatic lesions. CLE is a novel imaging technology that uses a low-power laser to obtain the in vivo histology of the gastrointestinal mucosa.30 In the study, a nCLE miniprobe was introduced through a 19-G FNA needle. Technical feasibility was achieved in 94% of the cases. Good to very good quality images were obtained in 56% of the cases.
THERAPEUTIC APPLICATIONS OF EUS TO PCLs
Perhaps the most widely utilized therapeutic application of EUS to PCLs is pseudocyst drainage. The advantages of EUS-guided drainage are as follows: (1) less invasiveness than surgery; (2) avoidance of local complications related to percutaneous drainage; and (3) real-time visualization of the pseudocyst and decreased bleeding rate by avoiding interposed blood vessels with the use of Doppler ultrasound compared with non-EUS-guided endoscopic drainage.32
In the technique, a linear endosonoscope with a therapeutic-sized accessory channel is used to visualize the pseudocyst. Vascular structures between the pseudocyst and needle path are readily identified and avoided with Doppler imaging. Under EUS guidance, the pseudocyst is punctured with an FNA needle or a puncture kit. A guidewire is then passed through the needle under fluoroscopic guidance. Several loops are formed with the guidewire within the pseudocyst cavity to provide greater security for maintaining wire access. The puncture site is dilated with a balloon catheter to 6–8 mm, and a double pigtail stent is inserted for drainage. When necessary, multiple stents or a nasocystic catheter may be inserted. To avoid the need to recannulate the pseudocyst, a “double-wire” approach may also be used.32
The reported treatment success rates for EUS-guided pseudocyst drainage are very high, ranging between 82% and 100%. The complications of EUS-guided pseudocyst drainage include pneumoperitoneum, bleeding, stent migration, perforation, and infection.33,34,35,36,37
EUS-guided pancreatic cyst ablation is another example of the therapeutic application of EUS to PCLs. Under EUS guidance, a cystic lesion is punctured, aspirated, and injected with a cytotoxic agent, which may result in the ablation of the cyst epithelium.38
Ethanol was the first cytotoxic agent to be used for this purpose. In a previous study, ethanol with concentrations ranging from 5% to 80% was injected into the PCLs of 25 patients under EUS guidance. Complete resolution of PCLs was observed in 8 patients. Histologic evidence of epithelial ablation was documented in 5 patients who underwent resection. No complication was reported.39 A prospective, randomized, multicenter trial comparing the lavage of PCLs with 80% ethanol to the lavage with saline was conducted. Ethanol lavage resulted in a greater mean percentage of cyst surface area decrease. The overall pancreatic cyst resolution rate was 33.3%. A review of the histology of 4 resected specimens demonstrated that no epithelial ablation occurred in 1 saline lavage case, whereas 50% to 100% epithelial ablation occurred in three ethanol lavage cases. The complication rates were similar in both groups.40 Long-term follow-up results of patients from this study showed that in 9 patients, follow-up CTs demonstrated no evidence of cyst recurrences performed after a median follow-up period of 26 months.41
EUS-guided ethanol lavage with paclitaxel injection (EUS-ELPI) for pancreatic cysts was recently introduced. To summarize the procedure, the cyst is aspirated under EUS guidance, lavaged with 99% ethanol, and injected with paclitaxel (concentration of 3 mg/mL). The volume of injected paclitaxel is the same as that of the aspirated cyst fluid.42 A study on 52 patients who underwent EUS-ELPI for PCLs was reported in 2011. There were 29 patients who showed complete response, 6 partial response, and 12 persistent PCLs. In 4 patients who underwent resection, histopathology revealed variable epithelial ablation extents of 0%, 25%, 40%, and 100%. A small cyst volume was the only independent factor associated with complete response. The reported complications were fever without bacteremia (n = 1), abdominal discomfort for 2 weeks (n = 1), pancreatitis (n = 1), pericystic spillage (n = 1), and splenic vein obliteration with collateral formation (n = 1).43
Another potential therapeutic application of EUS is EUS-guided radiofrequency ablation.44,45,46 This method is currently at the experimental level and can be later be applied as an ablation tool for benign tumors in the upper gastrointestinal tract and pancreas.
CONCLUSIONS
The application of EUS in the evaluation of PCLs is rapidly expanding. Novel cyst fluid markers for the diagnosis of PCLs and the differentiation of benign and malignant PCNs play critical roles in providing a highly accurate diagnosis. In the near future, several new applications using therapeutic EUS to manage cystic lesions of the pancreas are expected.
REFERENCES
- 1.Scheiman JM. Cystic lesion of the pancreas. Gastroenterology. 2005;128:463–9. doi: 10.1053/j.gastro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–7. doi: 10.1097/01.sla.0000124299.57430.ce. discussion 657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–53. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 5.Sand J, Nordback I. The differentiation between pancreatic neoplastic cysts and pancreatic pseudocyst. Scand J Surg. 2005;94:161–4. doi: 10.1177/145749690509400213. [DOI] [PubMed] [Google Scholar]
- 6.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–38. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 7.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 8.Sreenarasimhaiah J. Interventional endoscopic ultrasound: the next frontier in gastrointestinal endoscopy. Am J Med Sci. 2009;338:319–24. doi: 10.1097/MAJ.0b013e3181a44405. [DOI] [PubMed] [Google Scholar]
- 9.Goh BK, Tan YM, Thng CH, et al. How useful are clinical, biochemical, and cross-sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas? Results from a single institution experience with 220 surgically treated patients. J Am Coll Surg. 2008;206:17–27. doi: 10.1016/j.jamcollsurg.2007.06.312. [DOI] [PubMed] [Google Scholar]
- 10.Sahani DV, Kadavigere R, Saokar A, et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–84. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 11.Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 12.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, et al. Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol. 2011;20:e84–92. doi: 10.1016/j.suronc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Sahani D, Prasad S, Saini S, et al. Cystic pancreatic neoplasms evaluation by CT and magnetic resonance cholangiopan-creatography. Gastrointest Endosc Clin N Am. 2002;12:657–72. doi: 10.1016/s1052-5157(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Megibow AJ. Pancreatic neoplams. In: Gore RM, Levine MS, editors. Textbook of Gastrointestinal Radiology. 3rd ed. Philadelphia: Elsevier Saunders; 2008. pp. 1915–31. [Google Scholar]
- 15.Lim JH, Lee G, Oh YL. Radiologic spectrum of intraductal papillary mucinous tumor of the pancreas. Radiographics. 2001;21:323–37. doi: 10.1148/radiographics.21.2.g01mr01323. discussion 337-40. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Hong SS, Kim YJ, et al. Intraductal papillary mucinous neoplasm of the pancreas: differentiate from chronic pancreatits by MR imaging. Eur J Radiol. 2012;81:671–6. doi: 10.1016/j.ejrad.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 18.Penman ID, Lennon AM. EUS in the evaluation of pancreatic cysts. In: Hawes RH, Fockens P, Varadarajulu S, editors. Endosonography. 2nd ed. Philadelphia: Elsevier Saunders; 2011. pp. 166–177. [Google Scholar]
- 19.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, et al. Primary pancreatic cystic neoplasms revisited. Part III. Intraductal papillary mucinous neoplasms. Surg Oncol. 2011;20:e109–18. doi: 10.1016/j.suronc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Pais SA, Attasaranya S, Leblanc JK, et al. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol. 2007;5:489–95. doi: 10.1016/j.cgh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Kubo H, Chijiiwa Y, Akahoshi K, et al. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429–34. doi: 10.1111/j.1572-0241.2001.03794.x. [DOI] [PubMed] [Google Scholar]
- 22.Maimone S, Agrawal D, Pollack MJ, et al. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest Endosc. 2010;71:945–50. doi: 10.1016/j.gie.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. (198.e1-2).Clin Gastroenterol Hepatol. 2012;10:192–8. doi: 10.1016/j.cgh.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Cizginer S, Turner B, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024–8. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- 26.Pitman MB, Lewandrowski K, Shen J, et al. Pancreatic cysts: preoperative diagnosis and clinical management. Cancer Cytopathol. 2010;118:1–13. doi: 10.1002/cncy.20059. [DOI] [PubMed] [Google Scholar]
- 27.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–8. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–6. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol. 2008;24:631–7. doi: 10.1097/MOG.0b013e32830c91c7. [DOI] [PubMed] [Google Scholar]
- 31.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–60. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Seewald S, Ang TL, Teng KC, et al. EUS-guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21(Suppl 1):S61–5. doi: 10.1111/j.1443-1661.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, et al. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: results in 11 patients. JOP. 2006;7:616–24. [PubMed] [Google Scholar]
- 34.Antillon MR, Shah RJ, Stiegmann G, et al. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–43. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Kahaleh M, Shami VM, Conaway MR, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–9. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 37.Giovannini M, Pesenti C, Rolland AL, et al. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–7. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 38.Brugge WR. EUS-guided ablation therapy and celiac plexus interventions. In: Hawes RH, Fockens P, Varadarajulu S, editors. Endosonography. 2nd ed. Philadelphia: Elsevier Saunders; 2011. pp. 275–282. [Google Scholar]
- 39.Gan SI, Thompson CC, Lauwers GY, et al. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746–52. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 40.DeWitt J, McGreevy K, Schmidt CM, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–23. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 41.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–6. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 42.Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–42. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 43.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 45.Carrara S, Arcidiacono PG, Albarello L, et al. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321–6. doi: 10.1055/s-2007-995595. [DOI] [PubMed] [Google Scholar]
- 46.Varadarajulu S, Jhala NC, Drelichman ER. EUS-guided radiofrequency ablation with a prototype electrode array system in an animal model (with video) Gastrointest Endosc. 2009;70:372–6. doi: 10.1016/j.gie.2009.03.008. [DOI] [PubMed] [Google Scholar]


