Abstract
Objective:
Few studies have evaluated the risk of bacteremia and infectious complications after endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA). Therefore, we aimed to study the frequency of bacteremia and search for a method to potentially reduce bacterial infection after EUS-FNA. We also investigated the effect of taking proton pump inhibitors (PPIs) before examination on the occurrence of bacteremia.
Methods:
A total of 28 healthy adult dogs were randomly assigned into three groups: control group, povidone-iodine group and omeprazole group. The dogs in the povidone-iodine group were administered with 0.5% povidone-iodine solution (10 mL) to wash gastrointestinal mucosa, while the dogs in the omeprazole group were fed with 20 mg omeprazole orally twice a day for 3 days before the EUS-FNA procedure. Blood samples were collected for cultures before EUS examination, between EUS and FNA, and 5 min, 15 min and 30 min after FNA.
Results:
There were 3 true-positive cases of bacteremia in the control group while there was 1 true-positive case of bacteremia in each of the two experiment groups. The differences in the occurrences of bacteremia between the control group and both experiment groups were not statistically significant.
Conclusion:
There are no statistically significant differences in the frequencies of bacteremia between the two experiment groups and the control group. Therefore, washing of the gastrointestinal mucosa with 0.5% povidone-iodine solution may not reduce the risk of bacterial infection and taking the PPIs does not increase the risk of bacteremia after EUS-FNA.
Keywords: endoscopic ultrasonography, fine needle aspiration, bacteremia, povidone-iodine solution, blood culture, proton pump inhibitor
INTRODUCTION
Endoscopic ultrasonography (EUS) is a diagnostic tool used to visualize benign or malignant lesions of the gastrointestinal wall and surrounding organs; it is valuable for identifying the location of lesions, as well as confirming the diagnosis and interventional treatment on benign or malignant lesions of the esophagus, mediastinum, stomach, duodenum, cholesteropancreatic system and adrenal gland.1
Fine needle aspiration (FNA) extends the application of EUS to cytological diagnosis of lesions visualized under EUS. Also, EUS-FNA is an important diagnostic tool in the treatment of lung cancer and other mediastinal diseases, as well as in staging and diagnosing of lesions of the abdominopelvic cavity.2,3,4,5,6
Despite the increasing use of EUS-FNA, there are few studies on the risks of bacteremia and infectious complications after EUS-FNA, especially for examinations of the pancreas. Several studies have shown that prophylactic administration of antibiotics for patients undergoing EUS-FNA can reduce the frequency of bacteremia,7,8,9,10,11,12,13,14,15 but the American Society for Gastrointestinal Endoscopy (ASGE) advises the use of prophylactic antibiotics only for high-risk patients.16,17 Therefore, an evaluation of the frequency of bacteremia and methods to potentially reduce bacterial infection after EUS-guided FNA are needed. One study showed that washing of the gastrointestinal mucosa with povidone-iodine solution was an effective method for reducing the frequency of bacteremia following natural orifice transluminal endoscopic surgery (NOTES).18 This raises the possibility that washing of the gastrointestinal mucosa with povidone-iodine solution can also reduce the frequency of bacteremia after EUS-FNA.
Additionally, acid suppressive therapy, in the form of proton pump inhibitors (PPIs) such as omeprazole, is widely used in patients with upper gastrointestinal symptoms. PPIs suppress the production of gastric acid and increase the gastric pH, which weakens the inhibitory actions of the gastrointestinal tract against bacteria, possibly resulting in significant growth of bacteria in the digestive tract. In one reported case, a patient used acid-suppressing medications before endoscopic ultrasound-guided celiac plexus neurolysis developed a complicated retroperitoneal abscess.19 Therefore, we presume that application of PPIs before EUS-FNA procedure may increase the risk of bacteremia. It is unclear whether it is necessary to stop the PPIs before the procedure. Therefore, this study was designed to evaluate the frequency of bacteremia after EUS-FNA and the effect of washing the gastrointestinal mucosa with 0.5% povidone-iodine solution on the occurrence of bacteremia. We also evaluated the effects of taking the PPIs on the frequency of bacteremia.
MATERIALS AND METHODS
Animals
A total of 28 healthy adult dogs (14 males and 14 females) with no gastrointestinal diseases were randomly assigned into three groups: control group, povidone-iodine group, and omeprazole group. The dogs in the omeprazole group were fed with 20 mg omeprazole orally twice a day for 3 days prior to the experiment. All dogs were fasted for 12 hours and water was inhibited for 6 hours before the examination. The dogs in the povidone-iodine group were administered with 10-mL 0.5% povidone-iodine solution washing the gastrointestinal mucosa before EUS-FNA. The dogs in the control group and the omeprazole group were administered with 10-mL 0.9% saline solution. The dogs were anesthetized by 0.1 mL/kg Sumianxin intramuscularly. Blood samples were collected for cultures and immunoglobulin levels were measured in all dogs of the three groups at the time of performing EUS-FNA. Factors that may influence the frequency of bacteremia were recorded, including duration of EUS procedure, number of FNA passes, duration of FNA procedure and maximal depth of aspiration.
EUS-FNA procedure
The EUS-FNA procedure was performed by an experienced echo-endoscopist using linear-array echoendoscopes from Japanese Pentax EG-3630UA with a probe frequency of 5-7.5 MHz, diasonography with Japanese Hitachi EUB-6500 and 22-G Wilson-Cook fine aspiration needles. Each dog was laid on a bed after administration of anesthesia and a detained needle was placed into a femoral artery. Canine pancreas was punctured under EUS guidance in order to avoid blood vessels (Fig. 1). Next, a 10-mL negative pressure syringe was connected to EUS after stylet of the needle was put out. The needle is gently moved back and forth at the biopsy site under EUS guidance for more than 10 times. And Suction force on the syringe is then released slowly and the aspirated specimen is expelled by the pushing stylet. The puncture procedure was repeated until the worm-like materials were got and sufficient for the histological examination.
Figure 1.

Procedure of EUS-FNA. The arrow points to the needle tip.
Microbiological examination
Detained needles were placed into a femoral artery for all the dogs prior to the EUS procedure. The principles of aseptic technique were strictly observed during the entire examination process; 0.5% povidone-iodine solution was used to disinfect the skin three times after skin preparation. Blood samples were collected before EUS, immediately after routine EUS, and 5 min, 15 min and 30 min after EUS-FNA. Each sample was 10 mL in volume, except for the first sample, which was 15 mL. Blood was injected into aerobic or anaerobic blood culture flasks; the extra 5-mL blood from the first collection was used for immunoglobulin examination. The blood culture flasks were incubated at 35 °C for 5-7 days. The cultures were considered positive for bacteremia if bacterial growth was observed at least once in five times.
Statistical analysis
The data were analyzed by independent sample t-test and Fisher exact test using SPSS 16.0 software. P<0.05 was considered statistically significant.
RESULTS
Fundamental parameters
Ten dogs in the control group (6 females, 4 males; mean weight 18 kg (range 8-24 kg), 10 dogs in the povidone-iodine group (5 females, 5 males; mean weight 21 kg, range 15-25 kg), and 8 dogs in the omeprazole group (3 females, 5 males; mean weight 19 kg (range15-23 kg) were included in this study and underwent EUS-FNA. There were no differences between the control group and either of the two experiment groups in terms of weight, number of passes, duration of EUS, duration of FNA, depth of aspiration, or levels of IgG or IgM (Table 1).
Table 1.
Experimental parameters in three groups (Mean ± SD)

Bacterial culture results
Coagulase negative Staphylococcus, Escherichia coli, Bacillus subtilis, negative Bacillus, Gram-positive Bacillus, Corynebacteria, Enterococcus and Gram-positive cocci were isolated from cultures; coagulase negative Staphylococcus, Bacillus subtilis, Gram-positive Bacillus, Corynebacteria and Gram-positive cocci were regarded as contamination from the skin. True bacteremia was considered caused by Escherichia coli, Enterococcus and negative Bacillus (Table 2).20,21,22,23,24
Table 2.
Bacterium cultured in dogs with positive bacteremia
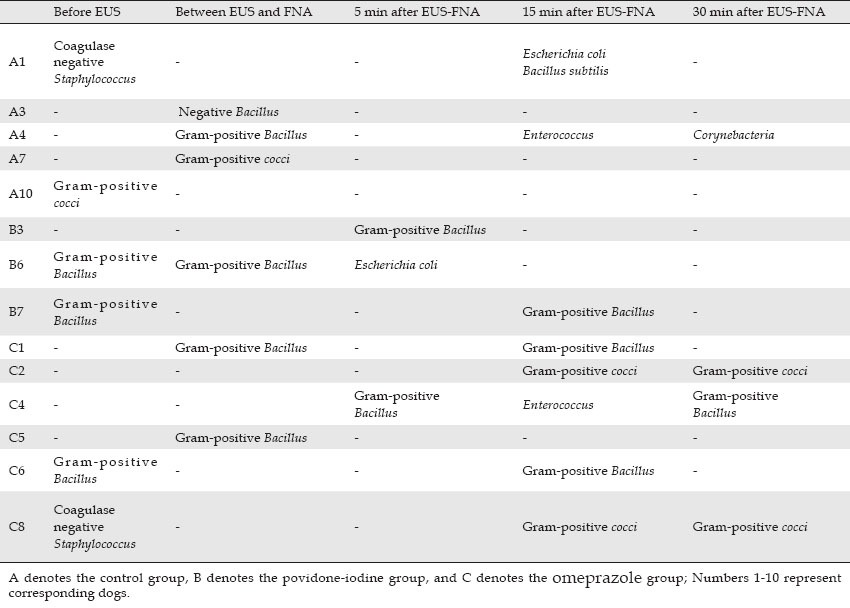
Four dogs in the control group, 3 dogs in the povidone-iodine group and 6 dogs in the omeprazole group exhibited bacteriu consistent with bacteremia. The contamination frequency of blood cultures was 12% (95%CI: 3.0%-29.0%), 10% (95%CI: 1.7%-18.3%), and 30% (95%CI: 15.8%-44.2%) in the control group, the povidone-iodine group, and the omeprazole group, respectively, and the overall frequency of the three groups was 16% (95%CI: 9.9%-22.1%) and in the total of all three groups, respectively.
Three dogs had true-positive bacteremia in the control group, with Escherichia coli or Enterococcus seen in 2 dogs 15 min after EUS-FNA and negative Bacillus in 1 dog between EUS and the FNA procedure. The frequency of bacteremia in the control group was 30% (95%CI: 7%-65%). In the povidone-iodine group, Escherichia coli was found in 1 dog 5 min after EUS-FNA, corresponding to a bacteremia frequency of 10% (95%CI: 0%-45%); Enterococcus was found in 1 dog 15 min after EUS-FNA in the omeprazole group, corresponding to a bacteremia frequency of 12.5% (95%CI: 0%-53%). There was no statistical significance in difference of the bacteremia frequency between the control group and either of the two experiment groups (P = 0.582, 0.383).
The control group was divided into two subgroups for further evaluation: true-positive bacteremia (Escherichia coli, Enterococcus and negative Bacillus culture results) subgroup and non-true-positive bacteremia subgroup (negative and contaminant blood culture results). We analyzed the potential factors influencing the frequency of bacteremia, including weight of dogs, duration of the EUS procedure, duration of the FNA procedure, number of times FNA completed, maximal depth of FNA and levels of immunoglobulin. However, the sample size in the experiment was small and the frequency of bacteremia was low, so effective analysis could not be performed (Table 3).
Table 3.
Potential factors influencing the frequency of bacteremia in the control group (Mean ± SD)

DISCUSSION
EUS-FNA is an important tool for the localization, diagnosis and interventional treatment of benign and malignant lesions of the esophagus, mediastinum, stomach, duodenum, cholangiopancreatic system and adrenal gland. EUS-FNA supplies cytological diagnosis of visual lesions under EUS. Owing to the increasing use of EUS-FNA, it is necessary to study potential infectious complications of the procedure. The process by which a 22- or 25-G needle penetrates into lesions through the gastric wall disturbs the integrity of the musosa and produces a pathway for the transmission of microorganisms that can result in transient bacteremia. If bacteria in the circulatory system settles on destroyed and abnormal cardiac valves, endocarditis may occur.25
In previous prospective studies of EUS-FNA, the frequency of bacteremia after EUS-FNA procedures was extremely low. In consideration of antibiotic resistance and the frequency of bacteremia, the ASGE advised that prophylactic administration of antibiotics was needed only for high-risk patients undergoing high-risk EUS-FNA procedures in order to reduce the occurrence of bacteremia, infection and related endocarditis.16,17 Nevertheless, EUS-FNA confers a potential risk of the development of bacterial endocarditis. The study by Sharon18 showed that povidone-iodine solution was an effective method for preventing bacteremia in NOTES. Povidone-iodine solution has broad sterilizing spectra, a long-lasting duration, and activity against viruses, bacteria, fungi and protozoa, and is effective against the previously-mentioned Escherichia coli and Enterococcus. If povidone-iodine solution effectively prevented the occurrence of bacteremia, it would be a safe and economic method for the prevention of infectious complications.
Acid-suppressing medications are increasingly used as clinical treatments for patients who are suffering from upper gastrointestinal diseases, many of whom may need to undergo an EUS-FNA procedure for definite diagnosis. Therefore, whether taking the PPIs will increase the incidence of bacteremia in these cases was a topic of concern.
In this study, 3 dogs in the control group, 1 dog in the povidone-iodine group and 1 dog in the omeprazole group developed true-positive bacteremia, excluding bacterial contamination. There was no statistically significant difference in the frequency of bacteremia between the povidone-iodine group and the control group (P = 0.582). Likewise, there was no statistically significant difference between the frequency of bacteremia in the omeprazole group and the control group (P = 0.383). There were no differences between the potentially influential parameters in the three groups, including weight of dogs, number of times FNA completed, duration of EUS, duration of FNA, depth of aspiration, or levels of IgG and IgM.
True-positive bacteremia occurred in 1 dog after the EUS procedure in the control group, which indicated that bacteremia was caused by the EUS procedure. Escherichia coli and Enterococcus were found in blood cultures of 2 dogs in the control group, Enterococcus in 1 dog in the omeprazole group, and Enterococcus in 1 dog in the povidone-iodine group 5 min after the FNA procedure, which suggested that the FNA procedure led to bacteremia, which generally lasted 5-15 min. In the present study, the total contamination rate was 16%, which is higher than that in other studies, which averaged about 3%.9,13,14,26 The possible reason is the higher amount of bacteria present in dogs’ hair than on adult human skin; furthermore, it is difficult to prepare and sterilize skin because of soft and fine hairs.
A study by Van de Mierop26 was the first reporting bacteremia after EUS-FNA. In this study, 3 in 15 patients who underwent EUS-FNA procedures developed transient bacteremia, as confirmed by blood cultures 15 and 30 min after the EUS-FNA procedure. In another prospective study by Barawi9 that included 100 patients, blood cultures were obtained 30 and 60 min after the EUS-FNA procedure; contaminated cultures were found in 6 patients without bacteremia or infectious complications, but because the blood collection was a little late, the possibility of missing the transient bacteremia should be considered. Levy's study13 showed 3 cases with bacteremia in 52 patients who underwent EUS-FNA. Janssen14 reported a 2% occurrence of bacteremia in the EUS group (n = 100), 4% in the EUS-FNA group (n = 50), and a total of 3% in the two groups. In our study, the frequency of bacterial growth was 17.9% (95%CI: 3.7%-32.1%), which is slightly higher than that in previous studies. The main reason for this finding is likely that more bacteria are present in dogs’ gastrointestinal tract and worse clearance of the gastrointestinal tract leads to an increased frequency of bacteria.
The potential factors influencing the frequency of bacteremia were not analyzed, owing to the small sample size and the low frequency of bacteremia. Therefore, potentially influential factors require further investigation.
In this study, only the frequency of bacteremia in the pancreas after an EUS-FNA procedure was evaluated. Therefore, it is necessary to study bacteremia after EUS-FNA of other organs. In the present study, the punctured pancreas was a normal organ without lesions, but the quality of lesions, such as cystic or solid lesions, may influence the occurrence of bacteremia. This factor should also be investigated in later studies.
In conclusion, there is no statistically significant difference in the frequency of bacteremia between either of the two experiment groups and the control group. For patients who are undergoing EUS-FNA, it is not necessary to wash the gastrointestinal mucosa with 0.5% povidone-iodine solution or stop use of the PPIs prior to the procedure.
REFERENCES
- 1.Bardales RH, Stelow EB, Mallery S, et al. Review of endoscopic ultrasound-guided fine-needle aspiration cytology. Diagn Cytopathol. 2006;34:140–75. doi: 10.1002/dc.20300. [DOI] [PubMed] [Google Scholar]
- 2.Nakazawa S. Recent advances in endoscopic ultrasonography. J Gastroenterol. 2000;35:257–60. doi: 10.1007/s005350050342. [DOI] [PubMed] [Google Scholar]
- 3.Pfau PR, Chak A. Endoscopic ultrasonography. Endoscopy. 2002;34:21–8. doi: 10.1055/s-2002-19394. [DOI] [PubMed] [Google Scholar]
- 4.Waxman I, Dye CE. Interventional endosonography. Cancer J. 2002;8(Suppl 1):S113–23. [PubMed] [Google Scholar]
- 5.Stanley MW. Endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol. 2003;120:309–10. doi: 10.1309/UKY9-XE21-EX59-NQLL. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MR. Endoscopic ultrasound-guided fine-needle aspiration. Cancer. 2004;102:203–6. doi: 10.1002/cncr.20486. [DOI] [PubMed] [Google Scholar]
- 7.O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–4. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 8.Wiersema M, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 9.Barawi M, Gottlieb K, Cunha B, et al. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189–92. doi: 10.1067/mge.2001.108966. [DOI] [PubMed] [Google Scholar]
- 10.Mierop FV, Bourgeois S, Leuven UG, et al. Bacteremia after EUS guided puncture: a prospective analysis [abstract] Gastrointest Endosc. 1999;49:AB100. [Google Scholar]
- 11.Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516–24. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;4:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MJ, Norton ID, Wiersema MJ, et al. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672–8. doi: 10.1067/mge.2003.204. [DOI] [PubMed] [Google Scholar]
- 14.Janssen J, Konig K, Knop-Hammad V, et al. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59:339–44. doi: 10.1016/s0016-5107(03)02707-x. [DOI] [PubMed] [Google Scholar]
- 15.Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–7. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 16.Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475–82. doi: 10.1067/s0016-5107(03)01883-2. [DOI] [PubMed] [Google Scholar]
- 17.ASGE STANDARDS OF PR ACTICE COMMITTEE. Banerjee S, Shen B, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791–8. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Bachman SL, Sporn E, Furrer JL, et al. Colonic sterilization for natural orifice translumenal endoscopic surgery (NOTES) procedures: a comparison of two decontamination protocols. Surg Endosc. 2009;23:1854–9. doi: 10.1007/s00464-008-0295-0. [DOI] [PubMed] [Google Scholar]
- 19.O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: results of a large case series. Endoscopy. 2009;41:593–7. doi: 10.1055/s-0029-1214868. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MP, Reller LB, Murphy JR, et al. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983;5:35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Jumaa PA, Chattopadhyay B. Pseudobacteraemia. J Hosp Infect. 1994;27:167–77. doi: 10.1016/0195-6701(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 22.Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–48. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 23.Thylefors JD, Harbarth S, Pittet D. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect Control Hosp Epidemiol. 1998;19:581–9. doi: 10.1086/647878. [DOI] [PubMed] [Google Scholar]
- 24.MacGregor RR, Beaty HN. Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med. 1972;130:84–7. [PubMed] [Google Scholar]
- 25.Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 1997;277:1794–801. [PubMed] [Google Scholar]
- 26.Van de Mierop F, Bourgeois S. Bacteremia after EUS guided puncture: a prospective analysis [abstract] Gastrointest Endosc. 1999;49:AB100. [Google Scholar]


